Abstract
Background
Over the last decade the age of trauma patients and injury mortality has increased. At the same time, many centers have implemented multiple interventions focused on improved hemorrhage control, effectively resulting in a bleeding control bundle of care. The objective of our study was to analyze the temporal distribution of trauma-related deaths, the factors that characterize that distribution and how those factors have changed over time at our urban level 1 trauma center.
Methods
Records at a urban Level 1 trauma center were reviewed. Two time periods (2005–2006 and 2012–2013) were included in the analysis. Mortality rates were directly adjusted for age, gender and mechanism of injury. The Mann-Whitney and chi square tests were used to compare variables between periods, with significance set at 0.05.
Results
7080 patients (498 deaths) were examined in 2005–2006, while 8767 patients (531 deaths) were reviewed in 2012–2013. The median age increased 6 years, with a similar increase in those who died. In patients that died, no differences by gender, race or ethnicity were observed. Fall-related deaths are now the leading cause of death. Traumatic brain injury (TBI) and hemorrhage accounted for > 91% of all deaths. TBI (61%) and multiple organ failure or sepsis (6.2%) deaths were unchanged, while deaths associated with hemorrhage decreased from 36% to 25% (p<0.01). Across time periods, 26% of all deaths occurred within one hour of hospital arrival, while 59% occurred within 24 hours. Unadjusted mortality dropped from 7.0% to 6.1% (p=0.01) and in-hospital mortality dropped from 6.0% to 5.0% (p<0.01). Adjusted mortality dropped 24% from 7.6% (95% CI: 6.9–8.2) to 5.8% (95% CI: 5.3–6.3) and in-hospital mortality decreased 30% from 6.6% (95% CI: 6.0–7.2) to 4.7 (95% CI: 4.2–5.1).
Conclusions
Over the same time frame of this study, increases in trauma death across the globe have been reported. This single-site study demonstrated a significant reduction in mortality, likely attributable to decreased hemorrhagic death. It is possible that efforts focused on hemorrhage control interventions (a bleeding control bundle) resulted in this reduction. These changing factors provide guidance on future prevention and intervention efforts.
Background
The federal government supports research to improve the general health of the nation and outcomes after disease and injury. As a result, life expectancy has increased by 11% from 70.8 to 78.8 (1970–2012)(1, 2), cancer mortality decreased (1991–2009) by 20%(3) and heart disease declined (2000–2010) by 31%.(4) However, during similar periods, injury-related mortality has risen. The past decade (2000–2010) has seen an increase in trauma-related mortality in the US by 22.8%(5) and worldwide (1990–2010) by 24%.(6) An increase in patient age, motor vehicle collisions by 46% and an increase in falls by 55% likely contributed to the global rise in trauma mortality.(7)
Internationally, injury-related mortality accounts for 1 in 10 deaths.(6, 7) In the United states, trauma-related injuries (unintentional + suicides + drug overdose) is the 3rd leading cause of death.(2) Between 2000 and 2010, traumatic injury increased from the leading cause of death among individuals younger than 43 years to the leading cause in those younger than 46.(5) Likewise, traumatic injury increased from the leading cause of life-years lost up to age 65 to the leading cause up to age 75.(8) Additionally, the mean age of trauma patients has increased.(9) Past studies have shown head injuries (42–52%) and hemorrhage (30–39%) to be leading causes of trauma-related deaths while multiple organ failure (MOF) trails at 7–11%.(9, 10)
Keeping with the tenets of a learning health care system(11) the objective of our study was to analyze mortality rates, the temporal distribution of the causes of trauma-related deaths, the factors that characterize that distribution and how those factors have changed over time at our urban level I trauma center. Since 2008, our center has focused on optimal resuscitation and stopping bleeding, employing multiple methods in the prehospital and hospital areas of care, resulting in a bleeding control bundle of care. None of these interventions were utilized prior to 2008. We hypothesized that from 2005–2006 in comparison to 2012–2013, the percentage of deaths would change, the distribution of cause-specific mortality would be different and that the time to death for trauma patients would be dissimilar.
Methods
The trauma registry, weekly Morbidity & Mortality reports, autopsy reports and electronic medical records at Memorial Hermann Hospital in Houston, TX were reviewed after receiving Institution Review Board approval. The Memorial Hermann Hospital is one of two level 1-trauma centers serving the greater Houston area and currently admits greater than 6000 injured patients a year.
Two time periods (2005–2006 and 2012–2013) were included in the analysis. These periods of time represent two years before and after implementation of major changes in control of bleeding and early resuscitation procedures.(12) Studies from our center and others have documented specific interventions for improved bleeding control, such as the use of pelvic binders, hemostatic dressings, extremity and junctional tourniquets, balloon occlusion of the aorta, minimizing crystalloid resuscitation, coagulation monitoring by TEG,(13) use of TXA in patients with increased fibrinolysis and prehospital and hospital balanced transfusion (damage control resuscitation) and rapid delivery of patients to the operating and interventional radiology suites.(12–27) These interventions are usually performed concurrently, but often analyzed separately. Few papers have examined the overall effects on hemorrhage related mortality of these often concurrent interventions.
The study included all deceased patients seen at Hermann Hospital between 2005–2006 and 2012–2013. Patients with primary burn injuries and pediatric age (<16) patients were excluded. Only patients declared dead in the hospital, including patients that were dead on arrival were included in this analysis. For each patient, data was collected on baseline characteristics, which included age, sex, mechanism of injury, cause of death, time of hospital arrival and time of death (same as discharge time). The time to death was calculated from time of hospital arrival to physician pronouncement of death or discharge time. At our weekly Morbidity and Mortality meetings, the trauma team, involved in the patient’s care reviewed each patient’s medical records to determine the primary cause of death. Autopsy data were also reviewed, but these were often not available at the time of the M&M conference. These data were recorded in each patient’s M&M report and entered into the trauma registry. Patients were classified into one of several groups: (1) head injuries- fatal brain injury; (2) hemorrhage- uncontrolled bleeding; (3) multiple organ failure and/or systemic infection (MOF+ Sepsis); (4) Respiratory Failure- inadequate exchange of gases by the lungs (Respiratory Fail); (5) Cardiac- sudden cardiac arrest; (6) Comorbid- presence of a significant secondary disease contributing to mortality; (7) Pulmonary Embolism- embolus lodging in pulmonary arteries (PE); (8) Other- primary cause of death not described by previous primary categories; (9) Unknown- missing sufficient data to determine cause of death (UNK). For patients with more than one factor contributing to death, each cause was counted separately. This more accurately represents the fact that multiple etiologies may have contributed to a patient’s death.
Penetrating injuries were defined as traumatic wounds that were a primary result of an object puncturing the skin and entering the underlying tissue. Blunt injuries were defined as injuries primarily resulting from the application of a non-penetrating mechanical force.
In hospital mortalities excludes all patients that were dead on arrival. Mortality rates were directly adjusted for age, gender and mechanism of injury using direct standardization, utilizing the data analysis program STATA 13.1. The trauma population from the two time periods was used as a standard population.
Results are expressed comparing 2005–2006 with 2012–2013. The Mann-Whitney rank sum and chi square tests, utilizing Fisher’s exact test, were used to compare variables between periods, with significance set at 0.05. Data is presented as medians and inter-quartile ranges (IQR). Analysis was conducted using STATA 13.1.
Results
We reviewed 15,874 trauma patients and 1029 deaths from the 4-year study period. 7080 patients including 498 deaths were examined in the early time period (2005–2006), while 8767 patients including 531 deaths were reviewed in the recent period (2012–2013). There was a 23% increase in the number of admissions in the recent period. The overall trauma population showed differences in age and gender over time but were similar in race/ethnicity distribution (Table 1). The median age of all patients increased by 6 years, 38 (24–52) to 44 (28–62) (p<0.01) years. The percent of male trauma patients seen at the trauma center decreased from 72% to 68% (p<0.01). In patients that died, no differences by gender, race or ethnicity were observed, however they differed in age. The median age of patients that died increased by 7 years from 46 (28–67) to 53 (32–73) (p<0.01) years. The median Injury Severity Score (ISS) was 26 (25–38) and did not change (p=0.09) (Table 1).
Table 1.
Patient Characteristics
| Total N= 1029 |
2005–2006 N= 498 |
2012–2013 N= 531 |
p | |
|---|---|---|---|---|
| Age | ||||
| All Trauma Patients, median (IQR) | 41 (26–57) | 38 (24–52) | 44 (28–62) | <0.01 |
| Trauma Deaths, median (IQR) | 50 (30–69) | 46 (28–67) | 53 (32–73) | <0.01 |
| Gender- Male | ||||
| All Trauma Patients, n (%) | 11008/15847 (69.5) | 5068/7080 (71.6) | 5940/8767 (67.8) | <0.01 |
| Trauma Deaths, n (%) | 737/1029 (71.6) | 364/498 (73.1) | 373/531 (70.2) | 0.35 |
| Race/Ethnicity (Trauma Deaths) | ||||
| Black, n (%) | 141/1029 (13.7) | 66/498 (13.3) | 75/531 (14.1) | 0.75 |
| Hispanic/Latino, n (%) | 211/1029 (20.5) | 102/500 (20.5) | 109/531 (20.5) | 1.00 |
| Other, n (%) | 57/1029 (5.54) | 25/498 (5.02) | 32/531 (6.03) | 0.57 |
| White, n (%) | 620/1029 (60.3) | 305/498 (61.2) | 315/531 (59.3) | 0.57 |
| Injury Severity Score, median (IQR) | 26 (25–38) | 26.5 (25–43) | 26 (25–36) | 0.09 |
IQR- Interquartile Ratio; ISS, Injury Severity Score
Age, gender, and mechanism of injury were found to be different among the overall trauma population. Thus, mortality rates were directly adjusted by these factors. Adjusted overall mortality (including those dead on arrival) dropped 24% from 7.6% (95% CI: 6.9–8.2) to 5.8% (95% CI: 5.3–6.3) (Table 2) between the two time periods. Adjusted in-hospital mortality (excluding dead on arrivals) decreased 30% from 6.6% (95% CI: 6.0–7.2) to 4.7% (95% CI: 4.2–5.1).
Table 2.
Mortality rates directly adjusted for age, gender and mechanism of injury
| Rates | 2005–2006 (N=7080) |
2012–2013 (N=8767) |
|---|---|---|
| Total Mortality, % (95% CI) | 7.6 (6.9–8.2) | 5.8 (5.3–6.3) |
| In-hospital Mortality, % (95% CI) | 6.6 (6.0–7.2) | 4.7 (4.2–5.1) |
CI- confidence interval
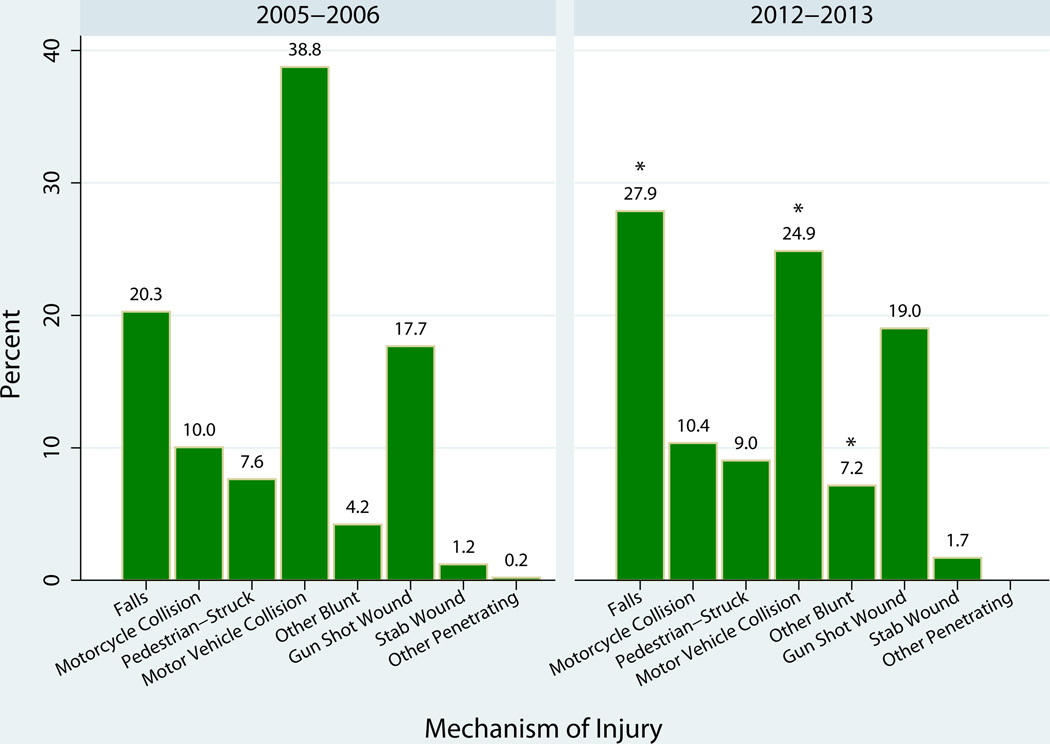
In 2005–2006 motor vehicle collisions (MVCs) represented the primary mechanism of injury, resulting in 38.8% of all mortalities (Figure 1). By 2012–2013, deaths resulting from MVCs dropped to 24.9% to become the 2nd leading mechanism of injury resulting in death (p<0.01). Taking its place as the leading mechanism of injury resulting in death, fall-related deaths increased from 20% to 28% (p<0.01). “Other blunt injuries” saw a change overtime from 4.2% to 7.2% (p=0.06), however this category included several miscellaneous groups of blunt injury. No other mechanisms of injury changed over time. Blunt injuries continued to comprise the majority of trauma related deaths and did not change (81% to 80%; p=0.56).
Figure 1.
Mechanism of injury among trauma deaths in 2006–2006 and 2012–2013.
There was a significant change in falls, motor vehicle collisions, and other blunt injuries, (p < 0.05).
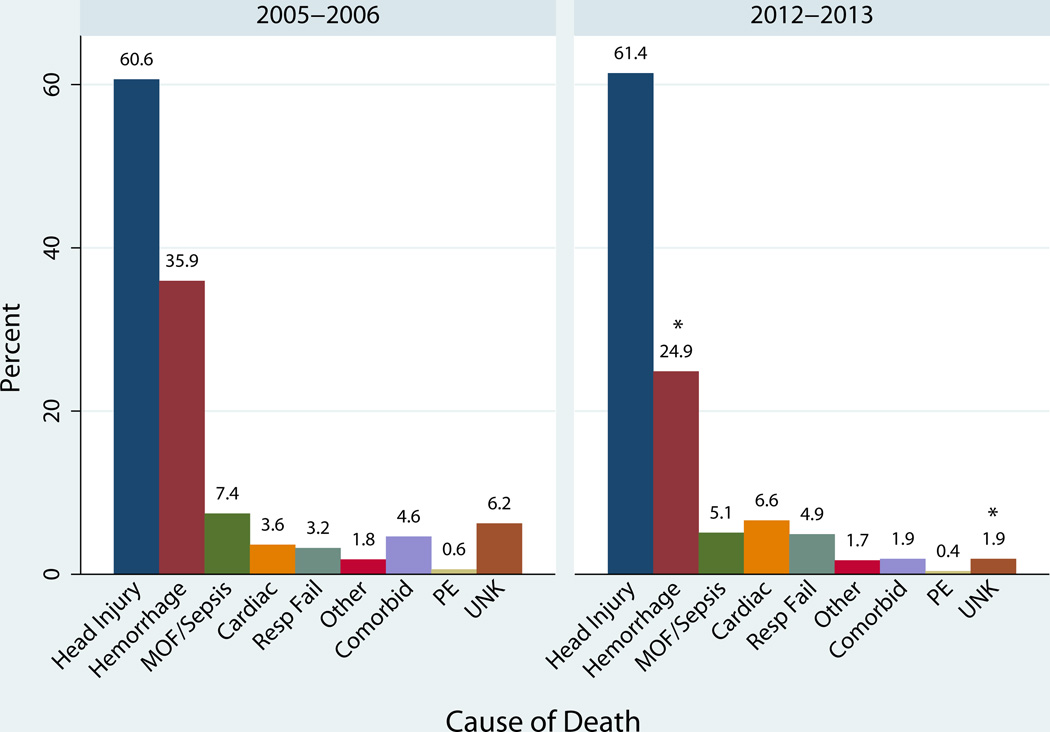
Head injuries and hemorrhage continued to comprise the leading causes of death with head injuries accounting for the majority of deaths (Figure 2). Deaths resulting from head injury (61%) (p=0.85) and multiple organ failure & sepsis (6.2%) (p=0.15) stayed constant. The head AIS increased from a median of 4 to 4.5 but this increase was not significant (p=0.19). Hemorrhage-related mortality decreased significantly from 36% to 25% (p<0.01).
Figure 2.
Cause of death in 2005–2006 and 2012–2013.
*Significant decrease in hemorrhage related deaths and unknown (p < 0.01). The cumulative percentage is greater than 100% due to patients with multiple contributing causes of death.
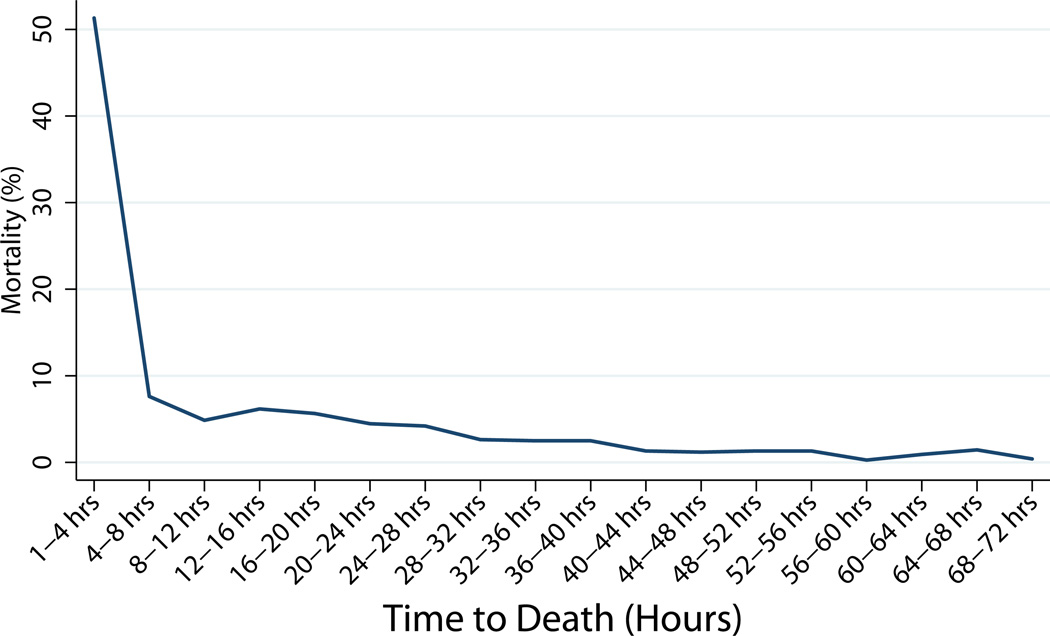
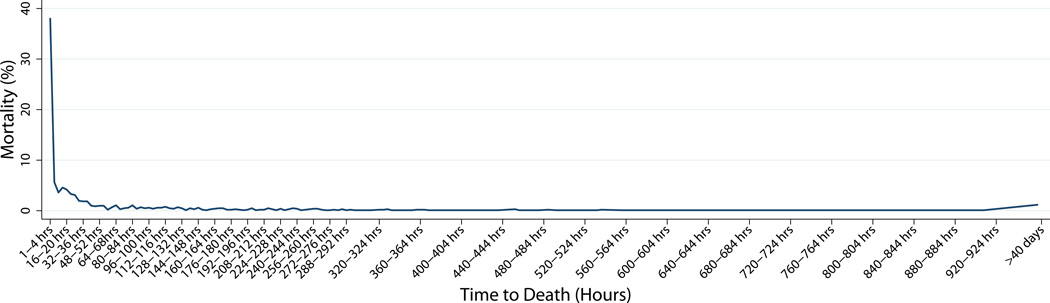
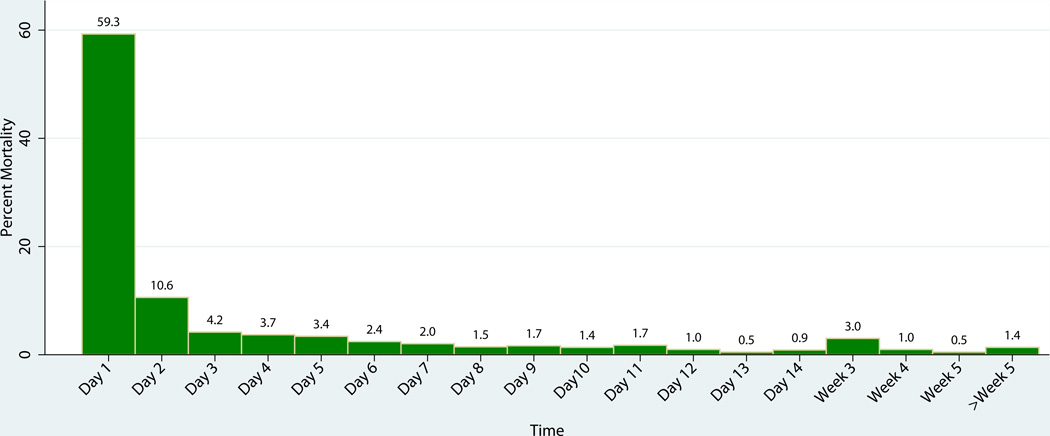
The median time to death (Table 3) decreased from 22.6 hours to 17.4 hours for patients with head injuries (p=0.02) and remained at 1.65 hours for hemorrhage, and 11 days for multiple organ failure. The overall time to death (14.6 hours) (p=0.95) did not change significantly over time, therefore we grouped the data from both groups to examine the time to death for all patients in the study. We found only one peak in the temporal distribution of deaths (including DOAs) (Figure 3). We found that 26% of all in-hospital deaths (including dead on arrival, DOAs) occurred within one hour of hospital arrival, while 59% occurred within 24 hours (Figure 4). The apparent increase in death at week 3 is due to a change in the interval on the x-axis. 78% of all deaths occurred by day 3, and 98% occurred within 30 days of injury. Concentrating on the first hour post injury, the primary cause of death changed from hemorrhage (60.3%) followed by head injury (37.5%) in 2005–2006 to mostly head injuries (52.7%) followed by hemorrhage (38%) in 2012–2013 (Table 4). In the first 8 hours, head injuries accounted for 47% of injuries and hemorrhage accounted for 50%. Between 8–48 hours, head injuries accounted for 83% of deaths while hemorrhage accounted for 17%. At the 8-hour time point, head injuries were associated with the majority of trauma injuries and continued to be a major contributor to death thereafter (Table 5). Hemorrhage was a major factor in deaths occurring soon after injury (1–8 hours) while MOF/sepsis was more important in later deaths (>7 days).
Table 3.
Time to Trauma Deaths Based on Cause of Death
| Total (N=1029) |
2005–2006 (N=498) |
2012–2013 (N=531) |
p | |
|---|---|---|---|---|
| Overall, med (IQR) | 14.6 (0.87, 78.5) | 13.0 (0.52, 80.3) | 16.2 (1.10, 77.9) | 0.95 |
| Head Injury, med (IQR) | 19.6 (2.75–78.3) | 22.6 (3.95–91.5) | 17.4 (2.08–54.3) | 0.02 |
| Hemorrhage, med (IQR) | 1.65 (0.22–12.4) | 1.52 (0.20–11.6) | 1.78 (0.24–15.2) | 0.61 |
| MOF + Sepsis, med (IQR) | 256 (122–456) | 287 (147–690) | 215 (101–406) | 0.09 |
| Respiratory Failure, med (IQR) | 170 (66–263) | 250 (184–621) | 109 (24–193) | 0.01 |
| Cardiac, med (IQR) | 35.9 (4.5–134) | 27.5 (2.1–172) | 35.9 (6.9–134) | 0.55 |
| Comorbid, med (IQR) | 124 (53–185) | 142 (66–255) | 102 (43–168) | 0.46 |
| Other, med (IQR) | 90.2 (19–199) | 75 (3.5–118) | 180 (34–199) | 0.35 |
| Unknown, med (IQR) | 0.08 (0.1–0.2) | 0.1 (0.1–0.3) | 0.04 (0.03–0.1) | 0.11 |
| PE, med (IQR) | 68 (13–313) | 313 (13–412) | 40 (12–68) | 0.25 |
med- median; IQR- interquartile ratio; MOF- multiple organ failure; PE- pulmonary embolism
Figure 3.
Temporal Distribution of trauma mortality showing a unimodal distribution in mortality.
a. 74% died in the first 72 hours.
b. An additional 26% died over the next 174 days. 12 people died after 40 days.
Figure 4.
Temporal distribution of trauma mortality in 2005–2006 & 2012–2013.
The x-axis changes from days to weeks after day 14. The apparent increase in death at week 3 is due to a change in the interval on the x-axis.
Table 4.
Cause of Death 1 hr and 24 hrs Post Injury for Major Causes of Death
| Total | 2005–2006 | 2012–2013 | p | |
|---|---|---|---|---|
| 1 hour post Injury | N=265 | N=136 | N=129 | |
| Head Injury, n (%) | 119/265 (44.9) | 51/136 (37.5) | 68/129 (52.7) | 0.02 |
| Hemorrhage, n (%) | 131/265 (49.4) | 82/136 (60.3) | 49/129 (38.0) | <0.01 |
| 24 hours post Injury | N=610 | N=302 | N=308 | |
| Head Injury, n (%) | 347/610 (56.9) | 158/302 (52.3) | 189/308 (61.4) | 0.03 |
| Hemorrhage, n (%) | 253/610 (41.5) | 147/302 (48.7) | 106/308 (34.4) | <0.01 |
Table 5.
Time to Death by Cause of Death
| <1 hr | 2 hr | 3 hr | 4 hr | 4–8 hr | 8–12 hr |
12–24 hr | Day 2 | Days 3–7 | Days 7–14 |
> 2 weeks |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head Injury, n (%) |
119/265 (45) |
20/62 (32) |
20/38 (53) |
13/26 (50) |
38/58 (66) |
30/37 (8) |
107/124 (86) |
87/109 (80) |
114/162 (70) |
51/88 (5) |
29/60 (48) |
| Hemorrhage, n (%) |
131/265 (50) |
38/62 (61) |
19/38 (50) |
14/26 (54) |
24/58 (41) |
7/37 (19) |
20/124 (16) |
19/109 (17) |
25/162 (15) |
7/88 (8) |
7/60 (12) |
| MOF + Sepsis, n (%) |
0/265 (0) |
0/62 (0) |
0/38 (0) |
0/26 (0) |
0/58 (0) |
0/37 (0) |
1/124 (1) |
3/109 (3) |
17/162 (11) |
20/88 (23) |
23/60 (38) |
| Resp Fail, n (%) |
4/265 (2) |
0/62 (0) |
0/38 (0) |
0/26 (0) |
1/58 (2) |
0/37 (0) |
0/124 (0) |
5/109 (5) |
10/162 (6) |
15/88 (17) |
7/60 (12) |
| Cardiac, n (%) |
7/265 (3) |
2/62 (3) |
4/38 (11) |
0/26 (0) |
4/58 (7) |
0/37 (0) |
5/124 (4) |
7/109 (6) |
13/162 (8) |
4/88 (5) |
7/60 (12) |
| Comorbid, n (%) |
0/265 (0) |
2/62 (3) |
0/38 (0) |
0/26 (0) |
0/58 (0) |
1/37 (3) |
2/124 (2) |
3/109 (3) |
13/162 (8) |
6/88 (7) |
6/60 (10) |
| Other, n (%) | 2/265 (1) |
0/62 (0) |
1/38 (3) |
1/26 (4) |
0/58 (0) |
0/37 (0) |
1/124 (1) |
3/109 (3) |
3/162 (2) |
6/88 (7) |
1/60 (2) |
| Unknown, n (%) |
36/265 (14) |
4/62 (6) |
0/38 (0) |
0/26 (0) |
0/58 (0) |
0/37 (0) |
0/124 (0) |
0/109 (0) |
1/162 (1) |
0/88 (0) |
0/60 (0) |
| PE, n (%) | 0/265 (0) |
0/62 (0) |
0/38 (0) |
0/26 (0) |
0/58 (0) |
0/37 (0) |
2/124 (2) |
0/109 (0) |
1/162 (1) |
1/88 (1) |
1/60 (2) |
Cumulative percentage is greater than 100% due to patients with multiple contributing causes of death. MOF- multiple organ failure; Resp Fail- respiratory failure; PE- pulmonary embolism
Discussion
Worldwide, injury-related deaths has increased by 24% (1990–2010)(6, 7) while US data shows a 23% increase in death rate due to trauma over the past decade (2000–2010).(5, 6) Dutton et al. (2010) in a similar single-site study showed an increase in mortality over time (1997–2008).(9) However the data at our single-site study demonstrates a significant reduction in adjusted overall and in-hospital mortality. While our data does not reflect the entire population of the greater Houston area, our patient population has increased 23.8% over the study period, without major changes in prehospital distribution of patients, or new adult Level 1 centers. The goal of our study was to describe the change in injury patterns over the last 8 years and in what population, if any, we have improved outcomes. In future studies, it would be worthwhile to examine which interventions may have resulted in the reduction in mortality in this population and whether such techniques can effectively impact mortality at other trauma centers.
Over the last decade, there have been significant clinical efforts, both throughout the country and at our center, attempting to improve outcomes after injury and to combat increasing trauma-related mortality. Resuscitation changes included the development of a damage control resuscitation protocol which called for decreased use of crystalloids and utilizing a balanced 1:1:1 plasma:platelet:RBC ratio.(12, 14–17) It is possible that concentrated efforts on improving resuscitation and multiple other hemorrhage control interventions resulted in the observed reduction in hemorrhage related mortality. For example, the PROMMT study found that a larger plasma and platelet ratio administered earlier in resuscitation resulted in a reduction in mortality among some patients in the first 24 hours post hospital admission.(28) The randomized PROPPR study showed decreased death from exsanguination when utilizing a 1:1:1 approach but no change in TBI deaths.(29) The use of damage control resuscitation has shown improved outcomes after significant blunt liver injury.(30) Likewise, adoption of hemorrhage-control techniques from the recent war, such as tourniquets and hemostatic dressings may have impacted trends in mortality in the civilian population.(18–23) One study in a civilian population show that a hemostatic dressing demonstrated improved hemorrhage control in 74% – 92% of bleeding patients hemorrhages.(20) The use of extremity and junctional tourniquets in both military and civilian populations has been shown control hemorrhage and improve mortality in several studies.(19, 22, 23) We have utilized TEG instead of conventional coagulation tests for many years, and feel they deliver faster and superior information to the bedside.(13) Additionally, it has been shown that in a trauma setting, resuscitative endovascular balloon occlusion of the aorta (REBOA) may be effective in controlling hemorrhage until definitive hemostasis can be achieved and is associated with improved survival.(24, 25) We have also focused on decreasing times to the operating room and interventional radiology suite.(26) TXA is used in patients with evidence of significant (≥ 3%) fibrinolysis on admission.(27) Finally, ratio driven resuscitation is utilized when patients are bleeding rapidly, and when hemorrhage control methods are becoming successful and hemorrhage is slowing, transition to goal directed resuscitation ensues.(31) These studies suggest that implementation of a multi-modal bleeding control bundle encompassing accurate identification of the bleeding patient, transfusion, hemorrhage control techniques, devices, coagulation monitoring and process improvement in civilian trauma patients may decrease death from hemorrhage (Table 6).(32) Our data, although retrospective seem to support this concept.
Table 6.
Bleeding Control Bundle of Care
| Identify the bleeding patient |
| Prehospital and hospital damage control resuscitation |
| Prehospital and hospital extremity and junctional tourniquets |
| Prehospital and hospital pelvic binders |
| Prehospital and hospital hemostatic dressings |
| Resuscitative endovascular balloon occlusion of the aorta |
| Coagulation monitoring with thromboelastrography |
| TXA for patients with significant fibrinolysis |
| Decreased time to operating room |
| Decreased time to interventional radiology |
| Goal directed resuscitation with blood products as bleeding slows |
There are clearly multiple limitations of these data. Due to constraints in retrospectively reviewing old paper and new electronic charts the incidence of hemorrhagic shock, laboratory data, crystalloid and transfusion amounts were not available. Therefore, we were unable to present hemorrhage adjusted mortality due to a lack of denominator. While age gender and mechanism did change over the study period, direct adjustment of these factors should have minimized the confounding effects to the observed reduction in mortality. However, it is likely that unaccounted elements may have influenced these changes as well. For example, it is possible that the reduction in motor vehicle collisions influenced the reduction in mortality. Additionally, the scene time was not recorded which may have impacted the time to death. These data only describe patients who died in our hospital and are therefore inadequate to tell the entire story of trauma deaths in the greater Houston region.
Trunkey first described the tri-modal distribution of trauma fatalities over 30 years ago.(33) The first peak in death distribution accounted for more than half of fatalities and occurred within 1 hour of injury. Deaths resulted primarily from central nervous system, cardiac or major vascular injuries. The second peak occurred within hours of injury and fatalities were largely a result of intracranial bleeds and exsanguination. The third peak occurred days to weeks later and was mostly a result of multiple organ failure or infection. This tri-modal distribution has guided the field of trauma for over 30 years. However, recent research has shown that this pattern of distribution no longer exists.(10, 34–38) In 1997 Meislin, et al.(35) described a bimodal distribution with 23% of deaths occurring within the first hour and 35% of deaths between 24 to 48 hours. Similarly, in 2005 Demetriades, et al.(34) showed only two peaks in fatalities, the first (50.2%) within one hour of injury and the second (18.3%) between 1–6 hours. Deaths occurring after one week only accounted for 7.6% of total fatalities. Our study supports a change from Trunkey’s tri-modal distribution towards a unimodal distribution (Figure 3) with 26% of deaths occurring within the first hour. The second and third peak described in 1983 has disappeared. The remaining 30% of deaths (from many different causes) are spread out somewhat evenly over the ensuing days to weeks that these seriously injured patients stay in the hospital.
Past research has shown that 46% of deaths from head injuries occurred within the first 24 hours post injury, compared to 83% of hemorrhagic deaths. Tishermans et al multicenter study showed that the median time to death was 29 hours among head injuries and 2 hours among hemorrhage.(39) Our single center study showed a very similar temporal distribution with 55% of head and 81% of hemorrhage related deaths occurring in the first 24 hours. The median time to death was 20 hours for head injuries and 1.7 hours for hemorrhagic injuries. Interestingly, we noted a significant reduction in the time to death over time for head injuries (Table 3). This may be a result of earlier withdrawal from life support in older patients dying from falls or that have a worse prognosis.
Like others, we found that the majority of deaths were concentrated very soon after injury with 59% of deaths occurring within the first day after injury and few deaths per day scattered somewhat evenly over the following days to weeks. This implies that efforts in controlling trauma mortality must continue to be concentrated on prehospital prevention and intervention or in the first hours after hospital admission. With the majority of deaths occurring so soon after injury, increased prevention efforts or reductions in mortality during this time period will show the greatest impact in reducing the overall mortality rate and addressing the increasing trend in mortality. It could be argued that concentrating on very early deaths is futile as their injuries are too severe and not amendable to effective intervention. However, we showed a significant reduction in mortality from hemorrhage as a proportion of early injuries (Table 4) even though the time to death did not change significantly over time. Much like the recent military data, reduction in hemorrhage related mortality appears to be an area in which impacts on mortality can be demonstrated.(40) When one considers that bleeding accounts for a substantial amount of early deaths, multimodal hemorrhage control methods should continue to be an area of focus for future improvement in clinical care and research.
Changes in the characteristics of the trauma population may have affected trends in mortality distribution. Many studies have shown changes in the age of patients dying from trauma-related injuries. In 2000, trauma was the leading cause of death in those 43 years of age and younger.(5) By 2010, trauma increased to become the leading cause of death in those 46 years of age and younger. This represents an aging population among trauma fatalities. We likewise observed an aging population of all trauma patients and among trauma deaths in our center. As the general US population continues to age, and as trauma becomes more of a problem with an older population, future intervention should be focused on this group. It is reassuring that despite the aging population at our center, efforts to decrease hemorrhage related mortality still seem to be effective.
Internationally, the leading mechanisms of injury are transportation related injuries, self-harm and falls.(6, 7) The majority (68%) of injury-related deaths were sustained by men and was highest in the younger age groups, with 52% of deaths occurring in men between the age of 10 to 24.(6, 7) The mechanism of injury in trauma deaths also seems to be changing over time in association with the increasing age of the patient population.(7) Studies have shown an increase in falls as a primary mechanism of injury in trauma-related deaths.(41, 42) This trend was also observed in our data and is likely a reflection of the aging trauma population. As falls are now the leading cause of death in our large urban trauma center, this further emphasizes the need for aggressive prevention efforts in this group and specialized care of this vulnerable population.(43)
Despite the increasing trend in injury related mortality, there is significantly less federal funding for trauma research as compared to other causes of death such as cancer, HIV and heart disease.(44) With adequate funding, these diseases have shown a reduction in mortality over time. As other major causes of death decline in mortality rates, and trauma increases to become a more prominent cause of death – particularly among the elderly – increased resources and efforts must be directed towards addressing this issue. In 1983, Trunkey documented a discrepancy between life years lost and trauma funding and Rhee documented that by 2012, this gap has actually expanded.(5, 33) In 2012, there was about 8 billion dollars funding research on cancer, 4 billion on heart disease, 4 billion on HIV/AIDS and only 700 million on Trauma.(5) In addition, there is ineffective lobbying for research and no institute at the National Institute of Heath (NIH) that is solely devoted to injury. Without such an institute and focused, coordinated effort, there is inadequate funding for trauma research, which results in the lack of high quality research that can rapidly change practice.(45)
Conclusion
Injury is a leading cause of mortality in the US and around the world. The dominant causes of death are TBI and hemorrhage. In this single center retrospective study, we demonstrated a reduction in hemorrhagic death rates, while TBI and MOF/sepsis rates remained unchanged. This change was likely associated with implementing a multimodal bleeding control bundle of care, rather than any one specific intervention. Adequate funding for high quality prehospital and early hospital based intervention studies will be important to verify and expand these results.
Acknowledgments
Financial Support: The Center for Translational Injury Research and The University of Texas Medical School at Houston- Office of the Dean. Dr. Scerbo was supported by a T32 fellowship from NIGMS (T32GM008792) while working on the manuscript.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969–2009. Am J Prev Med. 2014;46(2):e19–e29. doi: 10.1016/j.amepre.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief. 2014;(168):1–8. [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, et al. Increasing trauma deaths in the united states. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 6.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Years of Potential Life Lost (YPLL) Reports, 1999–2012 [Internet] [cited November 8, 2014]; Available from: http://webappa.cdc.gov/sasweb/ncipc/ypll10.html.
- 9.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma mortality in mature trauma systems: are we doing better? An analysis of trauma mortality patterns, 1997–2008. J Trauma. 2010;69(3):620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 10.Sauaia A, Moore F, Moore E, Moser K, Brennan R, Read R, et al. Epidemiology of Trauma Deaths: A Reassessment. J Trauma. 1995;38(2):185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Grumbach K, Lucey CR, Johnston SC. Transforming from centers of learning to learning health systems. JAMA. 2014;311(11):1109–1110. doi: 10.1001/jama.2014.705. [DOI] [PubMed] [Google Scholar]
- 12.Holcomb JB, Don J, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive truama patients. Ann Surg. 2012;256(3):476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 14.Duchesne JC, Barbeau JM, Islam TM, Wahl G, Greiffenstein P, McSwain NEJ. Damage control resuscitation: from emergency department to the operating room. Am Surg. 2011;77(2):201–206. doi: 10.1177/000313481107700222. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber M, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 16.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 17.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaway DW, Robertson J, Sztajnkrycer MD. Law Enforcement-applied Tourniquets: A Case Series of Life-saving Interventions. Prehosp Emerg Care. 2014;19(2):320–327. doi: 10.3109/10903127.2014.964893. [DOI] [PubMed] [Google Scholar]
- 19.Passos E, Dingley B, Smith A, Engels PT, Ball CG, Faidi S, et al. Tourniquet use for peripheral vascular injuries in the civilian setting. Injury. 2014;45(3):573–577. doi: 10.1016/j.injury.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Brown MA, Daya MR, Worley JA. Experience with chitosan dressings in a civilian EMS system. J Emerg Med. 2009;37(1):1–7. doi: 10.1016/j.jemermed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Rhee P, Brown C, Martin M, Salim A, Plurad D, Green D, et al. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64(4):1093–1099. doi: 10.1097/TA.0b013e31812f6dbc. [DOI] [PubMed] [Google Scholar]
- 22.Kragh JF, Littrel ML, Jones JA, Walters TJ, Baer DG, Wade CE, et al. Battle casualty survival with emergency tourniquet use to stop limb bleeding. J Emerg Med. 2011;41(6):590–597. doi: 10.1016/j.jemermed.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Kragh JF, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, et al. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7. doi: 10.1097/SLA.0b013e31818842ba. [DOI] [PubMed] [Google Scholar]
- 24.Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506–511. doi: 10.1097/TA.0b013e31829e5416. [DOI] [PubMed] [Google Scholar]
- 25.Moore LJ, Brenner ML, Kozar RA, Pasley JD, Wade CE, Baraniuk S, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523–532. doi: 10.1097/TA.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DA, Medina M, Cotton BA, Rahbar E, Wade CE, Cohen AM, et al. Are we delivering two standards of care for pelvic trauma? Availability to angioembolization after hours and on weekends increases time to therapeutic intervention. J Trauma Acute Care Surg. 2014;76(1):134–139. doi: 10.1097/TA.0b013e3182ab0cfc. [DOI] [PubMed] [Google Scholar]
- 27.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg. 2015;78(5):905–909. doi: 10.1097/TA.0000000000000612. discussion 9–11. [DOI] [PubMed] [Google Scholar]
- 28.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha B, Holcomb JB, Camp EA, del Junco DJ, Cotton BA, Albarado R, et al. Damage-control resuscitation increases successful nonoperative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78(2):336–341. doi: 10.1097/TA.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 31.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3058. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 32.Shafi S, Collinsworth AW, Richter KM, Alam HB, Becker LB, Bullock MR, et al. Bundles of care for resuscitation from hemorrhagic shock and severe brain injury in trauma patients - Translating knowledge into practice. J Trauma Acute Care Surg. 2016 doi: 10.1097/TA.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 33.Trunkey DD. Trauma. Accidental and intentional injuries account for more years of life lost in the U.S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Sci Am. 1983;249(2):28–53. [PubMed] [Google Scholar]
- 34.Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, et al. Trauma deaths in a mature urban trauma system: is "trimodal" distribution a valid concept? J Am Coll Surg. 2005;201(3):343–348. doi: 10.1016/j.jamcollsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Meislin H, Criss EA, Judkins D, Berger R, Conroy C, Parks B, et al. Fatal trauma: the modal distribution of time to death is a function of patient demographics and regional resources. J Trauma. 1997;43(3):433–440. doi: 10.1097/00005373-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Pang J-M, Civil I, Ng A, Adams D, Koelmeyer T. Is the trimodal pattern of death after trauma a dated concept in the 21st century? Trauma deaths in Auckland 2004. Injury. 2008;39(1):102–106. doi: 10.1016/j.injury.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Gunst M, Ghaemmagham V, Gruszecki A, Urban J, Frankel H, Shafi S. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Baylor Univ Med Cent) 2010;23(4):349–354. doi: 10.1080/08998280.2010.11928649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minei JP, Cuschieri J, Sperry J, Moore E, West MA, Harbrecht BG, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40(4):1129–1135. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, et al. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes consortium. Ann Surg. 2014;28 doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eastbridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): Implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 41.Rockett IRH, Reiger MD, Kapusta ND, Coben JH, Miller TR, Hanzlick RL, et al. Leading causes of unintentional and intentional injury mortality: United States, 2000–2009. Am J Public Health. 2012;102(11):e84–e92. doi: 10.2105/AJPH.2012.300960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu G, Baker SP. Recent increases in fatal and non-fatal injury among people aged 65 years and over in the USA. Inj Prev. 2010;16(1):26–30. doi: 10.1136/ip.2009.023481. [DOI] [PubMed] [Google Scholar]
- 43.Mangram AJ, Mitchell CD, Shifflette VK, Lorenzo M, Truitt MS, Goel A, et al. Geriatric trauma service: A one-year experience. J Trauma Acute Care Surg. 2012;72(1):119–122. doi: 10.1097/TA.0b013e318241f0ba. [DOI] [PubMed] [Google Scholar]
- 44.National Trauma Insttitue. The case for trauma funding [Internet] Available from: http://www.nationaltraumainstitute.org. [Google Scholar]
- 45.Holcomb JB, Hoyt DB. Comprehensive injury research. JAMA. 2015;313(14):1463–1464. doi: 10.1001/jama.2014.16802. [DOI] [PubMed] [Google Scholar]