Abstract
Background.
Glioma is the most common form of primary malignant brain tumor in adults, with approximately 4 cases per 100 000 people each year. Gliomas, like many tumors, are thought to primarily metabolize glucose for energy production; however, the reliance upon glycolysis has recently been called into question. In this study, we aimed to identify the metabolic fuel requirements of human glioma cells.
Methods.
We used database searches and tissue culture resources to evaluate genotype and protein expression, tracked oxygen consumption rates to study metabolic responses to various substrates, performed histochemical techniques and fluorescence-activated cell sorting-based mitotic profiling to study cellular proliferation rates, and employed an animal model of malignant glioma to evaluate a new therapeutic intervention.
Results.
We observed the presence of enzymes required for fatty acid oxidation within human glioma tissues. In addition, we demonstrated that this metabolic pathway is a major contributor to aerobic respiration in primary-cultured cells isolated from human glioma and grown under serum-free conditions. Moreover, inhibiting fatty acid oxidation reduces proliferative activity in these primary-cultured cells and prolongs survival in a syngeneic mouse model of malignant glioma.
Conclusions.
Fatty acid oxidation enzymes are present and active within glioma tissues. Targeting this metabolic pathway reduces energy production and cellular proliferation in glioma cells. The drug etomoxir may provide therapeutic benefit to patients with malignant glioma. In addition, the expression of fatty acid oxidation enzymes may provide prognostic indicators for clinical practice.
Key words: etomoxir, fatty acid oxidation, glioblastoma, glioma, metabolism
Glioma is the most common form of primary malignant brain tumor in adults. In >50% of cases, this tumor manifests as a grade IV astrocytoma (called glioblastoma or GBM), a highly malignant and invasive tumor with median patient survival of 12 months from diagnosis.1 Lower-grade gliomas increase in malignancy over time, with associated increases in mortality.2 There are no major heritable factors in the risk of glioma occurrence, and treatments including surgical resection, radiotherapy, and the chemotherapeutic drug temozolomide only minimally slow the course of this disease. There is a great need to develop novel targets for therapeutic intervention.
In the 1950s, Otto Warburg observed that tumors primarily metabolized glucose; instead of using the end product (pyruvate) to drive oxidative metabolism, the cells converted pyruvate into lactate and released it into the extracellular space. This dependence upon glycolysis instead of oxidative metabolism, (known as the Warburg effect) was first characterized in sarcomas and later shown in other cancers.3 Warburg hypothesized that this glycolytic switch was the initiating event during oncogenic transformation. Although it is now understood that cancers arise from genetic abnormalities affecting oncogene and tumor suppressor pathways, the possibility remains that targeting catabolic pathways required for cellular energy production may be a fruitful clinical strategy.
Like other tumors, gliomas have been thought to rely upon glycolysis for energy production, yet recent results from human NMR spectroscopy studies suggest that glucose contributes to <50% of acetyl-CoA production in gliomas.4 While the metabolic substrates preferred by these tumors have not been identified, other cancers have been shown to utilize alternative fuels for energy production and synthesis of raw materials.5 Fatty acid chains can be used to produce energy within a growing tumor;6 prostate and breast cancer cells in particular have been specifically shown to employ fatty acid oxidation as a metabolic strategy.7,8 Etomoxir, an inhibitor of fatty acid oxidation, has been shown to decrease oxygen consumption rates (OCRs) and impair ATP and NADPH production in the pediatric glioblastoma cell line SF188.9 Various substrates, including glucose, can be converted into fatty acids intracellularly; gliomas express fatty acid synthase (FASN), and expression of this enzyme increases with malignant grade.10
High levels of glycolysis have been primarily reported based upon the study of glioma cell lines that have adapted to culture conditions. It has recently been shown that patient-derived glioma cells cultured in the absence of serum retain their original characteristics and are more suitable for chemical and genetic screening.11,12 In this report, we show that fatty acid oxidation is in fact the primary catabolic pathway in human glioblastoma cells (hGBMs) maintained under such optimal culture conditions. Blocking this pathway reduces cellular respiratory and proliferative activity. Enzymes required for fatty acid oxidation are expressed and functional in human glioblastoma tissues, and upregulation of genes encoding enzymes in this pathway predicts worse survival in human patients with astrocytoma (although this effect is not significant in high-grade glioblastoma). Finally, we show that pharmacological inhibition of fatty acid oxidation prolongs survival in a syngeneic mouse model of malignant glioma in the context of a blinded, placebo-controlled preclinical trial. These findings present a new target that may aid in the clinical treatment of this disease.
Materials and Methods
REMBRANDT Database Search
Comparisons of patient survival on the basis of genotype were performed using 2 databases: the REpository of Molecular BRAin Neoplasia DaTa (REMBRANDT) and The Cancer Genome Atlas (TCGA), which provide coordinated molecular genetic data and clinical data from patients with brain tumors.13,14 All genes encoding enzymes in the fatty acid oxidation pathway were searched (Supplementary material, Table S1). Data mining and statistical analysis were performed with Project Betastasis software (http://www.betastasis.com/glioma/). Significant results yielded by Kaplan-Meier survival tests are reported as log-ranked P values for significance of difference in survival between groups. Previously published clinical and microarray data for low-grade glioma from the TCGA database14 were downloaded from https://genome-cancer.soe.ucsc.edu/proj/site/hgHeatmap/?datasetSearch=low+grade+glioma+TCGA, and specific transcripts were analyzed by 1-way ANOVA in SigmaPlot. Median gene expression level was used as the cutoff for comparing clinical outcomes.
Immunohistochemistry
All tissues were obtained with consent from patients under approval from Newcastle upon Tyne Hospitals NHS Foundation Trust. Twenty-eight samples identified as glioblastomas on the basis of clinical presentation and histological analysis were employed to investigate protein expression levels. Formalin-fixed, paraffin-embedded tissue blocks were obtained from the Cellular Pathology Department at the Royal Victoria Infirmary in Newcastle. Blocks were sectioned into 5 µm and dried overnight. The sections were placed in a 60°C oven for 30 minutes, moved directly into Histoclear, and then hydrated in decreasing concentrations of ethanol.
For antigen retrieval, cells were subjected to 0.01 M sodium citrate at 100°C for 10 minutes. Sections were then rinsed with phosphate-buffered saline (PBS). Nonspecific staining was blocked for 2 hours in PBS with 0.1% Triton X-100 and 5% donkey serum. Sections were incubated overnight at 4°C with appropriate primary antibodies and for 2 hours at room temperature with secondary antibodies (Supplementary material, Table S2). Sections were then co-stained with 1 µg/mL Hoechst. Coverslips were mounted over sections, and fluorescence microscopy was performed using a Zeiss Apoptome microscope with attached camera and Axiovision software.
Serum-free Primary Culture of hGBMs
hGBMs G144, G166, and GliNS2, which had been originally cultured in the lab of Professor Austin Smith, were obtained from a BioRep cryogenic storage facility (Milan, Italy). The cells were maintained in sterile, serum-free culture in NeuroCult Basal Medium (Stem Cell Technologies 05750) with NeuroCult Proliferation Supplement (Stem Cell Technologies 05753). NeuroCult Proliferation Medium was supplemented with 20 ng/mL bFGF (Peprotech 100-18) and 20 ng/mL EGF (Peprotech 100-15). This complete medium was used as the growth medium for cell culture. Cells were passaged every 4 days by dissociating with Accutase (Sigma A6964). In vitro experiments were performed with all 3 biological replicates. Fluorescence-activated cell sorting (FACS)-based mitotic profiling contained 6 technical replicates for each biological replicate, and Seahorse Analyzer experiments contained 5 technical replicates for each biological replicate.
Serum-free Primary Culture of Oncogenically Transformed Mouse NSCs
Neural stem cells (NSCs) were isolated from the adult wild-type C57B/6 mouse brain as previously described.15 The cells were oncogenically transformed in vitro as previously described.16 These glioma-initiating cells were maintained in serum-free growth media consisting of Dulbecco's modified Eagle's medium/F12 (Omega Scientific DM-25) supplemented with 2 mM glutamine, 1% N2 (Gibco), 20 ng/mL epidermal growth factor, and 20 ng/mL fibroblast growth factor-2 (Peprotech). In vitro experiments were performed with 3 biological replicates (each with 10 technical replicates) except for Seahorse Analyzer experiments, which were performed with 3 biological replicates (each with 5 technical replicates).
Extracellular Flux Analysis in Live Cells
OCRs were measured using the Seahorse XF24 Extracellular Flux Analyzer as described.17 hGBM cells, and oncogenically transformed mouse NSCs were plated in XF24 cell culture plates (Seahorse Bioscience) at 10^5 cells/well and incubated for 72 hours at 37°C with 5% CO2. One row of cells contained 10% fetal bovine serum (FBS). On the day of experimentation, each well was replaced with bicarbonate-free low-buffered medium (Seahorse Bioscience) with the following: no supplement, 5 mM glucose, 2 mM L-glutamine, or 1% FBS. Cells were incubated for 1 hour at 37°C with 0% CO2. Each time point included 5 minutes of rest, 1 minute of mixing, and 3 minutes of measuring. OCR measurements were normalized to cell counts and then compared using 2-tailed t tests in Excel. A similar protocol was used to evaluate cellular responses to 5 mM glucose and 5 mM 2-deoxyglucose (2-DG).
Analysis of Cellular Proliferation and Viability
To quantify the fractions of actively cycling cells in the population, we employed 2 methods: immunocytochemical labeling of KI67 and FACS-based mitotic profiling. For KI67 labeling, 13 mm glass coverslips were placed in 24-well plates and coated with 10 µg/mL laminin in Dulbecco's phosphate buffered saline (dPBS) for 2 hours at 37°C. hGBM cells were plated at a density of 10 000 cells per well on coated glass coverslips for 24 hours in growth medium. Cells were then treated with 10 µL of dPBS, 5 mM etomoxir, or 5 mM linoleic acid for a final concentration of 100 µM etomoxir (Sigma E1905) and 100 µM linoleic acid (Sigma L1376). Twenty-four hours after treatment, cells were fixed in 4% paraformaldehyde. Coverslips with attached cells were subjected to immunohistochemical staining as described above. In a separate experiment, TdT+ apoptotic cells were quantified using an enzymatic detection kit as directed (Chemicon S7160).
To perform mitotic profiling, hGBM cells were treated with dPBS, etomoxir, or linoleic acid. Twenty-four hours after treatment, cells were labelled with 2 µg/mL Hoechst in permeabilization solution and then subjected to FACS using LSRII equipment and ModFit software for mitotic profile analysis. For each experimental assay, each of 3 biological replicates was plated in triplicate for each treatment group. Groups were compared using 2-tailed t tests in Excel.
Inhibition of Fatty Acid Oxidation in Vivo
To assess the efficacy of inhibiting fatty acid oxidation in slowing tumor growth in vivo, we performed a blinded, placebo-controlled preclinical study in a mouse model of malignant glioma. All work on animals was performed in line with Home Office licensing. NSCs isolated from the adult mouse brain were oncogenically transformed in vitro (as described above) and then transplanted into the brain tissue of wild-type mice of the same genetic background (a syngeneic model). A total of 10^4 cells in 1 microliter were injected across 2 sites within striatum: AP + 1.0, ML −1.5, DV −3.5, and DV −3.0. Fourteen days after intracranial cell implantation, osmotic pumps were implanted subcutaneously. Pumps were loaded with either saline alone or 50 mg/mL etomoxir sodium-salt (Sigma E1905) in saline to achieve approximately 10 mg/kg of drug treatment per day. Once euthanasia criteria were reached, animals were transcardially perfused with 4% paraformaldehyde. Survival curves were compared between treatment groups using ANOVA in Prism.
Tissues were sectioned into 16 μm-thick slices for staining procedures. Tissues from all 26 animals were assessed by hemotoxylin & eosin (H&E) to confirm the presence of tumor, using an Olympus microscope with attached camera. For protein expression analysis (n = 9 animals per treatment group), immunohistochemistry was performed as described above. Fluorescence microscopy was performed using a Zeiss Apoptome microscope with attached camera and Axiovision software. Protein expression levels were compared between groups using 2-tailed t tests in Excel.
For MRI, mice were anesthetized by isoflurane/oxygen inhalation and imaged using the Rapid 33 mm volume coil with SA Instruments’ physiological monitoring system to maintain temperature and measure respiration. Parameters for the gradient echo multislice scan (GEMS) were: repetition time (TR) 500 ms, effective echo time (TE) 5.54 ms, matrix 256 × 256, field of view 20 × 20 mm, flip angle 20°, and slice thickness 1 mm, giving a T1 weighted sequence. The software Vnmr J (Varian/Agilent) Version 3.1A was used to calculate tumor volumes. One animal from each group was scanned.
Results
Expression of Fatty Acid Oxidation Enzymes in Human Glioma Tissues
We evaluated expression of enzymes involved in fatty acid oxidation in 28 samples of fixed human glioblastoma tissue (Fig. 1). Acyl-CoA dehydrogenases such as medium-chain acyl-CoA dehydrogenase (MCAD), which catalyzes the first oxidation step of fatty acids in the catabolic pathway, were observed in a fraction of cells in all tumor samples studied with variability between individuals (Figs 1 A–C and 2 W). Carnitine palmitoyl transferase 1a (CPT1a), which transports fatty acids across the mitochondrial membrane to be oxidized, and the trifunctional protein hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (HADHA), which catalyzes the final 3 steps in beta-oxidation, were also observed in all tumors (Fig. 1 D and E). A schematic showing each of the enzymes in this biochemical pathway is provided (Supplementary material, Fig. S1). Expression of enzymes in this pathway is not correlated with age or radiotherapy treatment (Supplementary material, Figs S2 and S3). Expression of the 3 acyl-CoA-dehydrogenase enzymes is correlated within individual glioblastomas, and MCAD expression is negatively correlated with tumor cell density (Supplementary material, Fig. S4).
Fig. 1.
Human glioblastomas express enzymes required for fatty acid oxidation Representative photomicrographs demonstrate staining in human glioblastoma tissue for medium-chain acyl-CoA dehydrogenase (MCAD, A), short-chain hydroxyacyl CoA dehydrogenase (SCHAD, B), very-long-chain acyl-CoA dehydrogenase (VLCAD, C), carnitine palmitoyl transferase 1a (CPT1a, D), and the trifunctional protein hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (HADHA, E). The majority of mitochondria labeled with the pan-mitochondrial marker succinate dehydrogenase (SDHA) are co-labeled with HADHA (F-J). A fraction of cells in human glioblastoma tissue identified with Hoechst (K,O,S) and expressing MCAD (L,P,T) co-label with the astroglial marker GFAP (M), the neural progenitor marker SOX2 (Q), and the glial progenitor marker OLIG2 (U). Representative abeled cells are shown in merged images (N,R,V). The fraction of cells expressing each of the fatty acid oxidation enzymes is shown (W), as well as the fraction of total cells (X) and MCAD+ cells (Y) co-labeled with each cell type. Scale bars are 10 µm.
Fig. 2.
Fatty acid oxidation is a primary contributor to aerobic respiration in primary-cultured hGBMs. The oxygen consumption rate (OCR) of primary-cultured serum-free hGBM cells was assessed using the Seahorse Analyzer (A). Baseline measurements were taken for cells in plain medium (empty circles), with glutamine (black triangles), with glucose (black squares), and exposed to 10% FBS for 72 hours prior to the experiment (grey diamonds). Cells were then treated with 100 µM linoleic acid (a polyunsaturated fatty acid), 100 µM etomoxir (an inhibitor of beta-oxidation), 2.0 µM FCCP (which induces maximal respiration), and 2.5 µM antimycin A (which inhibits aerobic respiration). Spare respiratory capacity, calculated by dividing basal OCR by maximal OCR, is shown (B). Cellular response to linoleic acid (light grey bars) and etomoxir (dark grey bars) is shown (C). The fraction of mitochondrial respiration dependent on fatty acids is shown (D). A similar experiment was conducted to evaluate responses to glucose and the glycolytic inhibitor 2-DG (E). Cellular responses are summarized (F), and the fraction of mitochondrial respiration dependent on glucose oxidation or glycolysis is shown (G). *indicates significant change in respiration, P < .05.
The enzymes appear to be localized to the mitochondria. Nearly all cells expressing the pan-mitochondrial marker succinate dehydrogenase (SDHA) express the fatty acid oxidation enzyme HADHA; these markers show strong co-labeling (Fig. 1 F–J). Mitochondria occasionally appeared not as puncta but as chains throughout the cytoplasm, as has been reported in other proliferative cell types.18
To identify the cell types within human glioblastoma tissue that are engaging in fatty acid oxidation, we colabeled the enzyme MCAD with cell type-specific antibodies, including the astroglial marker GFAP, the neural progenitor marker SOX2, and the glial progenitor marker OLIG2 (Fig. 1 K–V). MCAD is expressed in OLIG2+ cells to a greater extent than GFAP+ or SOX2+ (Fig. 1 X and Y), although this enzyme is present in multiple cell types within human glioblastoma tissue.
Differential Gene Expression and Survival Time in Human Glioma Patients
We next performed statistical analysis of glioma patient survival on the basis of gene expression.13,14 Kaplan-Meier survival plots were produced to compare survival among genetically defined glioma patients (Supplementary material, Fig. S5), particularly focusing on genes encoding fatty acid oxidation enzymes. We found that upregulation of 4 genes (of 9 total) in this metabolic pathway was associated with significantly worse survival in patients with low-grade astrocytoma (in both the REMBRANDT and TCGA databases). No significant differences in survival time for GBM patients were observed in either database.
Metabolic Fuel Requirements of Primary-Cultured Human Glioma Cells
To determine whether fatty acid oxidation enzymes expressed in brain tumor tissues are functional, further analysis was performed on primary-cultured hGBMs expanded under serum-free conditions.12 All experiments contained averaged data from 3 cultures: G144, G166, and GliNS2. The OCRs of these precharacterized cells were measured using the Seahorse Extracellular Flux Analyser.17 Cells were treated first with linoleic acid, a polyunsaturated fatty acid, then with etomoxir, an inhibitor of carnitine palmitoyl transferase I (the rate-limiting enzyme in fatty acid oxidation). The addition of linoleic acid stimulates OCR in hGBMs even in the presence of glucose or glutamine (Fig. 2 A–D). Cells also decrease OCRs in response to etomoxir, although this response is blunted in cells exposed to serum for 3 days (Fig. 2 C and D). A similar experiment was conducted on mouse glioma-initiating cells (NSCs). NSCs were isolated from normal adult wild-type mice and primary-cultured under serum-free conditions in vitro and then transformed oncogenically by introduction of several clinically relevant genetic lesions to reproduce the molecular and phenotypic characteristics of glioma. In these cells, linoleic acid significantly increased OCR; etomoxir had no effect in this experiment, either because cells switched to using glucose or because the mitochondria had already acquired sufficient fatty acids to supply the fatty acid oxidation pathway (Supplementary material, Fig. S6A–D).
The response of primary-cultured hGBMs to glucose and the glycolytic inhibitor 2-deoxyglucose (2-DG) was also tested using the Seahorse Analyzer. 2-DG significantly decreases OCR in non-serum-exposed cells, suggesting that glucose or its derivatives are otherwise being oxidized (Fig. 2 E–G). Meanwhile, glucose treatment significantly decreased OCR in serum-exposed cells, indicating a preference for anaerobic respiration when substrates are available (Fig. 2 E–G). A similar experiment was conducted on the mouse glioma-initiating cells (Supplementary material, Fig. S6E–G). Without serum exposure, these cells did not respond to either glucose or 2-DG. After serum exposure, however, these cells increased OCR in response to 2-DG.
The Role of Fatty Acid Oxidation in the Proliferation of Human Glioma Cells
To test whether fatty acid oxidation plays a role in cellular survival and proliferation, serum-free primary-cultured hGBMs were subjected to analysis after treatment with vehicle control, 100 µM etomoxir sodium salt, or 100 µM linoleic acid (Fig. 3 A–M). Etomoxir decreased KI67+ index, total cell count, and the fraction of cells in S+G2/M phase of the cell cycle, suggesting that this energy-producing biochemical pathway is important for the proliferation of human glioma cells. Inhibition of fatty acid oxidation with etomoxir also reduces proliferation and viability of mouse glioma-initiating cells (Supplementary material, Fig. S7).
Fig. 3.
Inhibition of fatty acid oxidation decreases proliferation in hGBMs but does not affect cellular survival. Sample photomicrographs of cells treated with phosphate-buffered saline (PBS) (A, D), 100 µM etomoxir (B, E), or 100 µM linoleic acid (C, F) are shown, stained with either TdT, a marker of apoptosis (A–C), or KI67, an S-phase cell cycle marker (D–F). Separate channels are shown to display Hoechst, a pan-nuclear marker ('), and TdT or KI67 (''). The fraction of TdT+ apoptotic cells did not change significantly in either treatment group (P > .05, G). The fraction of KI67+ proliferating cells decreased upon 24 hours treatment with etomoxir (P < .05, H) and increased upon 24 hours of treatment with linoleic acid (P < .01, H). The total cell count after 24 hours was significantly decreased in the presence of etomoxir (P < .01, I) but was unaffected in the presence of linoleic acid (P > .05, I). Shown are representative data from fluorescence-activated cell sorting-based analysis of the mitotic index of primary-cultured serum-free hGBM cells, performed after treatment with PBS (J), 100 µM etomoxir (K), or 100 µM linoleic acid (L). The fraction of cells in S + G2/M phase of the cell cycle decreased by 20% from control levels upon treatment with 100 µM etomoxir (P < .01, M); this fraction was unaffected by treatment with linoleic acid (P > .05, M).
Inhibiting Fatty Acid Oxidation in a Mouse Model of Malignant Glioma
To evaluate the efficacy of etomoxir in slowing tumor growth in vivo, we employed a mouse model of malignant glioma. The oncogenically transformed mouse NSCs used in the experiments described above were transplanted into immunocompetent wild-type mice of the same genetic background (a syngeneic model). This process reliably forms a high-grade glial tumor that reproduces the histological and clinical characteristics of human glioma.
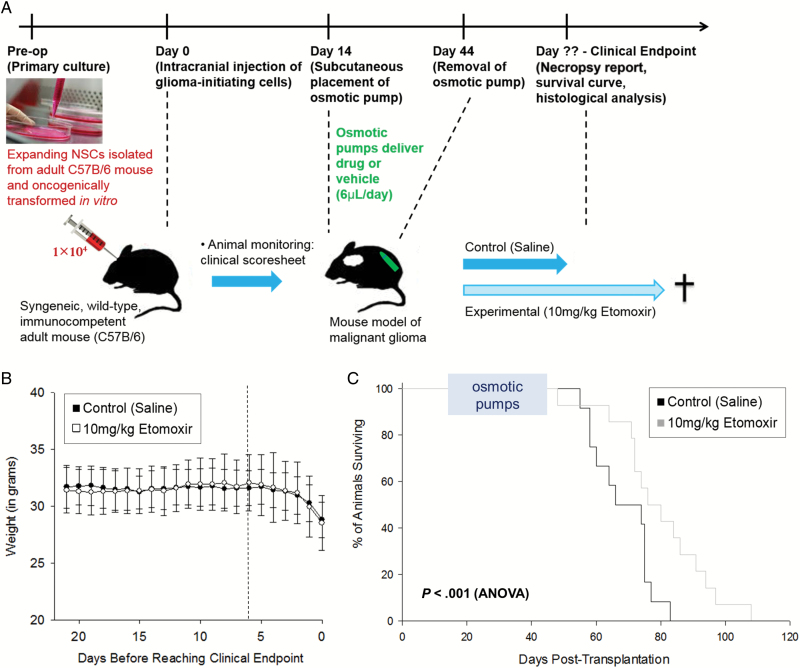
A blinded, placebo-controlled preclinical study was conducted to evaluate the efficacy of slowing glioma growth in vivo with etomoxir treatment. Fourteen days after cell implantation, when animals had only small regions of dysplasia, osmotic pumps were implanted to deliver drug or saline vehicle control for 30 days (Fig. 4 A). Animal weight and behavior were monitored daily until euthanasia criteria were reached (Supplementary material, Fig. S8), whereupon animals were found to have high-grade glial tumors. Mice in both treatment groups exhibited largely similar clinical symptoms during the last week of life leading up to clinical endpoint (Fig. 4 B, Supplementary material, Fig. S9). However, mice treated with 10 mg/kg etomoxir sodium salt survived significantly longer than their saline-treated cage mates (Fig. 4 C, Supplementary material, Table S3).
Fig. 4.
Inhibition of fatty acid oxidation by etomoxir prolongs survival time in a syngeneic mouse model of malignant glioma. Fourteen days after surgical implantation of glioma-initiating cells into the brains of wild-type adult mice, osmotic pumps were subcutaneously implanted to deliver drug or control substance for a period of 30 days in a blinded, placebo-controlled preclinical study (A). Animals were monitored until euthanasia criteria were met. Weight loss and other symptoms occurred primarily in the final 3 days of life (B) and were similar between the control and treatment groups (8.0 g vs 7.5 g, respectively, P > .05). Mice treated with 10 mg/kg etomoxir sodium salt lived significantly longer than animals treated with vehicle control (C, P < .001, ANOVA).
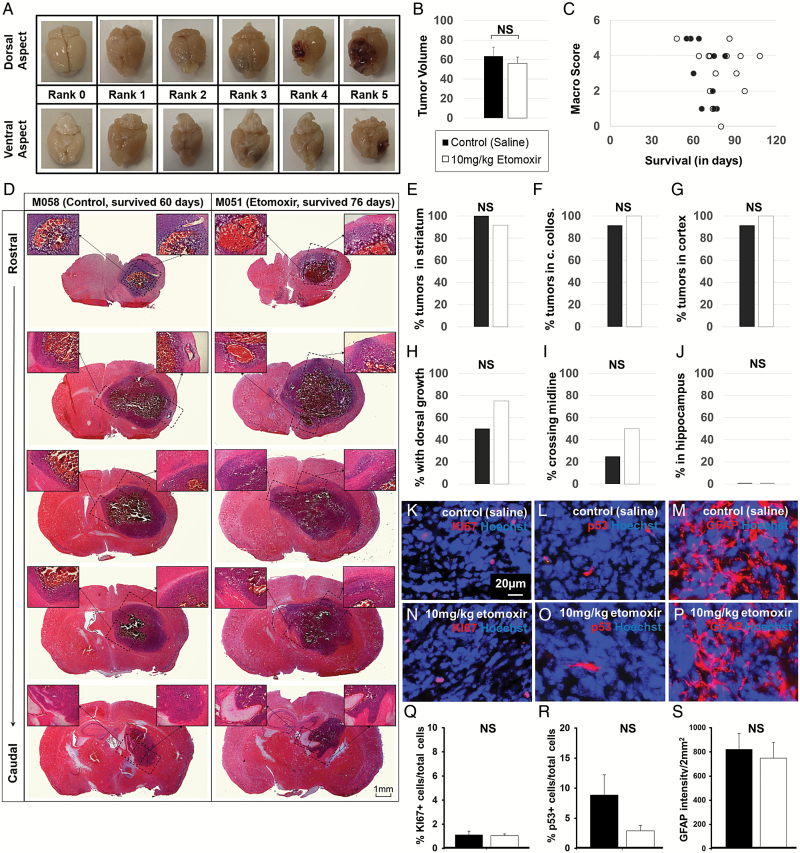
Upon tissue collection, brains were photographed to evaluate tumor severity on a macro scale; no differences in tumor size were observed between treatment groups at clinical endpoint, a finding that was subsequently confirmed by histopathological evaluation of H & E stained sections (Fig. 5 A–C). Sections from all animals were subjected to H & E staining procedures to assess tumor infiltration patterns throughout the brain and were not significantly different between treatment groups (Fig. 5 D–J). The postmortem tissues were evaluated by extrapolating criteria for human intracranial tumor grading set out by the 2007 WHO guidelines. The tumors were identified as high-grade anaplastic astrocytoma. Stereotypical reproduction of histological features was observed between animals at clinical endpoint. Hemorrhage was commonly observed in central regions of each tumor; examples of mitotic figures and apoptotic nuclei were also observed throughout the tumors. Tumors infiltrated tissues surrounding the striatal implantation site, including corpus collosum and cortex; some of the brain tissues demonstrated invasion of tumor cells across midline (Fig. 5 D–J). Mass effect was observed, analogous to tumor-related changes in human glioblastoma. Sections were also stained to assess KI67+ proliferation index, p53+ index, and GFAP+ glial content, none of which showed significant differences between treatment groups (Fig. 5 K–S).
Fig. 5.
No difference in tumor size, location, or phenotype was observed between treatment groups at clinical endpoint. Brains from the mice in the study were photographed to evaluate tumor severity. (A) No significant difference in tumor volume or macro score was observed between treatment groups at clinical endpoint (B–C), corresponding to similar tumor grading by histological assessment (D). Infiltration of tumors from the injection site (striatum, E) into corpus collosum (F), cortex (G), dorsal aspects of the brain (H), across midline (I), or into hippocampus (J) were not found to be significantly different between treatment groups (P > .05, chi-square test). Tumors were further evaluated to quantify KI67+ proliferation index (K, N), p53+ malignancy marker (L, O), and GFAP+ glial cell content (M, P) in control-treated animals (K-M) and etomoxir-treated animals (N–P). No difference in cellular phenotype was observed between groups (P > .05, 2-tailed t test).
The animals showed similar-sized tumors at clinical endpoint since all were subjected to the same euthanasia criteria. To evaluate tumor size over the entire experimental time-course, one animal in each treatment group was subjected to MRI over the course of the study (Fig. 6). The appearance of glioma using T1-weighted diffusion tensor imaging (without contrast agent) corresponded to the emergence of clinical symptoms (eg, weight loss) in both animals. Tumor growth occured over the course of approximately 2 months in control animals (Fig. 6 A–F) and slowed upon treatment with 10 mg/kg/day etomoxir (Fig. 6 G–L).
Fig. 6.
Emergence and progression of glioma were delayed upon treatment with the investigational drug etomoxir. Mouse 077 (control) and mouse 078 (etomoxir) were subjected to MRI to track tumor progression during the study. Coronal images throughout the brain are shown for Mouse 077 across the entire experimental time-course (A-E). At 46 days post cell implantation (DPI), an injection track was observed with no evidence of tumor (A). At 53 DPI, there was no evidence of tumor and no clinical symptoms (B). At 67 DPI, a 1.0 mm tumor was observed with no clinical symptoms (C). At 72 DPI, a 1.3 mm tumor was observed with approximately 5% loss of body weight the following day (D). At 75 DPI, a 5 mm tumor was observed, with approximately 15% loss of body weight and other symptoms signaling clinical endpoint (E). A zoom image of this time point is shown (F). Tumor growth was slowed upon treatment with 10 mg/kg/day etomoxir in mouse 078 (G-K). This animal first manifested tumor by MR at 95 DPI; at 97 DPI, this animal lost 15% body weight and reached clinical endpoint. A zoom image of this animal's brain at 75 DPI is shown to compare with the vehicle-treated animal at the same time point (L).
Discussion
The acquisition of a glycolytic strategy is considered a hallmark of oncogenic potential in the field of cancer biology.19 Reliance upon this biochemical pathway may be expected for cells within gliomas as they share morphological and phenotypic features with glial cells that are highly glycolytic and release lactate into the extracellular space.20 From a diagnostic imaging perspective, however, it was not evident that the Warburg effect was regularly manifested in gliomas. Between 35 and 40 percent of recurrent gliomas in human patients are not observed using imaging techniques based on glucose uptake (eg, FDG-PET), although these tumors can be observed by contrast MRI.21,22 Using NMR spectroscopy, Maher et al demonstrated that <50% of the acetyl-CoA pool was derived from blood-borne glucose; these results support the notion that additional substrates contribute to glioma bioenergetic flux.4 Therefore, excess glucose utilization, as predicted by the Warburg effect, may not be particularly characteristic of gliomas.
A number of studies have investigated the catabolic strategies of glioma cells, but technical difficulties have challenged efforts to accurately identify the metabolic fuel preferences of this tumor type. For example, a high lactate-to-pyruvate ratio has been identified in human glioma xenografts in rodents, a finding that seems to support the glycolytic nature of glioma.23 However, these results do not specifically establish that cells rely upon glycolysis for energy metabolism but do demonstrate that ATP levels remain high despite low hexokinase activity and high lactate secretion; one interpretation of this study could be that alternative substrates are being oxidized. Other researchers have shown that inhibition of glycolysis with dichloroacetate (DHA) decreases proliferation and increases rates of apoptotic cell death in primary-cultured glioma cells;24 however, this drug also decreases the activity of other metabolic pathways including fatty acid oxidation.25 Fatty acids have previously been shown to inhibit the growth of adult glioma cells cultured in the presence of serum,26 an effect thought to be mediated by changes in the redox state.27 However, the established cell lines and tumor xenografts used for these biochemical investigations have been exposed to serum, a common cell-culturing protocol that has been shown to cause genetic and phenotypic alterations to human glioma samples.12
In this study, we report that cells derived from human glioma and cultured under optimal conditions express fatty acid oxidation enzymes and are dependent upon fatty acid oxidation for aerobic respiration and proliferative activity. Figure 2 demonstrates that a majority of respiratory activity arises from fatty acid oxidation, while a smaller subset of respiratory activity arises from glucose oxidation. These findings are interesting in light of results in humans and suggest that much of the acetyl-CoA produced by malignant gliomas arises from nonglucose sources.28 Previous studies into the metabolic fate of 13C-glucose infused into human patients have demonstrated some role for this substrate in glioma cell respiration.29 However, radiolabeled fatty acids have not been infused for evaluation as a possible alternative metabolic substrate. We predict that such experiments, conducted in mouse models and human patients, will validate our findings by showing that fatty acids are metabolic substrates supporting glioma bioenergetics in vivo.
Our findings offer a novel target for the prognosis and treatment of malignant glioma. We tested a specific inhibitor of fatty acid oxidation, etomoxir (sodium salt), in the context of a blinded, placebo-controlled preclinical study design using a clinically relevant mouse model of malignant glioma.16 The promising results of etomoxir in slowing tumor growth and prolonging survival in this mouse model provide initial evidence for pursuing new therapeutic avenues to target fatty acid metabolism in the clinical treatment of astrocytomas.
Etomoxir has already been tested in phase I/II clinical trials for treating moderate congestive heart failure; this trial was discontinued because 4 patients (of 226 taking the drug) developed unacceptably high liver transaminase levels upon treatment, and the risk of such drastic side effects was deemed sufficient to negate the potential benefit of this drug for these patients.30 However, the treatment options and survival expectancy for patients with malignant glioma is much more dire, and the potential benefit in regard to risk is very different for patients with this diagnosis. This drug may therefore provide promise for clinical therapies aimed to slow the growth and progression of malignant glioma.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This research was supported by The Wellcome Trust Centre for Mitochondrial Research [G906919], the Engineering & Physical Sciences Research Council’s IDEAS Factory Sandpit [Newcastle Award, 2012], the Wellcome Trust and Engineering & Physical Sciences Research Council’s Innovative Engineering for Health Project Award (CANDO), and the Newcastle University Faculty of Medical Sciences Translational Research Fund.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
The authors would like to thank Mrs. Philippa Hepplewhite, Mrs. Debra Jones, Miss Rebecca Makin, Ms. Marie Strickland, Dr. John Yarham, and Mr. Ian Dimmick for technical support; Mr. Bill McMeekin and Dr. Swethajit Biswas at the Royal Victoria Infirmary for providing access to patient tissues; and Dr. Robert Rostomily (University of Washington, Seattle) for commenting on early versions of this manuscript. E.S. is grateful to Professor Rheba de Tornyay, Professor George Martin, and Ms. Luba Miagkova for their encouragement in pursuing this research.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17 (Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64 (6):479–489. [DOI] [PubMed] [Google Scholar]
- 3. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11 (2):85–95. [DOI] [PubMed] [Google Scholar]
- 4. Maher EA, Marin-Valencia I, Bachoo RM, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25 (11):1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35 8:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13 (4):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linher-Melville K, Zantinge S, Sanli T, Gerstein H, Tsakiridis T, Singh G. Establishing a relationship between prolactin and altered fatty acid beta-oxidation via carnitine palmitoyl transferase 1 in breast cancer cells. BMC Cancer. 2011;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlaepfer IR, Rider L, Rodrigues LU et al. . Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13 (10):2361–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807 (6):726–734. [DOI] [PubMed] [Google Scholar]
- 10. Tao B-B, He H, Shi X-h et al. . Up-regulation of USP2a and FASN in gliomas correlates strongly with glioma grade. J Clin Neurosci. 2013;20 (5):717–720. [DOI] [PubMed] [Google Scholar]
- 11. Lee J, Kotliarova S, Kotliarov Y et al. . Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9 (5):391–403. [DOI] [PubMed] [Google Scholar]
- 12. Pollard SM, Yoshikawa K, Clarke ID et al. . Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4 (6):568–580. [DOI] [PubMed] [Google Scholar]
- 13. Madhavan S, Zenklusen JC, Kotliarov Y, Sahni H, Fine HA, Buetow K. Rembrandt: helping personalized medicine become a reality through integrative translational research. Mol Cancer Res. 2009;7 (2):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG et al. . Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372 (26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoll EA, Habibi BA, Mikheev AM et al. . Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem Cells. 2011;29 (12):2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikheev AM, Stoll EA, Mikheeva SA et al. . A syngeneic glioma model to assess the impact of neural progenitor target cell age on tumor malignancy. Aging Cell. 2009;8 (4):499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu M, Neilson A, Swift AL et al. . Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292 (1):C125–C136. [DOI] [PubMed] [Google Scholar]
- 18. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16 (14):R551–R560. [DOI] [PubMed] [Google Scholar]
- 19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5):646–674. [DOI] [PubMed] [Google Scholar]
- 20. Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354 (1387):1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belohlavek O, Klener J, Vymazal J, Dbaly V, Tovarys F. The diagnostics of recurrent gliomas using FDG-PET: still questionable? Nucl Med Rev Cent East Eur. 2002;5 (2):127–130. [PubMed] [Google Scholar]
- 22. Chen W, Cloughesy T, Kamdar N et al. . Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46 (6):945–952. [PubMed] [Google Scholar]
- 23. Oudard S, Arvelo F, Miccoli L et al. . High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74 (6):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michelakis ED, Sutendra G, Dromparis P et al. . Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2 (31):31ra34. [DOI] [PubMed] [Google Scholar]
- 25. Bonnet S, Archer SL, Allalunis-Turner J et al. . A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11 (1):37–51. [DOI] [PubMed] [Google Scholar]
- 26. Tronstad KJ, Berge K, Flindt EN, Kristiansen K, Berge RK. Optimization of methods and treatment conditions for studying effects of fatty acids on cell growth. Lipids. 2001;36 (3):305–313. [DOI] [PubMed] [Google Scholar]
- 27. Tronstad KJ, Berge K, Dyroy E, Madsen L, Berge RK. Growth reduction in glioma cells after treatment with tetradecylthioacetic acid: changes in fatty acid metabolism and oxidative status. Biochem Pharmacol. 2001;61 (6):639–649. [DOI] [PubMed] [Google Scholar]
- 28. Mashimo T, Pichumani K, Vemireddy V et al. . Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159 (7):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marin-Valencia I, Yang C, Mashimo T et al. . Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell metabolism. 2012;15 (6):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holubarsch CJF, Rohrbach M, Karrasch M et al. . A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of 2 doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond). 2007;113 (4):205–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.