Summary
The past several years have seen major advances in the development of a safe and efficacious ricin toxin vaccine, including the completion of two Phase I clinical trials with two different recombinant A subunit (RTA)-based vaccines: RiVax™ and RVEc™ adsorbed to aluminum salt adjuvant, as well as a non-human primate study demonstrating that parenteral immunization with RiVax elicits a serum antibody response that was sufficient to protect against a lethal dose aerosolized ricin exposure. One of the major obstacles moving forward is assessing vaccine efficacy in humans, when neither ricin-specific serum IgG endpoint titers nor toxin-neutralizing antibody levels are accepted as definitive predictors of protective immunity. In this review we summarize ongoing efforts to leverage recent advances in our understanding of RTA-antibody interactions at the structural level to develop novel assays to predict vaccine efficacy in humans.
Keywords: toxin, biodefense, vaccine, protection, antibody, neutralizing, epitope
A. Perspective
The development of vaccines against lethal biological threat agents (biothreats) like ricin toxin poses unique challenges within the world of vaccinology [1,2]. Most notable among these is the need to assess vaccine worthiness when human efficacy trials are not feasible because of ethical and safety concerns. The Food and Drug Administration (FDA), which oversees vaccine regulatory concerns in the United States recognizes the inherent limitations associated with the development of medical countermeasures (MCM) for biodefense and has put forth guidelines for vaccines that rely on careful documentation of immune correlates in relevant animal models, preferably supported by descriptions of the mechanisms that underlie protection [3]. A persuasive case for vaccine licensure further necessitates validating specific responses observed in animal models to humans.
In this review we showcase recent advances in the development of ricin toxin subunit vaccines and highlight efforts to validate candidate vaccines using a combination of mouse and human immunology, structural biology and serology-based techniques. We argue that integrated approaches being used to develop a ricin toxin vaccine may serve as a prototype for other biothreat agents for which human efficacy trials are not feasible. This review will specifically focus on recent milestones in ricin vaccine development, notably Phase I clinical trials for two different subunit vaccines and immunity to aerosol challenge in a non-human primate model. The reader is referred to excellent recent reviews on other aspects of ricin, including incidence of human and animal exposures [4,5], biology of the toxin at the cellular and sub-cellular levels [6–8], animal models of ricin intoxication [9], immunity to ricin and preclinical vaccine development [10,11], and the design and development of post-exposure therapeutics [12].
B. Ricin toxin: Structure and function
Ricin is a member of the ribosome-inactivating protein (RIP) family of toxins found throughout the plant and microbial worlds [13,14]. Ricin toxin is found in the beans of the castor oil plant, Ricinus communis, which is ubiquitous in tropical and subtropical environments. While castor bean plants are often propagated as ornamentals in private and public garden spaces, they are most commonly grown for industrial applications. It is estimated that more than a million tons of castor beans are produced annually, with cultivation occurring mostly in India and Brazil. The oils extracted from the castor beans, notably ricinoleic acid, an unsaturated omega-9 fatty acid, are used in the production of industrial lubricants, cosmetics and even biofuels [15,16].
In its mature form, ricin is a 65 kDa glycoprotein consisting of two subunits, RTA and RTB, joined by a single disulfide bond [17–20]. RTA (267 amino acids) is an RNA N-glycosidase (EC 3.2.2.22) that selectively inactivates eukaryotic ribosomes through cleavage of a universally conserved ribosomal RNA element known as the sarcin-ricin loop (SRL) [21–23]. RTA assumes three distinct folding domains (residues 1–117, 118–210, and 211–267) [18,20] (Figure 1). The active site consists of a large, polar and solvent exposed cleft situated on one face of the molecule. Five residues, Tyr80, Tyr123, Arg180, Glu177, and Trp211 are critical for RTA’s RNA N-glycosidase activity [20,24–27]. One of the candidate ricin toxin subunit vaccines, RiVax, carries a point mutation in Tyr80 that attenuates RTA’s RNA N-glycosidase activity more than 1000 fold. The other candidate subunit vaccine, RVEc, is equally attenuated because of a deletion of folding domain 3 (residues 199–267).
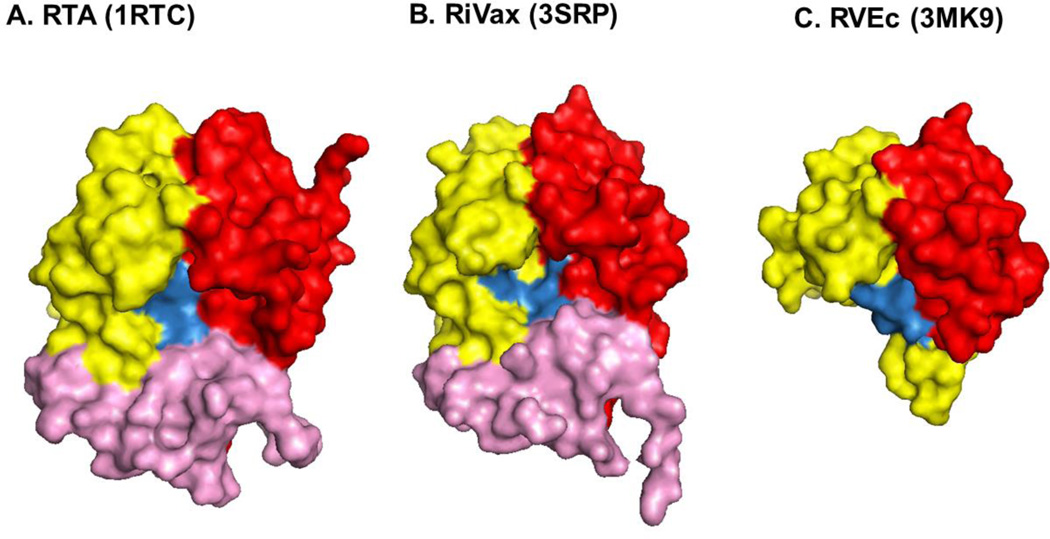
Figure 1. Structures of RTA, RiVax and RVEc.
The structures of recombinant (A) RTA, (B) RiVax and (C) RVEc are shown as surface representations generated using PyMOL from the PDB files indicated in parentheses and described in Table 1. RTA’s three folding domains (1–3) are colored in red, yellow and pink, respectively. The structures are orientated relative to RTA’s active site, which is highlighted in blue. The C-terminal truncation of RVEc relative to RTA and RiVax is evident by the absence of folding domain 3 (pink) and a slightly smaller “active site” region.
Ricin’s binding subunit, RTB is 262 amino acids in length and consists of two globular domains with identical folding topologies [18,28]. Domains 1 and 2 are themselves comprised of three homologous sub-domains (α, β, γ) that probably arose by gene duplication from a primordial carbohydrate recognition domain (CRD) [29]. Only sub-domains 1α and 2γ retain functional carbohydrate recognition activity [29,30]. Sub-domain 1α binds Gal and is considered a “low affinity” CRD, while sub-domain 2γ binds both Gal and GalNAc and is considered a “high affinity” CRD [20,31–33]. Both domains are involved in RTB’s ability to attach to cell surfaces [30,34].
The cytotoxic effects of ricin are well documented and have been recently reviewed [35,36]. Ricin is capable of attaching to and entering all mammalian cell types, including epithelial cells that line the respiratory and gastrointestinal tracts. Following attachment, the toxin is internalized into endosomes by clatherin-dependent and -independent mechanisms and then traffics, by a process known as retrograde transport, to the trans-Golgi network (TGN) and eventually the endoplasmic reticulum (ER). In the ER, RTA and RTB separate via a process involving protein disulfide isomerase (PDI) and ER degradation-enhancing α-mannosidase I-like protein 1 (EDEM1) [37–39]. RTA is then retrotranslocated across the ER membrane into the cytoplasm where refolding is facilitated by cytoplasmic chaperons (e.g., Hsc70) and possibly ribosomes themselves [6,40–42].
Once in the cytoplasm, RTA cleaves the N-glycosidic bond of a conserved adenosine residue within the SRL of 28S rRNA [23,43]. Hydrolysis of the SRL results in an immediate arrest in ribosome progression and a cessation in translation [22]. Damage to ribosomal RNA also activates the so-called ribotoxic stress response (RSR) involving stress-activated protein kinases (SAPK), leading to the production of pro-inflammatory cytokines and apoptosis-mediated cell death [44,45].
At the whole animal level, ricin is most toxic following injection or inhalation, with the latter route of exposure most relevant to the biodefense community [46,47]. The LD50 of ricin by aerosol exposure is estimated to be 4 µg/kg in mice [48] and 5.8 µg/kg in Rhesus macaques [46,49]. Mice and non-human primates succumb to 5–10 LD50 ricin exposure within 1–3 days; the ultimate cause of death has been attributed to respiratory failure. The recent Rhesus macaque studies conducted by Roy and colleagues underscore previous reports documenting the profound effects that ricin has on lung physiology, including massive fluid accumulation, inflammatory exudate, tissue edema, and hemorrhage [50,51]. For these reasons, eliciting a protective mucosal immune response against aerosolized ricin is considered one of the most significant challenges in ricin toxin vaccine development.
C. Preclinical studies of ricin toxin subunit vaccines, RiVax and RVEc
There are two candidate ricin toxin vaccines under development, RiVax™ and RVEc™. Both are recombinant, non-toxic derivatives of ricin’s enzymatic subunit, RTA (Table 1; Figure 1). RiVax, developed by Dr. Ellen Vitetta and colleagues at the University of Texas Southwestern (UTSW), is a full-length variant (267 amino acids) of RTA whose enzymatic activity has been eliminated (>1000 fold) through a single point mutation in a key active site residue (Y80). RiVax contains a second mutation at position 76 (V76M) as a means to eliminate RTA’s predilection to induce vascular leak syndrome (VLS) [26,52]. Structurally, RiVax is virtually identical to RTA (Table 1; Figure 1)[53].
Table 1.
Crystal structures relevant to ricin toxin vaccine development
| PDBa | Description | Reference |
|---|---|---|
| 2AAI | ricin | [20] |
| 1RTC | recombinant RTA | [105] |
| 3SRP | RiVax | [53] |
| 3MK9b | RVEc (RTA1–33/44–198) | [57] |
| Antibody-ricin Interactions | ||
| 4LGP | VHH E5-RTA complex | [97] |
| 4KUC | Fab 6C2-RTA complex | [102] |
Identification number assigned by the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Database (PDB);
, 3MK9 is a disulfide bond-stabilized variant of RVEc, as Compton and colleagues were unable to obtain X-ray crystallographic data of RVEc (1–33/44–198) itself.
RVEc, developed by investigators at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID), is a truncated version of RTA that lacks residues 199–267, as well as a small hydrophobic loop in the N-terminus (residues 34–43) (Table 1; Figure 1) [54–56]. RVEc was engineered to reduce RTA’s intrinsic propensity to aggregate and precipitate from solution. RVEc (also referred to as RTA1-33/44-198) lacks folding domain 3 and is only 188 residues in length. Despite the deletion/truncation, RVEc’s atomic structure is essentially superimposable onto RTA (Table 1: Figure 1)[57]. The fact that RVEc assumes RTA’s normal tertiary structure across folding domains 1 and 2 (despite the absence of folding domain 3) is certainly integral to the success of the vaccine, as a number of potent toxin-neutralizing antibodies are known to target conformation-dependent epitopes within folding domains 1 and 2 [58].
Over the past 10 years, both RiVax and RVEc have been subject to extensive preclinical testing, including efficacy and toxicity studies in mice and rabbits. Dr. Ellen Vitetta and colleagues demonstrated that RiVax elicits protective immunity against systemic and mucosal (aerosol and intragastric) challenges when administered to mice by the subcutaneous, intramuscular, intradermal or intraperitoneal routes [11,26,48,52,59,60]. RVEc is also highly efficacious in the mouse and rabbit models at stimulating systemic and mucosal immunity to ricin [55,56,61,62].
Although RVEc and RiVax have each been under investigation for more than a decade, it wasn’t until just recently that the two vaccines were compared to side-by-side in the same study [63]. Groups of mice were vaccinated by the subcutaneous route across a range of doses (0.3–10 µg) with RVEc and RiVax adsorbed to Alhydrogel. Animals were challenged with 10 LD50 ricin two weeks after the second or third vaccinations. The study revealed that in the mouse model the two vaccines were essentially identical. All vaccinated animals survived challenge, even groups that received only a prime boost regimen with the lowest dose of vaccine. Although RVEc was slightly more effective than RiVax at eliciting toxin-specific serum IgG titers and toxin-neutralizing activity (TNA) after only two immunizations, this trend was not apparent in a more recent study in which RiVax and RVEc were administered to mice by the intradermal routes [64]. While the two vaccines have not been tested head to head in the mouse model using the intramuscular route of vaccination to mimic the route by which the vaccines would be given to humans, there is no reason to expect that they would differ under such circumstances.
D. Outcome of Phase I clinical trials with RiVax and RVEc
The recent completion of two Phase I clinical trials in which RiVax and RVEc, each adsorbed to Alhydrogel, were administered to healthy volunteers suggest that the two vaccines perform similarly to each other in humans [65,66]. The two trials are summarized in Table 2. RiVax was adsorbed to 0.1% Alhydrogel and administered intramuscularly (i.m.) to groups of healthy adults (n=5 per group) at three different dosages (1, 10 or 100 µg) on days 0, 42 and 180. RVEc was adsorbed to 0.2% Alhydrogel and administered i.m. to groups of healthy adults (n=10 per group) at three doses (20, 50 and 100 µg) and on days 0, 28 and 56. In both studies the primary endpoints for assessing safety were occurrences of adverse events (AE). Immunogenicity was based on ricin-specific serum IgG levels determined by ELISA, as well as toxin-neutralizing activity (TNA) measured using mammalian cell-based cytotoxicity assays. Unfortunately, direct comparison of the two studies is slightly complicated because of different methodologies used to assess endpoint titers and TNA. Vitetta and colleagues report specific IgG (µg/ml) based on RiVax-coated ELISA plates, whereas Pittman and colleagues reported geometric mean endpoint titer (GMT) using ricin-coated plates. Vitetta and colleagues assessed TNA using human B cell lymphoma (Daudi) cells [26], while Pittman and colleagues employed mouse T cell lymphoma (EL4) cells [67]. Vitetta reported TNA as the reciprocal endpoint dilution that corresponded to the IC50; Pittman reported reciprocal endpoint dilution that conferred neutralizing activity above background. Despite these differences in study design and methodologies, there are obvious comparisons that can be made.
Table 2.
Comparison of RiVax and RVEc Phase I and NHP Studies
| Humans | R. macaques | ||
|---|---|---|---|
| RVEca | RiVaxa | RiVaxa | |
| Trial IDb |
NCT01317667 NCT01846104 |
NCT00812071 | |
| Group size (n=) | 10 | 5 | 12 |
| Groups (µg) | 20, 50, 100 | 1,10,100 | 100 |
| Vaccination-days | 0, 28, 56 | 0, 42, 180 | 0, 30, 60 |
| AEc | 2/30 | 1/14 (Grade III) | n.a. |
| Peak endpoint-day | 84 | 210 | 75 |
| Peak titers | 4500–4800d | 25–260 µg/ml | 1534 µg/ml |
| Peak TNA-day | 70 | n.r. | 110 |
| Peak TNA-titer | 73–78e | 20–24f | 1280f |
| Ab half life-day | 100 | 180 | n.r. |
| Reference | [65] | [66] | [47] |
Adsorbed to Alhydrogel;
clinicaltrials.gov identifier number(s);
Adverse events (AE). In the RiVax trial, s all volunteers experienced Grade I toxicities, one individual suffered Grade II headache and Grade III nausea. In the RVEc trial, one individual suffered shoulder injury related to vaccine administration, while another suffered rhabdomyolysis;
refers to GMT of reciprocal endpoint determined by ELISA using ricin-coated plates;
reciprocal toxin-neutralizing endpoint titers (IC50) established in Daudi cells;
reciprocal dilution of serum that conferred positive toxin-neutralizing activity in EL4 cells;n.a., not applicable, n.r., not reported.
Safety
RiVax was similar to RVEc in that the vast majority (80–100%) of study participants reported mild (grade I) systemic AE following vaccination. However, two subjects in the high dose RVEc group (100 µg) did experience grade IV elevation of creatine phosphokinase (CPK) levels after the first vaccination. The reasons for the elevated CPK levels in 2 of 10 subjects is unknown, but Pittman and colleagues make the case that the effects were unlikely to be related to RVEc antigen.
Seroconversion frequencies
Seroconversion frequencies between RiVax and RVEc at comparable dose levels were virtually identical. While the lowest dose of RiVax (1.0 µg) tested did not elicit seroconversion in any of the vaccinees, 4/5 individuals that received 10 µg RiVax and 5/5 that received 100 µg RiVax had seroconverted 2 weeks after the third vaccination. In the RVEc study, 10/10 (100%) and 9/10 (90%) individuals in the 20 and 50 µg groups, respectively, seroconverted after the third vaccination. It is interesting to note that the best seroconversion rates occurred in the middle (not highest) dose ranges. As a case in point, 10 days after the second vaccination, 9/10 individuals in the 20 µg dose RVEc group had seroconverted, whereas only 7/10 (70%) in the 50 µg group had seroconverted.
Peak toxin-specific serum IgG titers
In both the RiVax and RVEc clinical trials, peak ricin-specific serum IgG titers occurred 2–4 weeks after the third vaccination. Because of differences in reporting ricin-specific serum IgG levels between the two studies, a direct comparison of absolute titers is not possible.
TNA
TNA is the indicator most closely associated with immunity to ricin, and therefore a key measure of vaccine efficacy. Unfortunately, neither RiVax nor RVEc were particularly effective at eliciting TNA in human volunteers. In the RVEc study, only 40–50% of the vaccinated individuals in the 20 µg and 50 µg groups had detectable TNA in their sera after the third vaccination [67]. RiVax-vaccinated individuals (10 and 100 µg groups) fared slightly better in that all 8 vaccinated individuals had detectable TNA, although their actual titers were very low (e.g., 20–24) [66].
Ricin-specific IgG and TNA
A historically confounding issue in mouse studies and a pilot Phase I study with RiVax antigen alone (not adjuvanted) has been an apparent disconnect between ricin-specific endpoint titers and TNA [63,68]. For example, in the RiVax pilot Phase I trial, volunteer 13’s ricin-specific serum IgG levels were 4.7 µg/ml and toxin-neutralizing titers were 1.4 µg/ml [68]. Volunteer 7, on the other hand, had 22 µg/ml of ricin-specific serum IgG but only 1.7 µg/ml toxin-neutralizing titers. This disconnect was evident in the subsequent Phase I clinical trials. Pittman performed a Pearson correlation analysis examining ELISA units versus TNA from RVEc vaccinated individuals, which yielded an r value of 0.71 [65]. A similar analysis of RiVax-vaccinated individuals revealed an r value of 0.81, although that number was adjusted to eliminate a pair of outliers from the analysis [66]. The exact relationship between ricin-specific endpoint titers and toxin-neutralizing activity is an issue that needs to be resolved in future clinical studies.
Half-life of ricin-specific serum IgG
In both cases, toxin-specific serum IgG titers dropped steadily 2–4 weeks after the final vaccination, with half-lives between 100 and 180 days.
Passive transfer studies
Unfortunately, neither of the recent Phase I clinical trials included passive transfer studies to examine whether sera from humans vaccinated with RiVax or RVEc are sufficient to neutralize ricin in a mouse challenge model. Passive transfer studies were, however, done as part of a former pilot Phase I clinical trial with RiVax (without adjuvant), which demonstrated that high amounts (e.g., 25–60 µg) of human RiVax IgG antibodies were able to neutralize the effects of ricin in a mouse model[68].
Conclusions from RiVax and RVEc Phase I clinical trials
In summary, RiVax and RVEc were deemed comparatively safe and immunogenic in humans. However, toxin-specific antibody levels demonstrated short half-lives and neither vaccine proved particularly effective at eliciting toxin-neutralizing activity. While both these issues could potentially be resolved with more potent adjuvant(s) (see Section F), the ultimate success of the ricin vaccine program also necessitates more established surrogate and/or correlate markers of protection that can be used to assess vaccine efficacy in humans.
E. Vaccine-induced immunity to aerosolized ricin challenge in non-human primates
A recently completed RiVax vaccination/aerosol challenge study conducted in Rhesus macaques constitutes a milestone in the development of ricin subunit vaccine [47]. The study consisted of two cohorts of nine Rhesus macaques (Table 2). In each cohort six animals received 100 µg RiVax adsorbed to Alhydrogel and administered by intramuscular injection on days 0, 30 and 60. Three animals in each cohort served as controls and were not vaccinated. On day 110, the macaques were subject to aerosolized ricin challenge (1.5–6.5 LD50). Serum samples collected over the course of the study were examined for seroconversion, ricin-specific IgG levels and TNA.
Serum analysis studies indicated that all 12 RiVax-vaccinated animals had seroconverted two weeks after the second vaccination (day 45), with peak endpoint titers observed two weeks after the third immunization (1534 µg/ml RiVax-specific IgG; range 1190–1977µg/ml). Toxin-neutralizing titers achieved a geometric mean TNA endpoint titer of 427 (163–1132 range) two weeks after the third immunization. The TNA values increased an additional 3-fold (1280) in the ensuing month, indicating that toxin-neutralizing antibodies continued to mature for weeks after the third vaccination, a phenomenon that we have observed in mice [69]. Following aerosolized toxin challenge, control animals expired or were euthanized within 3 days, The RiVax-vaccinated animals all survived, except for one which died from complications of a secondary infection and not a direct consequence of ricin intoxication.
The study by Roy and colleagues is the first report in the literature demonstrating that parenteral vaccination of non-human primates with a recombinant, aluminum salts adsorbed RTA-based subunit vaccine is sufficient to confer protective immunity to an aerosolized ricin challenge. All previous vaccination/aerosol challenge studies were conducted in in mice, rats or rabbits [48,59,62]. The demonstration that Rhesus macaques can be rendered immune to inhaled ricin justifies the continued development of RiVax (and/or RVEc) for use in humans and downplays the need to identify alternative vaccine antigens. In our opinion, resources can now be allocated to better defining (i) correlates of immunity, (ii) optimizing vaccine formulations, (iii) defining route and optimal timing of vaccinations, and (iv) identifying compatible adjuvants [2].
The second important aspect of the study by Roy and colleagues is the demonstration that parenteral vaccination is sufficient to confer local immunity to a toxic agent like ricin, which is known to affect the respiratory epithelium and elicit massive pulmonary inflammation [46]. The suggestion from the study is that ricin was neutralized in the lung by toxin-specific serum IgG antibodies that had gained access to the respiratory compartment before or immediately following toxin exposure through transudation or possible FcR-mediated transport [70]. While a formal role for ricin-specific IgA in protection cannot be ruled out, parenteral vaccination is known not to stimulate a secretory antibody response. To our knowledge, there is only one other report that demonstrates in a non-human primate model that respiratory immunity to a biological toxin can be achieved through parenteral immunization. The other report entailed three intramuscular vaccinations of Rhesus monkeys with staphylococcus enterotoxin B (SEB) toxoid, which afforded protection against subsequent aerosol challenge [71]. Protective immunity correlated with circulating anti-SEB IgG antibody titers, further substantiating the importance of serum IgG in mucosal immunity to biothreat toxins.
Finally, some comparisons can be made regarding the relative magnitudes of the toxin-specific responses between the Rhesus macaques and human volunteers that received RiVax [47,66]. However, it must be underscored that the human vaccine study was conducted with freshly prepared RiVax antigen adsorbed to Alhydrogel on the day of vaccination (E. Vitetta, personal communication) [66], whereas the NHP study was conducted using a lyophilized glassy solid formulation of RiVax adsorbed to aluminum salts adjuvant [47,72]. The monkeys had peak toxin-specific serum IgG levels following RiVax vaccination that were on average 10 times greater than what was observed in RiVax-vaccinated humans (Table 2). At the time of challenge, however, toxin-specific serum IgG levels had declined to 267–499 µg/ml and were therefore just 2–3 times over the levels that were achieved in several of the humans that had received three vaccinations with RiVax (256–280 µg/ml). Because TNA in the monkeys were reported as endpoint titers, while the human samples were reported as micrograms per milliliter it is not possible to directly compare the two species in terms toxin-neutralizing antibodies [47,66]. This fact underscores the need for the field as a whole to establish standardized and validated assays so that critical parameters like toxin neutralization can be compared across species and across different clinical trials.
F. Key challenges moving forward in ricin toxin vaccine development
In addition to the need to establish standardized and universally accepted assays in the ricin research community, there are at least three additional challenges associated with the further development of a ricin toxin subunit vaccine.
The first pertains to subunit stability. RTA is purportedly highly unstable in solution and has a propensity to unfold and aggregate, a characteristic that would severely limit the shelf life of a candidate ricin vaccine [55,56,73]. In the case of RVEc, the issue of protein stability has been addressed at the genetic level through elimination of domains and loops involved in unfolding [56]. RVEc has been further engineered to include stabilizing disulfide bonds [74]. In the case of RiVax, the stabilization issue has been addressed through lyophilization technology [72]. RiVax adsorbed to aluminum hydroxide solutions in histidine or ammonium acetate buffers were lyophilized with trehalose as a stabilizing excipient. The lyophilized vaccine preparations were stable at 40°C for more than 15 weeks without an effect on immunogenicity or potency, as measured in the mouse model. While a rigorous systematic stability/potency study of lyophilized RiVax has not been reported in the literature, it is clearly efficacious because the lyophilized version of RiVax was administered to the Rhesus macaques that survived aerosol challenge [47].
The second major challenge associated with ricin toxin subunit vaccine development relates to adjuvanticity. It is apparent from the Phase I trials that neither RVEc nor RiVax adsorbed to Alhydrogel is sufficiently robust in humans, as evidenced by the fact that serum IgG titers were slow to peak and TNA were either not detectable or just above baseline even after a prime and two boosts [65,66]. Investigations into the effects of different “next generation” adjuvants like AS01 on RiVax and RVEc is clearly warranted [75–77]. Towards this goal we recently demonstrated that LT-IIa and LT-IIbT13I, two related ADP-ribosylating enterotoxins from E.coli are potent adjuvants when combined with RiVax and RVEc and administered to mice by the intradermal route [64,78]. It remains to be determined whether either of these adjuvants is active in humans.
The third obstacle associated with ricin toxin subunit vaccine development relates to assessing RiVax and RVEc’s efficacy in humans in the absence of human challenge studies, especially considering that the exact correlates of immunity to ricin remain poorly understood. On the one hand, the importance of toxin-neutralizing antibodies in protection against systemic and mucosal ricin exposure is indisputable. In just the past ten years, there have been more than a dozen reports demonstrating that passive administration of a toxin-neutralizing monoclonal antibody (mAb), polyclonal antibody (pAb) mixture or Fab fragments is sufficient to render mice impervious to a lethal dose of ricin administered by injection, ingestion or inhalation [79–88]. On the other hand, toxin-specific serum IgG levels, at least in mice, are not reliable indicators of immunity. We have observed that mice with similar or even identical toxin-specific endpoint titers, typically ranging from 1,000–10,000, experience different fates upon ricin challenge: some survive while others succumb at rates only marginally better than unvaccinated control animals, suggesting that the “quality” rather than the “quantity” is the primary factor in determining protection.
At the present time the sole measure of the “quality” of an anti-ricin antibody response is based on in vitro toxin-neutralizing activity (TNA) using mammalian cell-based cytotoxicity assays [89]. In dozens of experiments involving hundreds of mice conducted with RiVax and RVEc over the past three years, we have found that animals with detectable serum TNA are virtually guaranteed to survive a lethal dose ricin challenge [63,64,69,78]. In this respect, TNA can be considered an absolute correlate of protection, which Plotkin defines as “a quantity of a specific immune response to a vaccine that always provides near 100% protection” [90].
The problem is that TNA in and of itself may be too stringent a threshold by which to judge protection, because it is well documented in mice that survival following a lethal dose ricin challenge can occur in the absence of detectable TNA [63,64,69,78]. As a case in point, O’Hara and colleagues vaccinated groups of mice (n=10 per group) two times at monthly intervals with 0.3 microgram of RiVax or RVEc adsorbed to Alhydrogel, resulting in mean reciprocal endpoint titers that were 40–80,000 [63]. As it turns out all vaccinated animals survived 10xLD50 ricin toxin challenge toxin challenge, even though none had detectable TNA in their sera. We speculate that the levels of circulating toxin-neutralizing antibodies are simply below the limit of detection in our current cell-based assays [81]. Alternatively, we cannot formally rule out the possibility that a polyclonal mixture of “non-neutralizing” antibodies (as determined using current in vitro assays) may have the potential to inactivate ricin in vivo through mechanisms that have not yet been identified. For example, neutralization of anthrax toxin is modulated by FcR-mediated clearance though the action of specific IgG subclasses [91,92]. In the case of ricin toxin, the contribution of Fc-mediated neutralizing activity in vivo remains up for debate [84,87,93]. Indeed, very little is known about the exact mechanisms by which ricin is neutralized in vivo.
G. Establishing surrogate markers of immunity to ricin
The concept of serum antibody profiling or immune signature analysis is an emerging application in vaccinology and may be ideally suited to the ricin vaccine community [94–96]. Based on work from our laboratory, as well as several other laboratories, evidence suggests that, in the case of ricin, toxin-neutralizing antibodies constitute a small fraction (10%) of the antibody response elicited by RiVax and RVEc, and neutralizing antibodies target a very limited number of “hotspots” on the surface of RTA, which we refer to as epitope Clusters I-IV (Figure 2) [58,81,97]. So-called Cluster I was first identified by Lemley and colleagues as the target of the potent ricin-neutralizing mouse mAb UNIVAX 70 or simply R70 [98]. Peptide array analysis indicated that R70 recognizes a linear epitope corresponding to RTA’s α-helix B (residues 97–107) [99,100]. We identified a second murine mAb known as PB10 that binds the same peptide, and is as effective as R70 at neutralizing ricin in vitro and in vivo [81]. High resolution epitope mapping studies using a phage displayed peptide library demonstrated that PB10 and R70 recognize slightly different epitopes, even though they are focused on the same α-helix and bind the same peptide [101]. Recent X-ray crystallographic studies of toxin-antibody complexes have enabled atomic level resolution of epitopes within Cluster I. Zhu et al. solved the structure of RTA in complex with Fab fragments from a PB10-like mAb called 6C2 [102], which revealed antibody contact primarily with α-helix B (residues 97–107).
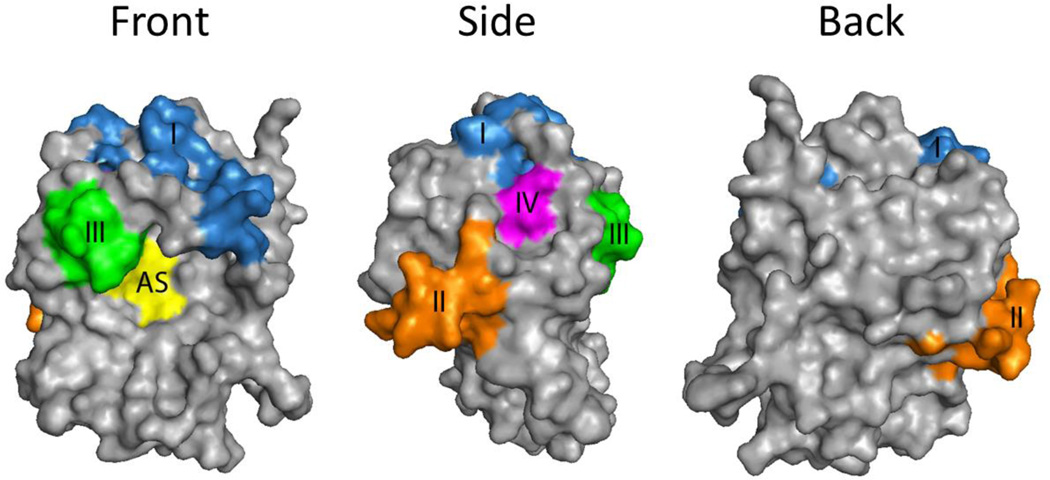
Figure 2. Four Proposed Neutralizing Epitope Clusters on RTA.
RTA orientated with respect to the active site (AS; yellow). The neutralizing clusters (I-IV), which are distributed across the surface of the protein, are color coded and described in the text.
To interrogate Cluster I in more detail, we screened a library of single domains camelid antibodies (VHH) from RiVax- and ricin toxoid-vaccinated alpacas [103]. Of the eleven anti-RTA VHHs identified in our first screen, seven were directed against Cluster I. Six were originally classified as Cluster I binders based on competitive ELISAs with PB10, while the seventh’s epitope was revealed through X-ray crystallography [97]. A second screen of the same VHH library identified an additional seven novel anti-RTA VHHs, six of which are Cluster I-specific (D. Vance, J. Tremblay, C. Shoemaker, N. Mantis, unpublished results). To date, seven X-ray crystal structures of Cluster I VHHs in complex with RTA have been solved [97] (M. Rudolph, D. Vance, M. Cassidy, Y. Rong, C. Shoemaker, and N. Mantis, manuscript submitted). All seven VHHs differ in the degree to which they make contact with β-strand h (residues 113–117), α-helix D (residues 150–157), and α-helix B (residues 97–107).
The other three epitope Clusters (II-IV) were originally defined based on our collection of “legacy” mAbs SyH7, IB2 and GD12 [58,79,81]. Cluster II is defined by SyH7, which recognizes a linear epitope situated within α-helix F (residues 187–198), Cluster III by IB2, which recognizes a discontinuous epitope involving α-helices C and possibly G (R. Toth, D. Weis, D. Volkin and N. Mantis, manuscript in preparation) and, finally, Cluster IV by GD12, which recognizes a linear epitope situated within α-helix E (residues 163–174). The identification of additional Cluster II- and III-specific mAbs, as well as numerous VHHs that target epitopes in or adjacent to those regions, has enabled us to define using X-ray crystallography, hydrogen deuterium exchange (HDX) and competition ELISAs, neutralizing and non-neutralizing epitopes within these two regions of RTA (D. Vance, M. Rudolph, G. Van Slyke, D. Weis, R. Toth, D. Volkin, C. Shoemaker, N. Mantis, manuscripts in preparation). Cluster IV is interesting because it was first identified as a target of toxin-neutralizing antibodies elicited in Hodgkin’s lymphoma patients who had received RTA-immunotoxin therapy [104]. However, GD12 remains the only mAb known that is directed against this region.
To begin to assess the utility of immune profiling analysis in ricin vaccine, sera from RiVax-vaccinated macaques and Phase I clinical trial volunteers were subjected to direct competition ELISAs with toxin-neutralizing mAbs directed against Cluster I (WECB2, PB10, R70) and Cluster II (PA1, SyH7) [47]. We reasoned that the degree to which an antiserum reduced binding of Cluster I and II-specific mAbs would serve as an indirect indicator of epitope utilization. Indeed, we observed that antisera from RiVax-vaccinated Rhesus macaques and humans reduced binding of Cluster I and II-specific mAbs by 10–80% depending on the specific mAb. Monkeys and humans had similar inhibitory profiles suggesting the antibody response is conserved between the two species. While these data are encouraging and suggestive of the possibility of serum antibody profiling being useful in assessing vaccine efficacy, the connection between mAb competition, toxin-neutralizing activity and protection has yet to be made.
One final point worth mentioning on the topic of B cell epitopes relates to how it is that RVEc is as effective as RiVax at eliciting protective immunity to ricin, considering that RVEc lacks RTA’s C-terminus (residues 199–267) and contains a deletion of residues 33–43. We would argue that the apparent immunodominant nature of Cluster I explains why RiVax and RVEc are essentially identical to each other. Specifically, β-strand h (residues 113–117), α-helix D (residues 150–157), and α-helix B (residues 97–107) are completely intact in RVEc. If Cluster I antibodies dominant the response to RiVax and RVEc vaccination, then one would not expect there to be a qualitative difference in the two vaccines. Indeed, that was exactly what we observed experimentally. If anything, we have suggested that the compact nature may actually focus the antibody response against α-helix B, although this has yet to be substantiated [63].
H. Expert Commentary and Five-year view
In summary, the past five years has seen major advances in terms of development of a ricin toxin subunit vaccine. Two clinical trials were completed with two different recombinant RTA-based vaccines, RVEc and RiVax [65,66]. In addition, a non-human primate study with RiVax demonstrated the vaccine’s potential to elicit immunity to aerosolized ricin challenge [47]. Finally, the first atomic level insights into the nature of neutralizing B cell epitopes on RTA emerged [97,102].
Key Issues.
We would argue that the success of a ricin vaccine will only occur if the research community continues along this multidisciplinary and highly integrated approach to vaccine development, as bulleted below:
It is imperative that the ELISA and TNA methodologies are standardized across institutions and that uniform criteria are applied to what constitutes neutralizing activity and protection;
It is critical that the relationship total toxin-specific antibody levels, TNA, and protective immunity be better defined;
It is essential that the quantitative thresholds associated with protection be established in mice and non-human primates, and validated in humans.
Novel approaches like serum antibody profiling may afford an alternative or at least complement cell based toxin-neutralizing assays;
A head-to-head comparison between RVEc and RiVax in non-human primates and/or humans should be conducted;
RVEc and/or RiVax should be tested in combination with new adjuvants in an effort to accelerate the onset and magnitude of TNA.
Acknowledgments
This work was supported in part by Contract No. HHSN272201400021C from the National Institutes of Allergy and Infectious Diseases.
We thank Dr. Michael Rudolph at the New York Structural Biology Center, and Drs. Ronald Toth IV, David Weis, David Volkin at the University of Kansas allowing us to cite unpublished results in this review. We thank Greta Van Slyke, Yinghui Rong, and Jennifer Westfall at the Wadsworth Center for thoughtful discussions.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Reisler RB, Smith LA. The need for continued development of ricin countermeasures. Adv Prev Med. 2012;2012:149737. doi: 10.1155/2012/149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe DN, Florence W, Bryant P. Current biodefense vaccine programs and challenges. Human vaccines & immunotherapeutics. 2013;9(7):1591–1597. doi: 10.4161/hv.24063. [DOI] [PubMed] [Google Scholar]
- 3.Approval of Biological Products When Human Efficacy Studies Are Not Ethical or Feasible: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=601&showFR=1&subpartNode=217.0.1.1.2.8)
- 4.Griffiths GD. Understanding ricin from a defensive viewpoint. Toxins (Basel) 2011;3(11):1373–1392. doi: 10.3390/toxins3111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worbs S, Kohler K, Pauly D, et al. Ricinus communis Intoxications in Human and Veterinary Medicine-A Summary of Real Cases. Toxins (Basel) 2011;3(10):1332–1372. doi: 10.3390/toxins3101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spooner RA, Lord JM. How Ricin and Shiga Toxin Reach the Cytosol of Target Cells: Retrotranslocation from the Endoplasmic Reticulum. Curr Top Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 7.Tesh VL. The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol. 2012;357:137–178. doi: 10.1007/82_2011_155. [DOI] [PubMed] [Google Scholar]
- 8.Tumer NE, Li XP. Interaction of ricin and Shiga toxins with ribosomes. Curr Top Microbiol Immunol. 2012;357:1–18. doi: 10.1007/82_2011_174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy CJ, Song K, Sivasubramani SK, Gardner DJ, Pincus SH. Animal Models of Ricin Toxicosis. Curr Top Microbiol Immunol. 2012 doi: 10.1007/82_2011_173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Hara JM, Yermakova A, Mantis NJ. Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol. 2012;357:209–241. doi: 10.1007/82_2011_193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallshaw JE, Vitetta ES. Ricin Vaccine Development. Curr Top Microbiol Immunol. 2012;357:259–272. doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- 12.Wahome PG, Robertus JD, Mantis NJ. Small-molecule inhibitors of ricin and Shiga toxins. Curr Top Microbiol Immunol. 2012;357:179–207. doi: 10.1007/82_2011_177. [DOI] [PubMed] [Google Scholar]
- 13.Mantis NJ. Ricin Toxin. In: Liu D, editor. Manual of Security Sensitive Microbes and Toxins. CRC Press; 2014. p. 1024. [Google Scholar]
- 14.Schrot J, Weng A, Melzig MF. Ribosome-inactivating and related proteins. Toxins (Basel) 2015;7(5):1556–1615. doi: 10.3390/toxins7051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Final report on the safety assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate. Int J Toxicol. 2007;26(Suppl 3):31–77. doi: 10.1080/10915810701663150. [DOI] [PubMed] [Google Scholar]
- 16.da Silva Nde L, Maciel MR, Batistella CB, Maciel Filho R. Optimization of biodiesel production from castor oil. Appl Biochem Biotechnol. 2006;129–132:405–414. doi: 10.1385/abab:130:1:405. [DOI] [PubMed] [Google Scholar]
- 17.Katzin BJ, Collins EJ, Robertus JD. Structure of ricin A-chain at 2.5 A. Proteins. 1991;10(3):251–259. doi: 10.1002/prot.340100309. [DOI] [PubMed] [Google Scholar]
- 18.Montfort W, Villafranca JE, Monzingo AF, et al. The three-dimensional structure of ricin at 2.8 A. Journal of Biological Chemistry. 1987;262(11):5398–5403. [PubMed] [Google Scholar]
- 19.Robertus JD, Piatak M, Ferris R, Houston LL. Crystallization of ricin A chain obtained from a cloned gene expressed in Escherichia coli. Journal of Biological Chemistry. 1987;262(1):19–20. [PubMed] [Google Scholar]
- 20.Rutenber E, Katzin BJ, Ernst S, et al. Crystallographic refinement of ricin to 2.5 A. Proteins. 1991;10(3):240–250. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- 21.Olsnes S, Fernandez-Puentes C, Carrasco L, Vazquez D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem. 1975;60(1):281–288. doi: 10.1111/j.1432-1033.1975.tb21001.x. [DOI] [PubMed] [Google Scholar]
- 22.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J. Biol. Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 23.Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262(17):8128–8130. [PubMed] [Google Scholar]
- 24.Monzingo AF, Robertus JD. X-ray analysis of substrate analogs in the ricin A-chain active site. J Mol Biol. 1992;227(4):1136–1145. doi: 10.1016/0022-2836(92)90526-p. [DOI] [PubMed] [Google Scholar]
- 25.Rutenber E, Robertus JD. Structure of ricin B-chain at 2.5 A resolution. Proteins. 1991;10(3):260–269. doi: 10.1002/prot.340100310. [DOI] [PubMed] [Google Scholar]
- 26.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20(27–28):3422–3427. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 27.Weston SA, Tucker AD, Thatcher DR, Derbyshire DJ, Pauptit RA. X-ray structure of recombinant ricin A-chain at 1.8 A resolution. J Mol Biol. 1994;244(4):410–422. doi: 10.1006/jmbi.1994.1739. [DOI] [PubMed] [Google Scholar]
- 28.Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. Faseb J. 1994;8(2):201–208. [PubMed] [Google Scholar]
- 29.Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326(6113):624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- 30.Swimmer C, Lehar SM, McCafferty J, Chiswell DJ, Blattler WA, Guild BC. Phage display of ricin B chain and its single binding domains: system for screening galactose-binding mutants. Proc. Natl. Acad. Sci U S A. 1992;89(9):3756–3760. doi: 10.1073/pnas.89.9.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J. Biol. Chem. 1979;254(19):9795–9799. [PubMed] [Google Scholar]
- 32.Newton DL, Wales R, Richardson PT, et al. Cell surface and intracellular functions for ricin galactose binding. J Biol Chem. 1992;267(17):11917–11922. [PubMed] [Google Scholar]
- 33.Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978;536(1):18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 34.Sphyris N, Lord JM, Wales R, Roberts LM. Mutational analysis of the Ricinus lectin B-chains. Galactose-binding ability of the 2 gamma subdomain of Ricinus communis agglutinin B-chain. J. Biol. Chem. 1995;270(35):20292–20297. doi: 10.1074/jbc.270.35.20292. [DOI] [PubMed] [Google Scholar]
- 35.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 36.Spooner RA, Lord JM. Ricin trafficking in cells. Toxins (Basel) 2015;7(1):49–65. doi: 10.3390/toxins7010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominska-Wojewodzka M, Gregers TF, Walchli S, Sandvig K. EDEM is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol Biol Cell. 2006;17(4):1664–1675. doi: 10.1091/mbc.E05-10-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokolowska I, Walchli S, Wegrzyn G, Sandvig K, Slominska-Wojewodzka M. A single point mutation in ricin A-chain increases toxin degradation and inhibits EDEM1-dependent ER retrotranslocation. Biochem J. 2011;436(2):371–385. doi: 10.1042/BJ20101493. [DOI] [PubMed] [Google Scholar]
- 39.Spooner RA, Watson PD, Marsden CJ, et al. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem J. 2004;383(Pt 2):285–293. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Argent RH, Parrott AM, Day PJ, et al. Ribosome-mediated folding of partially unfolded ricin A-chain. J Biol Chem. 2000;275(13):9263–9269. doi: 10.1074/jbc.275.13.9263. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Spooner RA, Allen SC, et al. Folding-competent and folding-defective forms of ricin A chain have different fates after retrotranslocation from the endoplasmic reticulum. Mol Biol Cell. 2010;21(15):2543–2554. doi: 10.1091/mbc.E09-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spooner RA, Hart PJ, Cook JP, et al. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105(45):17408–17413. doi: 10.1073/pnas.0809013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gluck A, Endo Y, Wool IG. The ribosomal RNA identity elements for ricin and for alpha-sarcin: mutations in the putative CG pair that closes a GAGA tetraloop. Nucleic Acids Res. 1994;22(3):321–324. doi: 10.1093/nar/22.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iordanov MS, Pribnow D, Magun JL, et al. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17(6):3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jandhyala DM, Thorpe CM, Magun B. Ricin and Shiga toxins: effects on host cell signal transduction. Curr Top Microbiol Immunol. 2012;357:41–65. doi: 10.1007/82_2011_181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pincus SH, Bhaskaran M, Brey RN, 3rd, Didier PJ, Doyle-Meyers LA, Roy CJ. Clinical and Pathological Findings Associated with Aerosol Exposure of Macaques to Ricin Toxin. Toxins (Basel) 2015;7(6):2121–2133. doi: 10.3390/toxins7062121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roy CJ, Brey RN, Mantis NJ, et al. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: Epitope-specific neutralizing antibodies correlate with protection. Proc Natl Acad Sci U S A. 2015;112(12):3782–3787. doi: 10.1073/pnas.1502585112. Demosntrates that RiVax adminstered by intramuscular injection to Rhesus monkeys confers protection against aerosol challenge.
- 48.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25(42):7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhaskaran M, Didier PJ, Sivasubramani SK, Doyle LA, Holley J, Roy CJ. Pathology of Lethal and Sublethal Doses of Aerosolized Ricin in Rhesus Macaques. Toxicol Pathol. 2013 doi: 10.1177/0192623313492248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown RF, White DE. Ultrastructure of rat lung following inhalation of ricin aerosol. Int. J. Exp. Path. 1997;78(4):267–276. doi: 10.1046/j.1365-2613.1997.300363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelmsen CL, Pitt ML. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Veterinary Pathology. 1996;33(3):296–302. doi: 10.1177/030098589603300306. [DOI] [PubMed] [Google Scholar]
- 52.Smallshaw JE, Ghetie V, Rizo J, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21(4):387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 53.Legler PM, Brey RN, Smallshaw JE, Vitetta ES, Millard CB. Structure of RiVax: a recombinant ricin vaccine. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 9):826–830. doi: 10.1107/S0907444911026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carra JH, Wannemacher RW, Tammariello RF, et al. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine. 2007;25(21):4149–4158. doi: 10.1016/j.vaccine.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 55. McHugh CA, Tammariello RF, Millard CB, Carra JH. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci. 2004;13(10):2736–2743. doi: 10.1110/ps.04897904. * Engineering more stable RTA subunit antigens.
- 56. Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein engineering, design & selection : PEDS. 2004;17(4):391–397. doi: 10.1093/protein/gzh043. Minimalist RTA subunit antigens prove effective in mice.
- 57.Compton JR, Legler PM, Clingan BV, Olson MA, Millard CB. Introduction of a disulfide bond leads to stabilization and crystallization of a ricin immunogen. Proteins. 2011;79(4):1048–1060. doi: 10.1002/prot.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA) Immunol Lett. 2014;158(1–2):7–13. doi: 10.1016/j.imlet.2013.11.009. *Identification of four toxin-neutralizing B cell epitope clusters on the surface of RTA.
- 59.Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine. 2010;28(32):5315–5322. doi: 10.1016/j.vaccine.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005;23(39):4775–4784. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 61.McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Progress in Biological Threat Agent Vaccine Development: A Repeat-Dose Toxicity Study of a Recombinant Ricin Toxin A-Chain (rRTA) 1-33/44-198 Vaccine (RVEc) in Male and Female New Zealand White Rabbits. Int J Toxicol. 2011 doi: 10.1177/1091581810396730. [DOI] [PubMed] [Google Scholar]
- 62.McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective Effect of Two Recombinant Ricin Subunit Vaccines in the New Zealand White Rabbit Subjected to a Lethal Aerosolized Ricin Challenge: Survival, Immunological Response and Histopathological Findings. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr274. [DOI] [PubMed] [Google Scholar]
- 63. O'Hara JM, Brey RN, 3rd, Mantis NJ. Comparative efficacy of two leading candidate ricin toxin a subunit vaccines in mice. Clin Vaccine Immunol. 2013;20(6):789–794. doi: 10.1128/CVI.00098-13. * comparision of RiVax and RVEc in mice.
- 64.Vance DJ, Greene CJ, Rong Y, Mandell LM, Connell TD, Mantis NJ. Comparative Adjuvant Effects of Type II Heat-Labile Enterotoxins in Combination with Two Different Candidate Ricin Toxin Vaccine Antigens. Clin Vaccine Immunol. 2015;22(12):1285–1293. doi: 10.1128/CVI.00402-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pittman PR, Reisler RB, Lindsey CY, et al. Safety and immunogenicity of ricin vaccine, RVEc, in a Phase 1 clinical trial. Vaccine. 2015;33(51):7299–7306. doi: 10.1016/j.vaccine.2015.10.094. **, Results of RVEc Phase I clinical trial.
- 66. Vitetta ES, Smallshaw JE, Schindler J. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol. 2012;19(10):1697–1699. doi: 10.1128/CVI.00381-12. Results of RiVax (adjuvanted) Phase I clinical trial.
- 67.Lindsey CY, Brown JE, Torabazar NR, Smith LA. EL4 cell-based colorimetric toxin neutralization activity assays for determination of neutralizing anti-ricin antibodies. Journal of AOAC International. 2013;96(1):147–154. doi: 10.5740/jaoacint.12-285. [DOI] [PubMed] [Google Scholar]
- 68. Vitetta ES, Smallshaw JE, Coleman E, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006;103(7):2268–2273. doi: 10.1073/pnas.0510893103. Results of pilot RiVax (unadjuvnted) Phase I clinical trial.
- 69.Vance DJ, Rong Y, Brey RN, 3rd, Mantis NJ. Combination of two candidate subunit vaccine antigens elicits protective immunity to ricin and anthrax toxin in mice. Vaccine. 2015;33(3):417–421. doi: 10.1016/j.vaccine.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rath T, Baker K, Pyzik M, Blumberg RS. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Front Immunol. 2014;5:664. doi: 10.3389/fimmu.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boles JW, Pitt ML, LeClaire RD, et al. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin Immunol. 2003;108(1):51–59. doi: 10.1016/s1521-6616(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 72.Hassett KJ, Cousins MC, Rabia LA, et al. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm. 2013;85(2):279–286. doi: 10.1016/j.ejpb.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piatak M, Lane JA, Laird W, Bjorn MJ, Wang A, Williams M. Expression of soluble and fully functional ricin A chain in Escherichia coli is temperature-sensitive. J Biol Chem. 1988;263(10):4837–4843. [PubMed] [Google Scholar]
- 74.Janosi L, Compton JR, Legler PM, et al. Disruption of the putative vascular leak peptide sequence in the stabilized ricin vaccine candidate RTA1->33/44–198. Toxins (Basel) 2013;5(2):224–248. doi: 10.3390/toxins5020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24(3):310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foged C, Rades T, Perrie Y, Hook S, editors. Subunit Vaccine Delivery. New York: Springer-Verlag; 2015. [Google Scholar]
- 78.Greene CJ, Chadwick CM, Mandell LM, et al. LT-IIb(T13I), a non-toxic type II heat-labile enterotoxin, augments the capacity of a ricin toxin subunit vaccine to evoke neutralizing antibodies and protective immunity. PLoS One. 2013;8(8):e69678. doi: 10.1371/journal.pone.0069678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neal LM, O'Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010;78(1):552–561. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Hara JM, Mantis NJ. Neutralizing monoclonal antibodies against ricin's enzymatic subunit interfere with protein disulfide isomerase-mediated reduction of ricin holotoxin in vitro. J Immunol Methods. 2013;395(1–2):71–78. doi: 10.1016/j.jim.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010;28:7035–7046. doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Hara JM, Whaley K, Pauly M, Zeitlin L, Mantis NJ. Plant-based expression of a partially humanized neutralizing monoclonal IgG directed against an immunodominant epitope on the ricin toxin A subunit. Vaccine. 2012;30(7):1239–1243. doi: 10.1016/j.vaccine.2011.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sully EK, Whaley KJ, Bohorova N, et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol. 2014;21(5):777–782. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pincus SH, Das A, Song K, Maresh GA, Corti M, Berry J. Role of Fc in antibody-mediated protection from ricin toxin. Toxins (Basel) 2014;6(5):1512–1525. doi: 10.3390/toxins6051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prigent J, Panigai L, Lamourette P, et al. Neutralising antibodies against ricin toxin. PLoS One. 2011;6(5):e20166. doi: 10.1371/journal.pone.0020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yermakova A, Mantis NJ. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB) Vaccine. 2011;29(45):7925–7935. doi: 10.1016/j.vaccine.2011.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yermakova A, Mantis NJ. Neutralizing activity and protective immunity to ricin toxin conferred by B subunit (RTB)-specific Fab fragments. Toxicon. 2013;72:29–34. doi: 10.1016/j.toxicon.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yermakova A, Vance DJ, Mantis NJ. Sub-Domains of Ricin's B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS One. 2012;7(9):e44317. doi: 10.1371/journal.pone.0044317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wahome PG, Mantis NJ. High-Throughput, Cell-Based Screens to Identify Small-Molecule Inhibitors of Ricin Toxin and Related Category B Ribosome Inactivating Proteins (RIPs) Curr Protoc Toxicol. 2013;Chapter 2(Unit2):23. doi: 10.1002/0471140856.tx0223s55. [DOI] [PubMed] [Google Scholar]
- 90.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 91.Abboud N, Chow SK, Saylor C, et al. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207(11):2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pohl MA, Rivera J, Nakouzi A, Chow SK, Casadevall A. Combinations of monoclonal antibodies to anthrax toxin manifest new properties in neutralization assays. Infect Immun. 2013;81(6):1880–1888. doi: 10.1128/IAI.01328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu CC, Yin J, Chau D, Cherwonogrodzky JW, Hu WG. Active immunity induced by passive IgG post-exposure protection against ricin. Toxins (Basel) 2014;6(1):380–393. doi: 10.3390/toxins6010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burbelo PD, Ching KH, Bush ER, Han BL, Iadarola MJ. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert review of vaccines. 2010;9(6):567–578. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Furman D, Davis MM. New approaches to understanding the immune response to vaccination and infection. Vaccine. 2015;33(40):5271–5281. doi: 10.1016/j.vaccine.2015.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakaya HI, Hagan T, Duraisingham SS, et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity. 2015;43(6):1186–1198. doi: 10.1016/j.immuni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rudolph MJ, Vance DJ, Cheung J, et al. Crystal structures of ricin toxin's enzymatic subunit (RTA) in complex with neutralizing and non-neutralizing single-chain antibodies. J Mol Biol. 2014;426(17):3057–3068. doi: 10.1016/j.jmb.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–421. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 99.Aboud-Pirak E, Hack D, Olson MA, et al. 1993 Medical Defense Bioscience Review. Baltimore, MD: US Army Medical Research and Material Command; 1993. Identification of a neutralizing epitope on ricin A chain and application of its 3D stucture to design peptide vaccines that protect against ricin intoxication. [Google Scholar]
- 100.Lebeda FJ, Olson MA. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int J Biol Macromol. 1999;24(1):19–26. doi: 10.1016/s0141-8130(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 101.Vance DJ, Mantis NJ. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin's enzymatic subunit. Toxicon. 2012;60(5):874–877. doi: 10.1016/j.toxicon.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu Y, Dai J, Zhang T, et al. Structural insights into the neutralization mechanism of monoclonal antibody 6C2 against ricin. J Biol Chem. 2013;288(35):25165–25172. doi: 10.1074/jbc.M113.480830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB. Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J Biol Chem. 2013;288(51):36538–36547. doi: 10.1074/jbc.M113.519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castelletti D, Fracasso G, Righetti S, et al. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin's lymphoma patients treated with an anti-CD25 immunotoxin. Clin. Exp. Immunol. 2004;136(2):365–372. doi: 10.1111/j.1365-2249.2004.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mlsna D, Monzingo AF, Katzin BJ, Ernst S, Robertus JD. Structure of recombinant ricin A chain at 2.3 A. Protein Sci. 1993;2(3):429–435. doi: 10.1002/pro.5560020315. [DOI] [PMC free article] [PubMed] [Google Scholar]