Abstract
Primary pulmonary angiosarcoma is a rare type of malignant vascular tumor with a very aggressive clinical course and a grim prognosis. To date, only a handful of cases have been reported in English literature. Its rarity and consequent low index of suspicion makes clinical diagnosis difficult. In this report we present three cases of primary pulmonary angiosarcoma with cough, hemoptysis, and progressive dyspnea to contribute to the sparse literature on this disease. A review is made of previous reports of primary pulmonary angiosarcomas, and the clinical characteristics, diagnosed method, treatment options, and prognosis of pulmonary angiosarcoma are also discussed.
Keywords: Angiosarcoma, biopsy, immunohistochemistry
Introduction
Angiosarcoma of the lung is a rare tumor that most often presents as metastatic disease. Primary pulmonary angiosarcoma is rarely reported. Because of the rarity of this disease, our understanding of primary pulmonary angiosarcoma is limited. In this report we present three cases of primary pulmonary angiosarcoma with a brief review, to contribute to the sparse literature on this disease.
Case Report
Case 1
A 59‐year‐old woman presented with increasing dyspnea and bloody sputum, which had occurred over a period of three months before admission. Her past medical history and physical examination were unremarkable, except for exposure to tuberculous infection 20 years ago. Computed tomography (CT) pulmonary angiography performed in another hospital revealed a filling defect in the right upper pulmonary artery. She was initially sequentially treated for pulmonary thromboembolism with low molecular weight heparin and warfarin. However, as her shortness of breath worsened, she was admitted to our hospital for further investigation. At admission, her vital signs were stable. A clinical respiratory examination revealed decreased respiratory sounds in the bilateral lung zone. Chest CT showed bilateral pleural effusion and multiple nodules with scattered areas of ground glass opacity in the lung parenchyma (Fig 1a). Pleural fluid was hemorrhagic and exudative by Light's criteria. Repeated cytologic tests of pleural effusion were negative for tumor cells. No extrapulmonary lesions were identified by positron emission tomography (PET) scan; therefore, metastatic disease was excluded. The patient underwent video‐assisted thoracoscopy surgery (VATS), which revealed diffuse small nodular lesions on the parietal pleura with vacuolar changes on the left lung (Fig 1c). Thoracoscopy biopsy revealed malignant cell infiltration (positive for vimentin, CD31, CD34; negative for S100, thyroid transcription factor‐1 [TTF‐1]/Napsin‐A/carcinoembryonic antigen, cytokeratin [CK]7, CK5, CK20, caudal type homeobox 2, gross cystic disease fluid protein‐15, D2‐40, metastatic carcinoma [MC], calretinin, and periodic acid–Schiff–diastase), consistent with pulmonary primary angiosarcoma (Fig 1b,d). The patient had to abandon further chemotherapy because of physical weakness. After two months of supportive therapy, she died as a result of respiratory failure.
Figure 1.
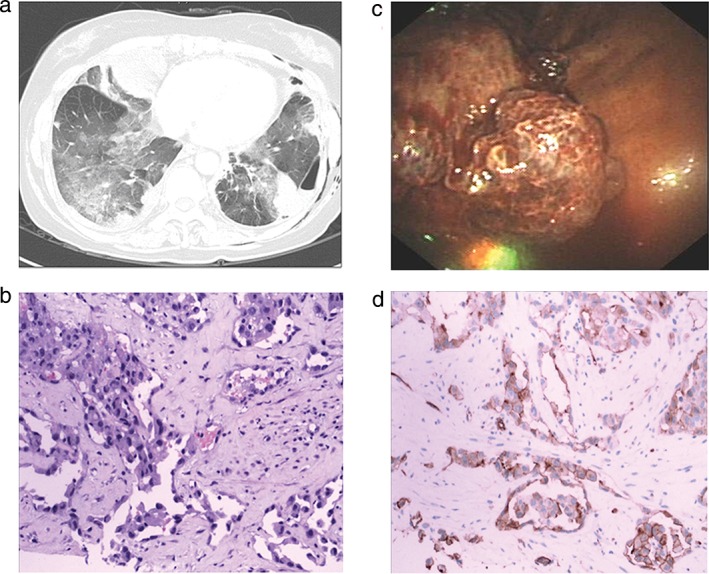
(a) Thoracic computed tomography scan revealed multiple nodules with scattered areas of ground glass opacity in the lung parenchyma. (b) Specimen stained with hematoxylin and eosin showed histological findings of epithelioid angiosarcoma. (c) Video‐assisted thoracoscopy revealed diffuse small nodular lesions on parietal pleura with vacuolar changes on the left lung. (d) Histological findings of epithelioid angiosarcoma with CD31.
Case 2
A 32‐year‐old non‐smoking female with no past medical history presented to our emergency department with a two‐month history of productive cough, intermittent hemoptysis, and progressive dyspnea on exertion. During physical examination, the patient was anxious and appeared ill. She was tachycardic to 130/minute; her BP was 100/70 mmHg; her respiratory rate 32/minute; and her pulse oximetry 90% on room air. A chest examination revealed a reduction in respiratory sounds in the bilateral lower lung zone. Chest CT revealed bilateral pleural effusion, multiple nodules, ground‐glass opaque lesions, and large pericardial effusion (Fig 2a). A PET scan failed to reveal any tumors in other locations. Laboratory tests for infection, blood dyscrasia, and connective tissue disease were all negative. After introduction of a pericardium tube, 1000 mL of hematic fluid was drained. A cytological study of the pericardium effusion did not reveal any definite results. The patient underwent a bronchoscopy and transbronchial biopsies; however, these did not contribute to a diagnosis. A left medical thoracoscopy with pleural biopsies was performed and showed multiple visceral pleural flat nodules of a dark red color (Fig 2c). Histologic examination of the pleura biopsy specimen showed vascular spaces lined by malignant endothelial cells, some containing vesicular nuclei, which is suggestive of high‐grade angiosarcoma (Fig 2b,d). The patient was treated with intermittent Endostar and supportive therapy for two months with little improvement in her shortness of breath. She died as a result of respiratory failure after four months.
Figure 2.
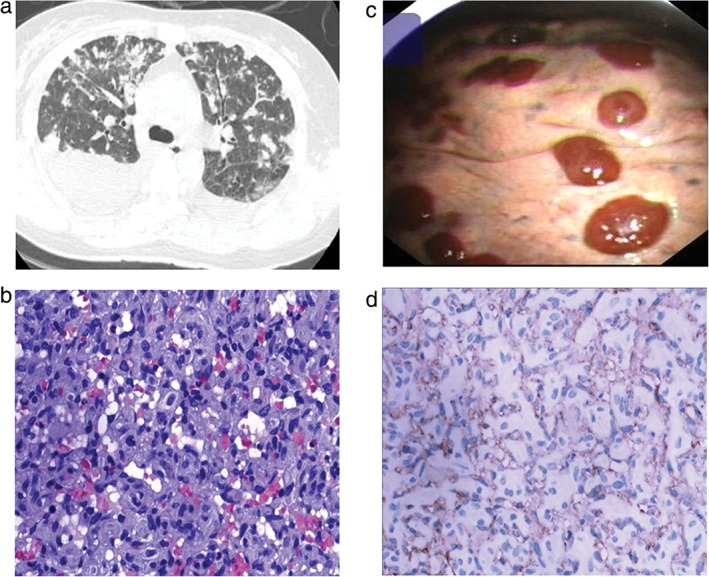
(a) Computed tomography of the chest revealed bilateral pleural effusion, multiple nodular, ground glass opaque lesions and large pericardial effusion. (b) Hematoxylin and eosin showed vasoformative architecture in the myxoid matrix, with intracytoplasmic lumina. (c) Medical thoracoscopy showed multiple visceral pleural flat nodules of a dark red color. (d) Immunohistochemical findings of epithelioid angiosarcoma with CD31.
Case 3
A 69‐year‐old, chronic smoking male presented with cough, bloody sputum, and weight loss of a five‐month duration. He had a history of hypertension and diabetes. He had consulted a general physician, and chest CT had shown multiple nodules in the central and peripheral portions of both lungs. Many of the nodules were surrounded by a halo of ground‐glass haziness (Fig 3a). He had initially been treated for pneumonia with various antibiotics in another medical center but had remained symptomatic. He was admitted to our hospital for further investigation of his condition. The patient's general condition was fair. Inspiratory crackles and wheezing could be heard bilaterally. Other physical findings were negative. Laboratory data were normal, except for a moderate degree of anemia (hemoglobin, 11 g/dL). To explore the origin of hemoptysis and make a definite diagnosis, the inferior lobe and lingular segment of the superior lobe of the left lung were resected by VATS. A histological pathology examination of the resected specimen revealed well‐formed anastomosing vessels. The vasoformative areas consisted of channels lined by high‐grade epithelioid cells, with abundant eosinophilic cytoplasm and significant nuclear atypia (Fig 3b). On immunohistochemical testing, the tumor cells stained positive for endothelial markers factor VIII, CD31, and CD34 (Fig 3c,d) and negative for CK7 and TTF. A PET scan identified no extrapulmonary lesions. A diagnosis of primary epithelioid angiosarcoma was made. After a month of supportive therapy, the patient died as a result of multiple organ failure.
Figure 3.
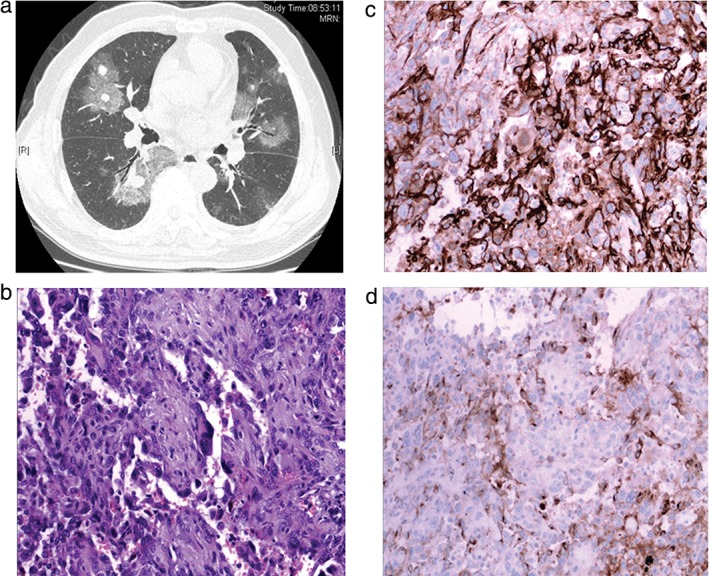
(a) Computed tomography of the chest showed multiple nodules in the central and peripheral portions of both lungs. Many of the nodules were surrounded by a halo of ground‐glass haziness. (b) Well‐formed anastomosing vessels. The vasoformative areas consist of channels lined by high‐grade epithelioid cells, with abundant eosinophilic cytoplasm and significant nuclear atypia. (c,d) Immunohistochemical stain showed tumor cells positive for CD31 and VIII.
Discussion
Primary pulmonary angiosarcoma is a rare type of malignant vascular tumor characterized by the proliferation of tumor cells with endothelial features. To the best of our knowledge, only 28 cases, including our patients, have been reported in English literature (Table 1).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The mean age of the reported cases was 54.7 years (range 23–85). Of these cases, 18 (64.3%) were men and 10 (35.7%) were women.
Table 1.
Reported cases of pulmonary angiosarcoma
| No. | Publication year | Author | Age/gender | Symptoms | Image | Diagnosis | Therapy | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 19541 | Benjamin & Towne | 48/M | Blood sputum, shortness of breath, cough | Pleural fluid, multiple nodules | Autopsy | None | 12 days |
| 2 | 19832 | Spragg et al. | 75/M | Progressive dyspnea, weakness | Diffuse infiltration | Autopsy | None | 5 days |
| 3 | 19873 | Palvio et al. | 59/M | Chest pain and hemoptysis | Consolidated upper and middle lobes | Thoracotomy | Pneumonectomy | 1 week |
| 4 | 19874 | Ott et al. | 60/M | Shortness of breath, chest pain, cough | Mass involving all three lobes of the right lung with mediastinal invasion | Open lung biopsy | Palliative operation | 2 months |
| 5 | 19885 | Segal et al. | 72/F | Malaise and dyspnea | Bilateral alveolar infiltration | Open lung biopsy | None | 2 days |
| 6 | 19976 | Sheppard et al. | 60/M | Hemoptysis | Diffuse ground glass pattern throughout both lung fields | Autopsy | None | 2 days |
| 7 | 20017 | Atasoy et al. | 50/M | Chest pain, malaise, hoarseness | Hypodense mass with soft tissue attenuation in the LUL | NA | Chemotherapy | 9 months |
| 8 | 20038 | Kojima et al. | 25/M | Chest pain, cough | A solid mass in the left hilar lesion with mediastinal invasion | Bronchoscopic biopsy | Radiation therapy and interleukin‐2 | > 1 year |
| 9 | 20049 | http://www.ncbi.nlm.nih.gov/pubmed/?term=Maglaras%20GC%5bAuthor%5d&cauthor=true&cauthor_uid=15909614 | 46/M | Hemoptysis | Nodular infiltrates and bilateral hemothorax | Thoracotomy | Chemotherapy | 1 month |
| 10 | 200510 | Pandit et al. | 79/F | Shortness of breath, chest pain and cough | Multiple nodules in both lungs | Open wedge biopsy | None | 18 months |
| 11 | 200611 | Ozcelik et al. | 62/M | Cough, hemoptysis, chest pain, fatigue, weight loss | A nodular lesion in the posterior segment of the RUL | Thoracotomy | Surgery, chemotherapy and radiation therapy | 5 months |
| 12 | 200612 | Patsios et al. | 76/F | Hemoptysis | Soft tissue opacity | Thoracotomy | Surgery | 1 month |
| 13 | 200813 | Wilson et al. | 56/M | Hemoptysis | A LUL mass | Lobectomy | Surgery, chemotherapy and radiation therapy | 39 months |
| 14 | 200914 | Campione et al. | 85/F | Cough and dyspnea | Right pleural effusion | Open wedge biopsy | None | 3 months |
| 15 | 200915 | Kuroda et al. | 43/M | Cough | A mass in the LLL | Lobectomy | Surgery | 15 months |
| 16 | 201016 | Chen et al. | 50/M | Hemoptysis | Multiple nodules in the peripheral of both lungs | Open lung operation | Surgery and chemotherapy | > 6 months |
| 17 | 201017 | Wan Musa et al. | 23/M | Chest pain | Left pleural effusion with consolidation at the lingula area | CT guided biopsy | Chemotherapy | 2 weeks |
| 18 | 201118 | Judy et al. | 45/M | Weight loss, anorexia, hemoptysis, fatigue | Bilateral pulmonary nodules | Thoracotomy | Chemotherapy | 5 months |
| 19 | 201219 | Kakegawa et al. | 45/M | Cough, bloody sputum | A nodule in the left main bronchus, atelectasis of the LUL | Thoracotomy | Surgery | > 12 months |
| 20 | 201220 | Yang et al. | 41/M | Productive cough | A solitary pulmonary nodule with bilobulation in the LUL | Thoracoscopic wedge resection | Surgery | < 1 week |
| 21 | 201321 | Obeso Carillo et al. | 56/F | Hemoptysis | A peripheral nodule in the LLL | Intraoperative biopsy of the pleural lesions | Surgery and chemotherapy | 7 months |
| 22 | 201321 | Gerado et al. | 35/F | Bone and joint pain | A solid lesion in the RLL | Thoracotomy | Radical tumor resection | 6 months |
| 23 | 201422 | Treglia et al. | 54/F | Symptomless | A mass in the inferior lobe of the left lung | Lobectomy | None | NA |
| 24 | 201523 | Tanaka et al. | 48/M | Dyspnea on exertion | Nodules involving the lower segments (S8, S9, S10) of the left lung | Bronchoscopy biopsy | Surgery and chemotherapy | 1 year |
| 25 | 201524 | Shimabukuro et al. | 79/F | Bloody sputum | Consolidation and GGO in the right middle and lower lobes and GGO in the left S1 + 2 and S8 | Surgical resections | Palliative operation | 3 months |
| 26 | 2016 | Yanhong et al. | 59/F | Dyspnea and bloody sputum | Pleural effusion and multiple nodules with GGO in lung parenchyma | Video‐assisted thoracoscopy | None | 2 months |
| 27 | 2016 | Yanhong et al. | 32/F | Productive cough, hemoptysis and dyspnea | Pleural effusion, multiple nodules, GGO lesions, and large pericardial effusion | Medical thoracoscopy | Intermittent Endostar | 2 months |
| 28 | 2016 | Yanhong et al. | 69/M | Cough, bloody sputum and weight loss | Multiple nodules in the central and peripheral portions of both lungs | Video‐assisted thoracoscopy. | None | 1 month |
F, female; GGO, ground‐glass opacity; LLL, left lower lobe; LUL, left upper lobe; M, male; NA, not available; RLL, right lower lobe; RUL, right upper lobe.
There are no symptoms specific to pulmonary angiosarcoma, as the presentation is similar to symptoms found in all lung cancers. Of the 28 cases, hemoptysis and/or hemosputum (16 cases) were the most frequently observed symptoms at the first visit, followed by cough (13) and dyspnea (12).
Reported chest radiograph findings ranged from solitary lesions to multiple nodular densities, with or without pleural effusion. Among the 28 cases, multiple lesions were the most common presentation, occurring in up to 50% (14/28) of patients. Multiple lesions could be expressed as nodules, ground glass opacities, and halo signs. Solitary lesions accounted for 39.3% (11/28). In addition, three cases were described as pulmonary consolidation. Compared with patients with solitary lesions, patients with multiple lesions had a poorer prognosis because of the rapid progression of multiple lesions and less effective clinical treatment. Particular attention should be paid to the three cases with pulmonary consolidation. Median survival in these patients was similar to patients with multiple lesions. Therefore, primary pulmonary angiosarcoma showing multiple infiltration or consolidation suggests a prognostic indicator for an unfavorable outcome.
Early diagnosis of primary pulmonary angiosarcoma is uncommon because of the non‐specific respiratory manifestations and consequent low index of suspicion. Definitive diagnosis is made on the basis of histopathological and immunohistochemical findings. The proliferation of variably sized vascular channels with irregular branching, epithelioid‐like endothelial lining of vascular spaces, abundant cytoplasm with pleomorphic nuclei and prominent nucleoli, and mitotic figures in atypical fashion are typical pathological features of pulmonary angiosarcoma. Immunohistochemical examination is essential to confirm diagnosis. Among the various immunohistochemical markers used for classification, CD31, CD34, and factor VIII‐related antigen are considered specific for diagnosis.
Among the 28 reported patients with primary pulmonary angiosarcoma, bronchoscopy was performed in 13 patients, but only two cases were definitively diagnosed (positive diagnostic rate 15%). Open lung biopsy was performed in 19 patients (including 11 of the patients who were not definitely diagnosed after bronchoscopy) and a definitive diagnosis was obtained. The total positive diagnostic rate of open lung biopsy was 100%. Percutaneous needle aspiration cytology (PNAC) and pleural biopsy are also effective in the diagnosis of pulmonary angiosarcoma, especially for patients with peripheral pulmonary nodules. Of the 28 patients, four were diagnosed by PNAC and pleural biopsy. In addition, two patients were diagnosed after autopsy; one article did not mention the diagnosis method. Our analysis suggests a low positive diagnostic rate by bronchoscopic biopsy for primary pulmonary angiosarcoma, regardless of the type of radiologic features. Possible reasons for infrequent diagnosis by bronchoscopic biopsy include the fact that the tumors involved few bronchi and the atypical pathological features of primary pulmonary angiosarcoma are difficult to recognize with a small bronchoscopic biopsy sample size. Once clinically suspected, medical thoracoscopy/VATS or open lung biopsy are more effective methods of diagnosis.
There is no standard treatment regimen specifically for pulmonary epithelioid angiosarcoma. Surgical resection, radiation, and chemotherapy have all been attempted. Surgery has been the mainstay for locally confined disease.8 Several previous studies have shown that angiosarcoma is radiosensitive.25 Chemotherapy has also been reported to be effective. Two chemotherapeutic combinations have demonstrated partial and full effects: doxorubicin/ifosfamide and docetaxel/gemcitabine.13 Systemic administration of high doses of recombinant interleukin 2 (rIL‐2) also seems to have been effective.3 However, rIL‐2 therapy is usually used with radiotherapy or chemotherapy; therefore, the potential efficacy of rIL‐2 alone cannot be assessed. Although many attempts have been made to treat primary pulmonary angiosarcoma, none have proven effective. Of the 28 reported cases, 91% of patients with solitary lesions (10/11) were treated actively, including radiotherapy (1 case), chemotherapy (1), surgery combined with chemotherapy and radiotherapy (3), and surgery (5 cases). The median overall survival was seven months. Only five cases of 14 with multiple lesions accepted chemotherapy, radiotherapy, and palliative surgery. The remaining patients (9/14) had to abandon further therapy because of physical weakness. The median overall survival of patients with multiple lesions was two months. All three cases of our report showing pulmonary consolidation received surgery or chemotherapy. The survival times were one week, two weeks, and six months. Compared with patients with solitary lesions, patients with multiple lesions had a poorer prognosis.
In summary, primary pulmonary angiosarcoma is a very rare type of malignant vascular tumor and its clinicopathologic features are not well known. Definitive diagnosis is made by histopathological and immunohistochemical findings. Once clinically suspected, medical thoracoscopy/VATS or open lung biopsy are reliable diagnostic methods. To date, there have been few reports of an effective treatment for this condition. Surgical resection, radiation, and chemotherapy have all been attempted; however, these tumors have a very aggressive clinical course and a grim prognosis.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank Dr. Wang Zheng (Peking Hospital) for her expert assistance with pathology. This work was supported by the Beijing Nova program (No. 2010B012) and the National Natural Science Foundation of China (No. 31570890 and No. 81000029).
Reference
- 1. Benjamin C, Towne VW. Case records of the Massachusetts General Hospital: Weekly clinicopathological exercises: Case 40191. N Engl J Med 1954; 250: 837–41. [DOI] [PubMed] [Google Scholar]
- 2. Spragg RG, Wolf PL, Haghighi P, Abraham JL, Astarita RW. Angiosarcoma of the lung with fatal pulmonary hemorrhage. Am J Med 1983; 74: 1072–6. [DOI] [PubMed] [Google Scholar]
- 3. Palvio DH, Paulsen SM, Henneberg EW. Primary angiosarcoma of the lung presenting as intractable hemoptysis. Thorac Cardiovasc Surg 1987; 35: l05–7. [DOI] [PubMed] [Google Scholar]
- 4. Ott RA, Eugene J, Kollin J, Kanas RJ, Conston DE, Chi JC. Primary pulmonary angiosarcoma associated with multiple synchronous neoplasms. J Surg Oncol 1987; 35: 269–76. [DOI] [PubMed] [Google Scholar]
- 5. Segal SL, Lenchner GS, Cichelli AV, Promisloff RA, Hofman WI, Baiocchi GA. Angiosarcoma presenting as diffuse alveolar hemorrhage. Chest 1988; 94: 214–6. [DOI] [PubMed] [Google Scholar]
- 6. Sheppard MN, Hansell DM, Du Bois RM, Nicholson AG. Primary epitheloid angiosarcoma of the lung presenting as pulmonary hemorrhage. Hum Pathol 1997; 28: 383–285. [DOI] [PubMed] [Google Scholar]
- 7. Atasoy C, Fitoz S, Yigit H, Atasoy P, Erden I, Akyar S. Radiographic, CT, and MRI findings in primary pulmonary angiosarcoma. Clin Imaging 2001; 25: 337–40. [DOI] [PubMed] [Google Scholar]
- 8. Kojima K, Okamoto I, Ushijima S et al. Successful treatment of primary pulmonary angiosarcoma. Chest 2003; 124: 2397–400. [DOI] [PubMed] [Google Scholar]
- 9. Maglaras GC, Katsenos S, Kakadelis J et al. Primary angiosarcoma of the lung and pleura. Monaldi Arch Chest Dis 2004; 61: 234–6. [DOI] [PubMed] [Google Scholar]
- 10. Pandit SA, Fiedler PN, Westcott JL. Primary angiosarcoma of the lung. Ann Diagn Pathol 2005; 9: 302–4. [DOI] [PubMed] [Google Scholar]
- 11. Ozcelik C, Onat S, Yaldiz M, Ozcelik Z. Primary epithelioid angiosarcoma of the lung presenting as pulmonary hemorrhage. Asian Cardiovasc Thorac Ann 2006; 14: 69–71. [DOI] [PubMed] [Google Scholar]
- 12. Patsios D, de Perrot M, Tsao MS, Weisbrod G. Epithelioid angiosarcoma of the lung: A rare late complication of Lucite plombage. Br J Radiol 2006; 79: e36–9. [DOI] [PubMed] [Google Scholar]
- 13. Wilson R, Glaros S, Brown RK, Michael C, Reisman D. Complete radiographic response of primary pulmonary angiosarcomas following gemcitabine and taxotere. Lung Cancer 2008; 61: 131–6. [DOI] [PubMed] [Google Scholar]
- 14. Campione A, Forte G, Luzzi L, Comino A, Gorla A, Terzi A. Pulmonary angiosarcoma presenting as spontaneous recurrent hemothorax. Asian Cardiovasc Thorac Ann 2009; 17: 84–5. [DOI] [PubMed] [Google Scholar]
- 15. Kuroda N, Hamaguchi N, Inoue K et al. Application of immunocytochemistry to the diagnosis of primary epithelioid angiosarcoma of the lung. Med Mol Morphol 2009; 42: 250–3. [DOI] [PubMed] [Google Scholar]
- 16. Chen YB, Guo LC, Yang L et al. Angiosarcoma of the lung: 2 cases report and literature reviewed. Lung Cancer 2010; 70: 352–6. [DOI] [PubMed] [Google Scholar]
- 17. Wan Musa WR, Abdulwakil Elraied MA, Phang KS et al. Primary epithelioid angiosarcoma of the lung presenting as left‐sided shoulder pain. Ann Acad Med Singapore 2010; 39: 658–9. [PubMed] [Google Scholar]
- 18. Judy BF, Predinal JD, Mittal J, Deshpande C, Singhall S. Metastatic primary pulmonary angiosarcoma. Surg Sci 2011; 2: 130–3. [Google Scholar]
- 19. Kakegawa S, Kawashima O, Ibe T et al. A case of primary angiosarcoma of the lung presenting as a hemorrhagic bronchial tumor. Ann Thorac Cardiovasc Surg 2012; 18: 347–51. [DOI] [PubMed] [Google Scholar]
- 20. Yang CF, Chen TW, Tseng GC, Chiang IP. Primary pulmonary epithelioid angiosarcoma presenting as a solitary pulmonary nodule on image. Pathol Int 2012; 62: 424–8. [DOI] [PubMed] [Google Scholar]
- 21. Obeso Carillo GA, García Fontán EM, Cañizares Carretero MA, Pérez Pedrosa A. Primary pulmonary angiosarcoma, an exceptional neoplasm with a poor prognosis: Reports of two cases and review of the literature. Gen Thorac Cardiovasc Surg 2013; 61: 643–7. [DOI] [PubMed] [Google Scholar]
- 22. Treglia G, Cardillo G, Graziano P. A rare case of primary pulmonary epithelioid angiosarcoma detected by (18)F‐FDG PET/CT. Clin Nucl Med 2014; 39: 450–2. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka H, Yorita K, Takahashi N, Usuma Y, Nakamura K, Kataoka H. Primary pulmonary angiosarcoma: A case report. Pathol Int 2015; 65: 554–7. [DOI] [PubMed] [Google Scholar]
- 24. Shimabukuro I, Yatera K, Noguchi S et al. Primary pulmonary angiosarcoma presenting with hemoptysis and ground‐glass opacity: A case report and literature review. Tohoku J Exp Med 2015; 237: 273–8. [DOI] [PubMed] [Google Scholar]
- 25. Sasaki R, Soejima T, Kishi K et al. Angiosarcoma treated with radiotherapy: Impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys 2002; 52: 1032–40. [DOI] [PubMed] [Google Scholar]


