Abstract
Background
Perineural invasion has been found in several types of human tumors, and is associated with poor prognosis; however, few studies have examined perineural invasion in lung cancer. We evaluated the relationship between autonomic nervous densities, pathological risk grading, and prognosis in patients with lung adenocarcinoma (LADC).
Methods
Neural fiber expression was examined by immunofluorescence in resected lung specimens in control patients (n = 30), and low‐risk (n = 22), and high‐risk LADC patients (n = 43). The nerve densities of normal lung tissue and abnormal lung tissues in the tumor and surrounding tissues were evaluated by a semi‐quantitative score method.
Results
Increased sympathetic fibers mainly infiltrated the paratumoral area, while increased parasympathetic fibers were largely restricted to the tumor (paratumor vs. tumor, P = 0.000 in high, P = 0.034 in low; each). In addition, high‐risk patients presented the highest density of neural fibers, followed by low‐risk and control patients (P = 0.000; each). In Kaplan–Meier survival analysis, the densities of sympathetic fibers in paratumoral tissue and parasympathetic fibers in the tumor, respectively, correlated with poor recurrence‐free survival in patients who were not treated with adjuvant therapy (P < 0.001; each). Further multivariate analysis showed that these two factors were associated with poor prognosis in all LADC patients (P = 0.024 sympathetic fibers; P = 0.037 parasympathetic fibers).
Conclusion
These findings reveal a positive correlation between nervous infiltration and risk of poor prognosis in patients with LADC.
Keywords: Autonomic nerve, lung adenocarcinoma, parasympathetic nerve, prognosis, sympathetic nerve
Introduction
Non‐small‐cell lung cancer (NSCLC) is one of the leading causes of cancer‐related death worldwide.1 Lung adenocarcinoma (LADC) is the most common subtype of NSCLC (63.6%), followed by squamous cell (27.4%), and large cell carcinomas (3.6%).2
Nerves are a common characteristic of the tumor microenvironment.3 They are believed to be an indicator of aggressive tumor behavior and have been shown to correlate with poor clinical outcomes in certain extrapulmonary cancers.4 In addition to the metastatic spread route provided by the nerves, the discovery of a neuroendocrine phenomenon, in which nerves provide positive signals to the tumor in the tumor microenvironment, may partially explain the promotion of tumor growth and progression.5 However, few studies of autonomic nerves have been published. Whether autonomic nerves infiltrate human LADC and associate with pathological risk grading and prognosis has not been examined in detail. The aims of the present study were to examine sympathetic adrenergic neural fibers and parasympathetic cholinergic neural fibers in surgically resected lung specimens of 65 LADC patients and 30 non‐cancer patients, focusing on the association of nerve density and cancer pathological risk grading, and the prognostic value of nervous infiltration in human LADC.
Methods
Patient characteristics
Sixty‐five patients with local recurrence or distant metastases after surgical treatment for LADC and 30 patients without cancer who underwent pulmonary surgeries at the Department of Respiratory Diseases, the First Affiliated Hospital, School of Medicine, Zhejiang University between January 1, 2011 and December 31, 2014 were recruited. None of the patients received preoperative thoracic radiation or chemotherapy. Each patient was radiologically examined for recurrence every three months. The non‐cancer patients harbored pulmonary tuberculosis (25 patients), granuloma (3), lung abscess (1), and hamartoma (1). Clinicopathological information, including age, gender, smoking status, pathological tumor node metastasis (pTNM) staging, pathological grade, pathological differentiation, treatment, and prognosis were obtained from patient records. All cancer patients were classified into two groups according to pathological risk: low‐risk cancer (pathological grade I or II, or moderately, well‐to‐moderately or well differentiated; n = 22) or high‐risk cancer (pathological grade III or IV, or poorly or moderately‐to‐poorly differentiated; n = 43; Table S1). The 30 non‐cancer patients were considered the control. Local tumor recurrence was defined as tumor relapse within the same lung or hemithorax. Distant metastases were defined as neoplastic lesions that developed in other organs (outside the lung) or remote lymph nodes. pTNM staging and pathological grade, as well as local recurrence and distant metastasis were determined by the seventh edition clinical TNM staging classification of NSCLC.6
Human samples
Formalin‐fixed paraffin‐embedded surgically resected human lung specimens were serially sectioned (thickness 5 mm). For each block, one section was stained with hematoxylin and eosin (H&E) to evaluate tissue viability and the location of the normal area surrounding the cancer. Adjacent normal tissue ≥ 3 cm from the tumor was considered paratumoral tissue. A further two consecutive sections were stained for tyrosine hydroxylase (TH) and neurofilament (NF), and vesicular acetylcholine transporter (VAChT) and NF, respectively. Nerve score evaluation was performed in a blinded fashion. Two independent pathologists from the Department of Pathology, the First Affiliated Hospital, School of Medicine, Zhejiang University reviewed all the sections of both H&E and immunofluorescence (IF) staining. Disagreements were resolved by discussion.
Histopathology and immunofluorescence
Tyrosine hydroxylase is a useful marker for sympathetic materials in both the central and peripheral sympathetic neurons, and the adrenal medulla. VAChT is a neurotransmitter transporter responsible for loading ACh into secretory organelles in neurons labeling parasympathetic materials. NF is a neuron‐specific cytoskeletal subunit. As TH and VAChT are not nerve specific, co‐expression of NF is necessary to confirm neural specificity. Here, combinations of TH and NF and VAChT and NF, respectively, were used to identify TH+ sympathetic adrenergic neural fibers and VAChT+ parasympathetic cholinergic neural fibers.
Hematoxylin and eosin staining was conducted using standard procedures. For IF analyses, lung sections were fixed with acetone or methanol and incubated in hydrogen peroxide to quench endogenous peroxidase. The sections were then deparaffinized with xylene and rehydrated in a graded alcohol series, followed by antigen retrieval in heat‐treated Tris‐ethylene‐diamine‐tetraacetic acid buffer solution (Vector, Burlingame, CA, USA). Nonspecific binding was blocked by protein block (Dako, Carpinteria, CA, USA). Sections were incubated with a mouse antibody to TH (1:200, Millipore, Billerica, MA, USA), or a rabbit antibody to VAChT (1:5000, MBL International, Woburn, MA, USA), together with a mouse antibody to NF (1:200, LabVision, Burlingame, CA, USA) overnight, followed by secondary biotinylated goat antibody to mouse or rabbit immunoglobulin G (Vector). The signal was amplified by Vectastain Elite ABC Kit (Vector) and visualized by Tyramide Signal Amplification kit for TRITC (PerkinElmer, Hopkinton, MA, USA). DAPI (4′,6‐diamidino‐2‐phenylindole) was used for nuclear staining.
Statistical analysis
The authors semi‐quantitatively scored TH+ and VAChT+ nerve specific fibers in the whole section in the control group, and in the tumor and remaining normal lung tissues surrounding the tumor in the cancer groups, respectively. A semiquantitative score was established as follows: 0 = no neural fibers (0 fiber per field), 1 = scarce number of neural fibers (1–3 fibers per field), 2 = scarce to moderate neural fibers (4–6 fibers per field), 3 = moderate neural fibers (7–9 fibers per field), and 4 = numerous neural fibers (≥ 10 fibers per field). Pearson chi‐square or Fisher's exact tests were conducted in certain clinical characteristics. A nonparametric analysis of variance was performed to identify statistical significance among the three groups (Kruskal–Wallis test), or between the two groups (Mann–Whitney U test). A Spearman test was conducted to detect the correlation between nervous infiltration and pathological stage, differentiation, and risk grading. A prognostic examination with recurrence‐free interval as the endpoint was then performed. The recurrence‐free interval was defined as the interval between the date of surgical resection and the first evidence of either local recurrence or distant metastasis after surgery, by 31 December 2015. Kaplan–Meier was used to assess the recurrence‐free survival (RFS) curve and statistical significance was calculated by log‐rank test. In addition, multivariate analysis, which included pathological stage, adjuvant therapy, pathological differentiation, distant metastasis, local tumor recurrence, number of sympathetic fibers in paratumoral lung tissue, number of parasympathetic fibers in the tumor, and family history of cancer, was conducted. All analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
The institutional review board approved the study and individual consensus was obtained from each patient.
Results
Patient characteristics
The median follow‐up was 15.8 months (range 3–40.2). Postoperative local recurrence (n = 45, 69.2%) or distant metastasis (n = 30, 46.2%) was detected in the cancer patients from 0.9 to 32.5 months. The median duration from surgery to local recurrence or distant metastasis was 27.6 months in low‐risk and 24.8 in high‐risk patients. The presence of postoperative local recurrence was higher than distant metastasis, but was not statistically significant (P = 0.078 high risk, P = 0.069 low risk). Regrettably, mortality data were not recorded in the current study because many patients were lost to follow‐up after recurrence or metastasis. Postoperative adjuvant therapy included maintenance chemotherapy in 38 (58.5%) patients and molecular‐targeted agent therapy in two (3.1%) with epidermal growth factor receptor mutations. Further patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Clinical characteristics | Results | P | ||
|---|---|---|---|---|
| Control (n = 30) | Low‐risk (n = 22) | High‐risk (n = 43) | ||
| Age, years | 0.191 | |||
| Mean (SD) | 60.3 (12.8) | 55.4 (8.8) | 59.8 (12.0) | |
| Median (range) | 62 (38–82) | 54.5 (36–74) | 61 (34–81) | |
| Gender | 1.000 | |||
| Male (%) | 20 (66.7) | 10 (45.5) | 23 (53.5) | |
| Female (%) | 10 (33.3) | 12 (54.5) | 20 (46.5) | |
| Smokers (%) | — | 8 (36.4) | 20 (46.5) | 0.434 |
| Hypertension (%) | 5 (16.7) | 6 (27.3) | 14 (32.6) | 0.318 |
| Arrhythmia (%) | 2 (6.7) | 3 (13.6) | 6 (14.0) | 0.599 |
| Pathological stage | 0.000 | |||
| IA (%) | — | 6 (27.3) | 1 (2.3) | — |
| IB (%) | 5 (22.7) | 0 (0) | ||
| IIA (%) | 8 (36.4) | 8 (18.6) | ||
| IIB (%) | 3 (13.6) | 2 (4.7) | ||
| IIIA (%) | 0 (0) | 11 (25.6) | ||
| IIIB (%) | 0 (0) | 2 (4.7) | ||
| IV (%) | 0 (0) | 19 (44.2) | ||
| Pathological differentiation | 0.000 | |||
| Poorly (%) | — | 0 (0) | 3 (7.0) | — |
| Moderately to poorly (%) | 0 (0) | 34 (79.1) | ||
| Moderately (%) | 13 (59.1) | 6 (14.0) | ||
| Well to moderately (%) | 3 (13.6) | 0 (0) | ||
| Well (%) | 6 (27.3) | 0 (0) | ||
SD, standard deviation.
Autonomic nervous infiltration in different groups
Autonomic nervous infiltration was present in 96.9% cancer patients (100% in high risk, 90.9% in low risk patients). To ascertain whether both TH+ and VAChT+ neural fibers contributed equally to the increased number of fibers, we performed further individual assessment of each branch of the autonomic nervous system. We found that TH+ neural fibers were present in 100% (n = 43) of high‐risk patients and 77.3% (n = 17) of low‐risk patients, and were predominantly localized in the normal lung tissue surrounding the tumor (paratumor vs. tumor; P = 0.000). By contrast, VAChT+ parasympathetic cholinergic neural fibers, which were largely restricted to the tumor, were detected in 95.3% (n = 41) of high‐risk and 81.8% (n = 18) of low‐risk patients (paratumor vs. tumor; P = 0.034).
Sympathetic nervous infiltration in different groups
Immunofluorescence showed that high TH+ nerve densities in paratumoral lung tissues were strongly associated with a higher risk of cancer (Fig 1a,b). A semi‐quantitative score of the results was performed, which revealed that TH+ score was increased in the high‐risk cancer group. The lowest average and median TH+ scores were found in the control (0.7 and 1, respectively), followed by the low‐risk (1 each) and high‐risk groups (2 each). Further assessment suggested that there was a statistically significant difference in the TH+ score among different groups (all, P = 0.000; low vs. control, P = 0.026; high vs. control, P = 0.000; high vs. low, P = 0.001; Table 2). Of the 22 patients in the low risk group, 5 (22.7%), 10 (45.5%), 3 (13.6%), 4 (18.2%), and 0 patients presented scores of 0–4, respectively. In the high‐risk group, 3 (7%), 5 (11.6%), 17 (39.5%), 13 (30.2%), and 5 (11.6%) patients presented a TH+ score of 0–4, respectively (Fig 1c). The percentage of patients with a TH+ score ≥ 3 varied significantly among the groups (P < 0.001): one (3.3%) in the control, four (18.2%) in the low risk, and 18 (41%) in the high risk group, respectively (Fig 1d).
Figure 1.
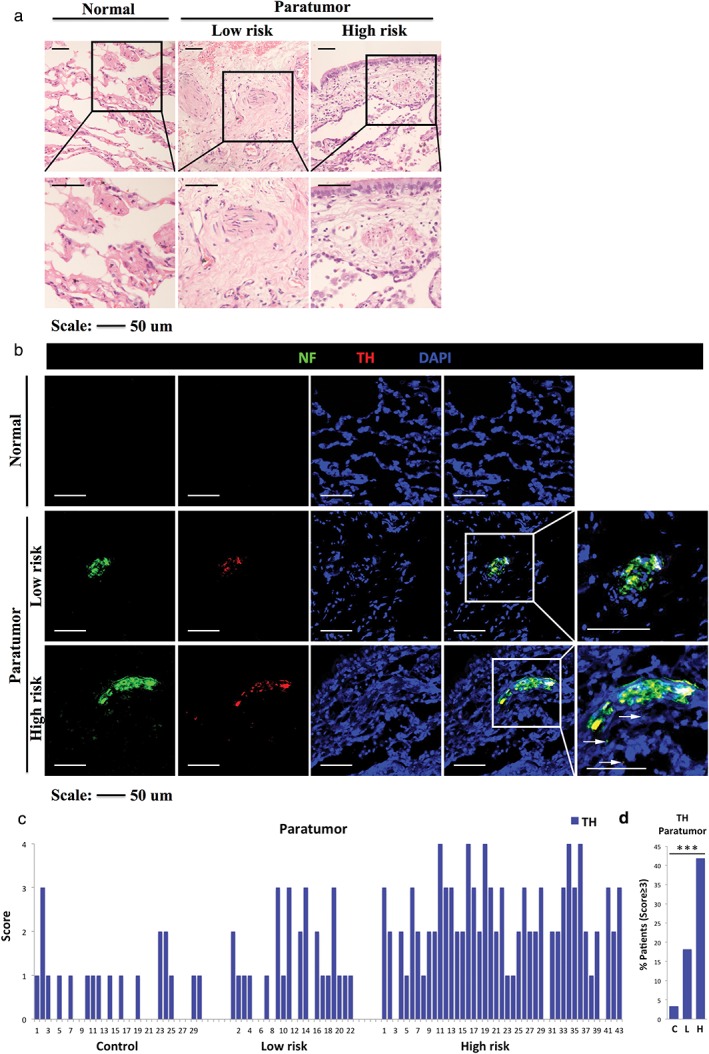
(a) Representative hematoxylin and eosin (H&E) images of normal lung tissue in the control (left), paratumoral lung tissue in the low‐risk (middle) and high‐risk groups (right). Boxed area shows a higher magnification of H&E images (similar size as [b]). (b) Almost consecutive frozen sections stained with immunofluorescence for neurofilament (NF; a specific nerve marker), tyrosine hydroxylase (TH; an adrenergic marker) and 4′,6‐diamidino‐2‐phenylindole (DAPI) of normal lung tissue in the control (score = 0), and paratumoral lung tissue in the low‐risk (score = 2) and high‐risk groups (score = 4). Boxed area shows higher magnification of infiltrating nerves. Arrows show tiny nerve fibers. (c) Score of immunostained TH+ neural fibers in normal lung tissue in the control (n = 30), in paratumoral lung tissue in the low risk (n = 22) and high‐risk groups (n = 43). (d) Percentage of cancer patients with TH+ score ≥ 3 in the control, low‐risk and high‐risk groups. ***P < 0.001. Scale bar, 50 μm.
Table 2.
Nerve score characteristics
| Group | Results | P | |||
|---|---|---|---|---|---|
| Control (n = 30) | Low‐risk (n = 22) | High‐risk (n = 43) | ALL/HL/HC/LC | Paratumor vs. tumor | |
| Paratumor, TH | 0.000 | 0.000Hth | |||
| 0 (%) | 14 (46.7) | 5 (22.7) | 3 (7.0) | 0.001HL | — |
| 1 (%) | 13 (43.3) | 10 (45.5) | 5 (11.6) | 0.000HC | |
| 2 (%) | 2 (6.7) | 3 (13.6) | 17 (39.5) | 0.026LC | |
| 3 (%) | 1 (3.3) | 4 (18.2) | 13 (30.2) | ||
| 4 (%) | 0 (0) | 0 (0) | 5 (11.6) | ||
| Tumor, TH | 0.160 | 0.034Lth | |||
| 0 (%) | 14 (46.7) | 10 (45.5) | 10 (23.3) | 0.125HL | — |
| 1 (%) | 13 (43.3) | 10 (45.5) | 29 (67.4) | 0.094HC | |
| 2 (%) | 2 (6.7) | 2 (9.1) | 4 (9.3) | 0.984LC | |
| 3 (%) | 1 (3.3) | 0 (0) | 0 (0) | ||
| 4 (%) | 0 (0) | 0 (0) | 0 (0) | ||
| Paratumor, VAChT | 0.171 | 0.000Hvt | |||
| 0 (%) | 15 (50.0) | 12 (54.5) | 15 (34.9) | 0.109HL | — |
| 1 (%) | 14 (46.7) | 9 (40.9) | 23 (53.5) | 0.137HC | |
| 2 (%) | 0 (0) | 1 (4.5) | 5 (11.6) | 0.784LC | |
| 3 (%) | 1 (3.3) | 0 (0) | 0 (0) | ||
| 4 (%) | 0 (0) | 0 (0) | 0 (0) | ||
| Tumor, VAChT | 0.000 | 0.034Lvt | |||
| 0 (%) | 15 (50.0) | 7 (31.8) | 2 (4.7) | 0.001HL | — |
| 1 (%) | 14 (46.7) | 8 (36.4) | 10 (23.3) | 0.000HC | |
| 2 (%) | 0 (0) | 4 (18.2) | 17 (39.5) | 0.038LC | |
| 3 (%) | 1 (3.3) | 3 (13.6) | 11 (25.6) | ||
| 4 (%) | 0 (0) | 0 (0) | 3 (7.0) | ||
HC, high‐risk compared with the control group; HL, high‐risk compared with the low‐risk group; Hth/Lth, mean tyrosine hydroxylase (TH) nerve score in the paratumoral area compared with that in the tumor in the high/low risk group, respectively; Hvt/Lvt, mean vesicular acetylcholine transporter (VAChT) nerve score in the tumor compared with that in the paratumoral area in the high/low risk group, respectively; LC, low‐risk compared with the control group.
Cholinergic nervous infiltration in different groups
Immunofluorescence staining demonstrated that a drastic increase of VAChT+ nerve density in the tumor was associated with an increased cancer risk (Fig 2a,b), which was confirmed following evaluation of the VAChT+ score within the tumor. Similar to the TH+ score, control patients presented the lowest average and median VAChT+ scores (0.6 and 0.5, respectively), followed by low‐risk (1 each) and high‐risk patients (2 each). Our results suggested that there was a statistically significant difference in the VAChT+ score between the different groups (all P = 0.000; low vs. control P = 0.038; high vs. control P = 0.000; high vs. low P = 0.001; Table 2). In the low risk group patients, 7 (31.8%), 8 (36.4%), 4 (18.2%), 3 (13.6%) and 0 presented VAChT+ scores of 0–4, respectively. Of the 43 patients in the high risk group, 2 (4.7%), 10 (23.3%), 17 (39.5%), 11 (25.6%) and 3 patients (7%) presented scores of 0–4, respectively (Fig 2c). There was a significant difference in the proportion of patients with a VAChT+ score ≥ 3 (P < 0.01): 1 (3.3%) in the control, 3 (13.6%) in the low‐risk, and 14 (32.6%) in the high‐risk group (Fig 2d).
Figure 2.
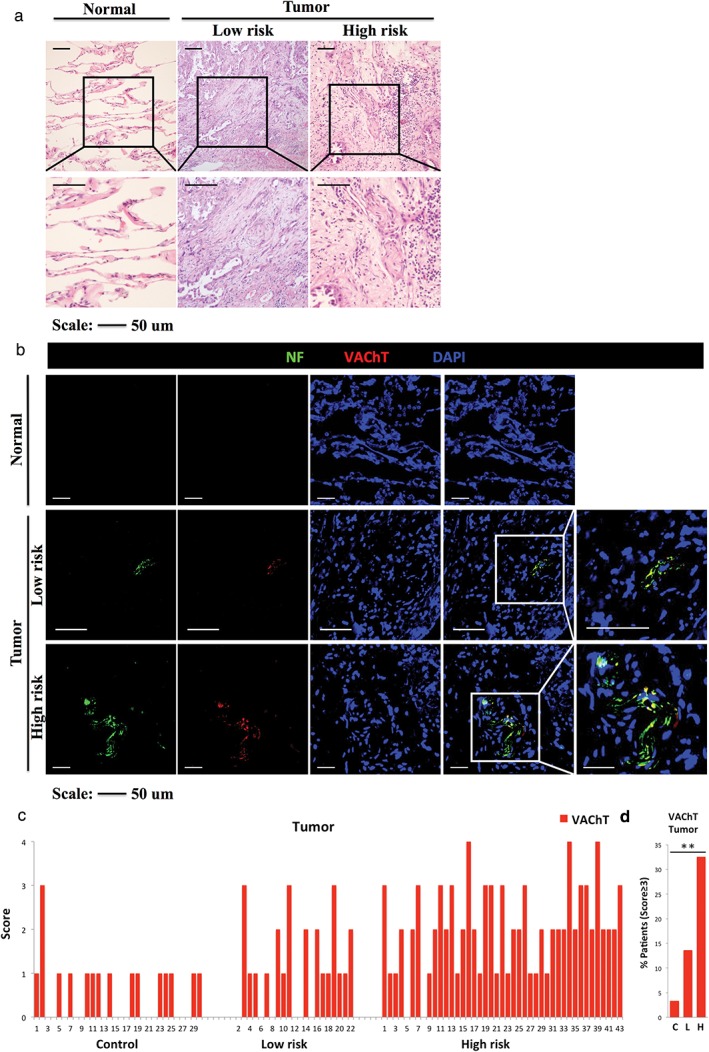
(a) Representative hematoxylin and eosin (H&E) images of normal lung tissue in the control (left), and tumor in the low‐risk (middle) and high‐risk groups (right). Boxed area shows a higher magnification of H&E images (similar size as [b]). (b) Almost consecutive frozen sections stained with immunofluorescence for neurofilament (NF; a specific nerve marker), vesicular acetylcholine transporter (VAChT; a cholinergic marker) and 4′,6‐diamidino‐2‐phenylindole (DAPI) of normal lung tissue in the control (score = 0), and the tumor in the low‐risk (score = 2) and high‐risk groups (score = 4). Boxed area shows higher magnification of infiltrating nerves. (c) Score of immunostained VAChT+ neural fibers in normal lung tissue in the control (n = 30) and in the tumor in the low‐risk (n = 22) and high‐risk groups (n = 43). (d) Percentage of cancer patients with VAChT+ score ≥ 3 in the control, low‐risk and high‐risk groups. **P < 0.01. Scale bar, 50 μm.
Correlation between nerve infiltration and clinicopathological characteristics
Spearman correlation analysis showed that the densities of sympathetic fibers in the remaining normal lung tissue surrounding the tumor and parasympathetic fibers in the tumor, respectively, strongly correlated with pathological risk grading (P = 0.000 and P = 0.001, respectively). However, there was no significant correlation between nerve infiltration and pathological stage (P = 0.572 for sympathetic fibers, P = 0.391 for parasympathetic fibers) or pathological differentiation (P = 0.146 for sympathetic fibers, P = 0.106 for parasympathetic fibers).
Correlation between nerve infiltration and postoperative recurrence‐free survival
Our study has revealed that the number of TH+ neural fibers in normal tissues surrounding the tumor was positively correlated with low RFS. A TH+ score ≥ 3 at diagnosis was associated with a higher recurrence rate in all cancer patients (P < 0.001; Fig 3a) and patients not treated with postoperative adjuvant therapy (P < 0.001; Fig 3b), but not patients treated with postoperative adjuvant therapy (P = 0.239; Fig 3c).
Figure 3.
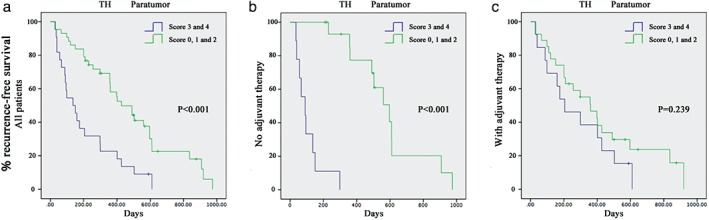
(a) Recurrence‐free survival of cancer patients with high (tyrosine hydroxylase [TH+] nerve score ≥ 3) and low (TH+ nerve score < 3) adrenergic nerve densities in paratumoral lung tissue. P < 0.001 in all patients. (b) P < 0.001 in the patients who were not treated with postoperative adjuvant therapy. (c) P = 0.239 in patients treated with postoperative adjuvant therapy.
A VAChT+ score ≥ 3 at diagnosis showed a stronger trend toward an association with a high rate of recurrence in all cancer patients (P = 0.003; Fig 4a), and patients not treated with postoperative adjuvant therapy (P < 0.001; Fig 4b). However, it was not associated with poor survival in patients treated with adjuvant therapy (P = 0.170; Fig 4c).
Figure 4.
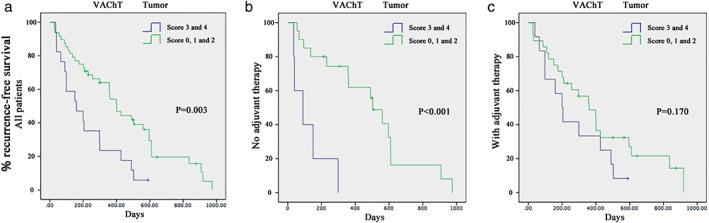
(a) Recurrence‐free survival of cancer patients with high (vesicular acetylcholine transporter [VAChT+] nerve score ≥ 3) and low (VAChT+ nerve score < 3) cholinergic nerve densities in tumor. P = 0.003 in all patients. (b) P < 0.001 in the patients who were not treated with postoperative adjuvant therapy. (c) P = 0.170 in patients treated with postoperative adjuvant therapy.
Multivariate analysis showed that pathological stage (P = 0.007), adjuvant therapy (P = 0.002), family history of cancer (P = 0.048), number of sympathetic fibers in paratumoral lung tissue (P = 0.024), and number of parasympathetic fibers in the tumor (P = 0.037) were factors associated with poor postoperative RFS. In contrast, pathological differentiation (P = 0.193), local tumor recurrence (P = 0.303), and distant metastasis (P = 0.09) did not contribute to a poor prognosis.
Discussion
Our group examined neural fibers in human resected lung specimens, revealing a large prevalence of innervation in the tumor and surrounding normal tissue (96.9%, n = 63). This study focused on these increased nerves, their relationship to tumor pathological risk grading, and their prognostic value for disease recurrence.
The autonomic nervous system has two separate divisions, the sympathetic and parasympathetic nervous systems, which usually function in opposition to each other. To date, there have been few studies of autonomic nerves in lung cancer. On the one hand, several lines of evidence have shown that the sympathetic nervous system is strongly correlated with lung tumor progression. In vivo experiments have demonstrated that activation of the sympathetic nervous system plays a critical role in promoting lung metastases but not growth of the primary tumor. Sloan et al. demonstrated that isoproterenol, which is a β‐adrenergic receptor agonist, induced 30‐fold metastasis to the lung in primary breast cancer in mice.7 Another two β‐adrenergic receptor agonists, propranolol and nadolol, have been subsequently reported to have a similar impact in rats.8, 9 In contrast, inhibition of the sympathetic nervous system can effectively protect mice against lung metastases. Vegas et al. indicated that antalarmin, a corticotropin‐releasing factor receptor antagonist, significantly reduced the development of pulmonary metastases in melanoma in mice.10 More recently, the administration of carvedilol, a β1, β2 and α‐adrenergic receptor antagonist, has been reported to prevent smokers and nicotine addicts from developing primary pulmonary tumors.11 On the other hand, the cholinergic system is essentially based on acetylcholine (Ach) as a mediator. Many epithelial and endothelial cells in airways express non‐neural origin Ach, promoting normal cell proliferation and conferring susceptibility to lung carcinoma.12 Lung cancers have also been found to secret Ach through an autocrine cholinergic loop to stimulate tumor growth.13, 14, 15 In addition to Ach, VAChT is another essential component of the cholinergic system. Research on in vitro culture and in vivo nude mice models has suggested that vesamicol, a VAChT antagonist, can induce potent apoptosis of human bronchioalveolar carcinoma.16 These findings suggest the possibility that the cholinergic system may contribute to tumor progression through certain transmitter substances.
Currently, the most essential factors for pathological risk grading of resected NSCLC are TNM staging and pathological differentiation. According to the latest epidemiological data on prognosis published in 2015 by the International Association for the Study of Lung Cancer, advanced pTNM staging represented a rapid decline in median survival time, from 33 months in stage IB to 7.3 in stage IV. The longest one‐year survival was reported in stage I (91–100%), followed by stage II (80–87%), stage III (48–58%), and stage IV (27%). Similarly, the longest two‐year survival was reported in stage 1 (68–93%), with a significant decrease by stage IV (8%).2, 17, 18, 19 These data suggest that high TNM staging is a strong indicator of poor survival. In addition to TNM staging, pathological differentiation is also thought to have important prognostic value. Patients with diseases with poor tumor differentiation were more often in a higher stage at diagnosis, with a greater risk of recurrence or metastasis, and a shorter survival time after diagnosis.20 In a study by Chung et al. of 96 cases, well or moderately differentiated lung cancer patients had lower rates of lymph node metastasis, local recurrence, and shorter survival compared with poorly differentiated.5
The relationship between nerves and pathological risk grading of cancer was not studied in detail until 2013 when Magnon et al. found evidence linking dense innervation and high pathological risk grading in prostate cancer.4 However, whether a similar phenomenon exists in lung cancer is unknown. Yilmaz et al. reported that the prevalence of perineural invasion did not correlate with pTNM staging in a review of 289 patients with NSCLC.21 In the 53 patients who had perineural invasion, the highest prevalence was found in pathological grade II (47.2%, n = 25), followed by grade I (28.3%, n = 15), and grades III–IV (24.5%, n = 13). In the same study, patients with perineural invasion (71.7%, n = 38) tended to have tumor cells with moderate to poor differentiation, which was confirmed at a higher percentage (85.7%) by Hsieh et al. in a study of 108 cases.1 Perineural invasion includes the invasion of both sympathetic and parasympathetic nerve fibers. Our results for a relationship between the prevalence of individual nerve fibers and pTNM staging were similar to Yilmaz et al.’s study for both nerve fibers (P = 0.572 for sympathetic fibers, P = 0.391 for parasympathetic fibers); however, our results for a relationship between prevalence and pathological differentiation were not (P = 0.146 for sympathetic fibers, P = 0.106 for parasympathetic fibers). In addition, our findings strongly suggest that nerve densities are paralleled with pathological risk grading (P = 0.000 for sympathetic fibers, P = 0.001 for parasympathetic fibers). These results indicate that although nerve infiltration is not associated with either pTNM staging or pathological differentiation, it is paralleled with cancer risk assessment based on a combination of these two important factors, and, therefore, may have a potential role in predicting cancer risk. Regarding the mechanism, on the one hand, we can hypothesize that cancer cells release substances like growth factors, which stimulate nerves in both tumors and neighboring lung tissue.22 On the other hand, nerves provide survival signals to tumors or provide a gateway toward hematogenous spread.5 To the best of our knowledge, this is the first study in which the individual density of sympathetic or parasympathetic neural fibers and their relationship with pathological risk grading in human lung cancer have been explored.
Perineural invasion has been determined as a poor prognostic factor in prostate, pancreatic, breast, and rectal cancers.4, 23, 24, 25 However, the prognostic relevance of perineural invasion in lung cancer remains controversial. In some studies, perineural invasion was concluded as an independent predictive factor for poor survival.1, 26, 27, 28, 29 Nevertheless, many studies have not determined whether nerves are related to cancer survival.30, 31 Discrepancies between these results may be explained in part by small sample sizes that are not sufficient to detect moderate predictive effects, different grading criteria among pathologists, and inconsistent grouping systems among the pathological grades analyzed. To further ascertain whether nerve infiltration contributed to a poor prognosis, Kaplan–Meier survival analysis and multivariate analysis were performed. Although our results revealed that nerve densities play a prognostic role in LADC patients, we hypothesized that adjuvant therapy may eliminate this prognostic indication. Therefore, further Kaplan–Meier survival analysis was conducted with patients separated into two groups: those who received postoperative adjuvant therapy and those who did not. Our findings confirmed this conjecture. Nerve densities were closely correlated with disease‐free interval in patients who did not receive postoperative adjuvant therapy. The high density of sympathetic neural fibers in the paratumoral area (P < 0.001), or the high density of parasympathetic neural fibers in the tumor (P < 0.001) at diagnosis, respectively, was related to higher recurrence rates. However, such significant differences were not detected in patients who were treated with postoperative adjuvant therapy (P = 0.239 in the sympathetic nerves, P = 0.170 in the sympathetic nerves, respectively). Multivariate analysis revealed that density of sympathetic fibers in paratumoral lung tissue (P = 0.024), density of parasympathetic fibers in the tumor (P = 0.037), adjuvant therapy (P = 0.002), pathological stage (P = 0.007) and family history of cancer (P = 0.048) were factors associated with poor RFS. All of these findings indicate that nerve infiltration is a dependent prognostic factor, regardless of adjuvant therapy, and, therefore, could be a prognostic indicator for LADC patients.
Our study had some limitations. The small sample size and semi‐quantitative score method might limit the reproducibility of our findings. Quantitative studies with a large series are required to evaluate the value of nerve infiltration in LADC.
In this study, we found that infiltration of autonomic nerves was upregulated in tissues from patients with LADC, and revealed a positive correlation between infiltration of autonomic nerves and prognosis in these patients. Our findings indicate that the density of infiltrated autonomic nerves may serve as a potential parameter for pathological risk grading and as a biomarker for prognosis in patients with LADC.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Clinical and pathological characteristics of patients with lung adenocarcinoma.
Acknowledgments
This study was supported by research funding from the National Natural Science Foundation of China (No. 81472171) and the major project of Science and Technology Department of Zhejiang Province, China (No. 2012C13022‐2).
References
- 1. Hsieh CP, Fu JY, Liu YH et al. Prognostic factors in resectable pathological N2 disease of non‐small cell lung cancer. Biochem J 2015; 38: 329–35. [DOI] [PubMed] [Google Scholar]
- 2. Rami‐Porta R, Bolejack V, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015; 10: 990–1003. [DOI] [PubMed] [Google Scholar]
- 3. Ayala GE, Dai H, Tahir SA et al. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res 2006; 66: 5159–64. [DOI] [PubMed] [Google Scholar]
- 4. Magnon C, Hall SJ, Lin J et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341: 1236361. [DOI] [PubMed] [Google Scholar]
- 5. Chung CK, Zaino R, Stryker JA, O'Neill M Jr, DeMuth WE Jr. Carcinoma of the lung: Evaluation of histological grade and factors influencing prognosis. Ann Thorac Surg 1982; 33: 599–604. [DOI] [PubMed] [Google Scholar]
- 6. Groome PA, Bolejack V, Crowley JJ et al. The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 694–705. [DOI] [PubMed] [Google Scholar]
- 7. Sloan EK, Priceman SJ, Cox BF et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010; 70: 7042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shakhar G, Ben‐Eliyahu S. In vivo beta‐adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol 1998; 160: 3251–8. [PubMed] [Google Scholar]
- 9. Ben‐Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and beta‐adrenoceptors. Neuroimmunomodulation 2000; 8: 154–64. [DOI] [PubMed] [Google Scholar]
- 10. Vegas O, Garmendia L, Arregi A, Beitia G, Azpiroz A. Effects of antalarmin and nadolol on the relationship between social stress and pulmonary metastasis development in male OF1 mice. Behav Brain Res 2009; 205: 200–6. [DOI] [PubMed] [Google Scholar]
- 11. McCarty MF, O'Keefe JH, DiNicolantonio JJ. Carvedilol and spirulina may provide important health protection to smokers and other nicotine addicts: A call for pertinent research. Mo Med 2015; 112: 72–5. [PMC free article] [PubMed] [Google Scholar]
- 12. Saracino L, Zorzetto M, Inghilleri S, Pozzi E, Stella GM. Non‐neuronal cholinergic system in airways and lung cancer susceptibility. Transl Lung Cancer Res 2013; 2: 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spindel ER. Cholinergic targets in lung cancer. Curr Pharm Des 2016; 22: 2152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao Q, Gu X, Zhang C, Lu Q, Chen H, Xu L. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial‐mesenchymal transition (EMT) in non‐small cell lung cancer (NSCLC). Cancer Biol Ther 2015; 16: 634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spindel ER. Muscarinic receptor agonists and antagonists: Effects on cancer In: Fryer AD, Christopoulos A, Nathanson MM. (eds). Handbook of Experimental Pharmacology, Muscarinic Receptors , Vol. 208 Springer‐Verlag, Berlin, Heidelberg: 2012; 451–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lau JK, Brown KC, Thornhill BA et al. Inhibition of cholinergic signaling causes apoptosis in human bronchioalveolar carcinoma. Cancer Res 2013; 73: 1328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstraw P, Chansky K, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 18. Eberhardt WE, Mitchell A, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2015; 10: 1515–22. [DOI] [PubMed] [Google Scholar]
- 19. Rami‐Porta R, Bolejack V, Giroux DJ et al. The IASLC lung cancer staging project: The new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014; 9: 1618–24. [DOI] [PubMed] [Google Scholar]
- 20. Sun Z, Aubry MC, Deschamps C et al. Histologic grade is an independent prognostic factor for survival in non‐small cell lung cancer: An analysis of 5018 hospital‐ and 712 population‐based cases. J Thorac Cardiovasc Surg 2006; 131: 1014–20. [DOI] [PubMed] [Google Scholar]
- 21. Yilmaz A, Duyar SS, Cakir E et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non‐small cell lung cancer. Eur J Cardiothorac Surg 2011; 40: 664–70. [DOI] [PubMed] [Google Scholar]
- 22. Panuncio A, Vignale R, Lopez G. Immunohistochemical study of nerve fibres in basal cell carcinoma. Eur J Dermatol 2003; 13: 250–3. [PubMed] [Google Scholar]
- 23. Ozaki H, Hiraoka T, Mizumoto R et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today 1999; 29: 16–22. [DOI] [PubMed] [Google Scholar]
- 24. Margolese RG, Lasry JC. Ambulatory surgery for breast cancer patients. Ann Surg Oncol 2000; 7: 181–7. [DOI] [PubMed] [Google Scholar]
- 25. Shirouzu K, Isomoto H, Kakegawa T. Prognostic evaluation of perineural invasion in rectal cancer. Am J Surg 1993; 165: 233–7. [DOI] [PubMed] [Google Scholar]
- 26. Kiliçgün A, Turna A, Sayar A, Solak O, Urer N, Gürses A. Very important histopathological factors in patients with resected non‐small cell lung cancer: Necrosis and perineural invasion. Thorac Cardiovasc Surg 2010; 58: 93–7. [DOI] [PubMed] [Google Scholar]
- 27. Sayar A, Turna A, Solak O, Kiliçgün A, Urer N, Gürses A. Nonanatomic prognostic factors in resected nonsmall cell lung carcinoma: The importance of perineural invasion as a new prognostic marker. Ann Thorac Surg 2004; 77: 421–5. [DOI] [PubMed] [Google Scholar]
- 28. Demir A, Gunluoglu MZ, Kara HV, Buyukpinarbasili N, Dincer SI. Prognostic factors in resected T3 non‐small cell lung carcinoma: Perineural invasion as a new prognostic factor. Thorac Cardiovasc Surg 2008; 56: 93–8. [DOI] [PubMed] [Google Scholar]
- 29. Filosso PL, Ruffini E, Asioli S et al. Adenosquamous lung carcinomas: A histologic subtype with poor prognosis. Lung Cancer 2011; 74: 25–9. [DOI] [PubMed] [Google Scholar]
- 30. Poncelet AJ, Cornet J, Coulon C et al. Intra‐tumoral vascular or perineural invasion as prognostic factors for long‐term survival in early stage non‐small cell lung carcinoma. Eur J Cardiothorac Surg 2008; 33: 799–804. [DOI] [PubMed] [Google Scholar]
- 31. Park C, Lee IJ, Jang SH, Lee JW. Factors affecting tumor recurrence after curative surgery for NSCLC: Impacts of lymphovascular invasion on early tumor recurrence. J Thorac Dis 2014; 6: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical and pathological characteristics of patients with lung adenocarcinoma.


