Abstract
Background.
The optimal therapeutic approach for patients with AIDS-related primary central nervous system lymphoma (AR-PCNSL) remains undefined. While its incidence declined substantially with combination antiretroviral therapy (cART), AR-PCNSL remains a highly aggressive neoplasm for which whole brain radiotherapy (WBRT) is considered a standard first-line intervention.
Methods.
To identify therapy-related factors associated with favorable survival, we first retrospectively analyzed outcomes of AR-PCNSL patients treated at San Francisco General Hospital, a public hospital with a long history of dedicated care for patients with HIV and AIDS-related malignancies. Results were validated in a retrospective, multicenter analysis that evaluated all newly diagnosed patients with AR-PCNSL treated with cART plus high-dose methotrexate (HD-MTX).
Results.
We provide evidence that CD4+ reconstitution with cART administered during HD-MTX correlates with long-term survival among patients with CD4 <100. This was confirmed in a multicenter analysis which demonstrated that integration of cART regimens with HD-MTX was generally well tolerated and resulted in longer progression-free survival than other treatments. No profound differences in immunophenotype were identified in an analysis of AR-PCNSL tumors that arose in the pre- versus post-cART eras. However, we detected evidence for a demographic shift, as the proportion of minority patients with AR-PCNSL increased since advent of cART.
Conclusion.
Long-term disease-free survival can be achieved in AR-PCNSL, even among those with histories of opportunistic infections, limited access to health care, and medical non-adherence. Given this, as well as the long-term toxicities of WBRT, we recommend that integration of cART plus first-line HD-MTX be considered for all patients with AR-PCNSL.
Key words: AIDS, brain tumor, HAART, lymphoma, methotrexate
AIDS-related primary CNS lymphoma (AR-PCNSL) has long been regarded as an end-stage manifestation of HIV infection that is typically associated with CD4+ cell counts less than 50 cells/mm3.1 AR-PCNSL is notable for its aggressive nature and prognosis that is markedly inferior to PCNSL arising in the immunocompetent. The median survival for AR-PCNSL patients rarely exceeds 3 months.2 , 3 Since the early 1990s it has been recognized that progress in AR-PCNSL would await significant advances in control of HIV.4
The development of combination antiretroviral therapy (cART), also known as highly active antiretroviral therapy (HAART), has been transformative, resulting in a marked decline in incidence of new diagnoses of AIDS and in AIDS-related deaths. Greater than 3 decades after the first cases, HIV infection is no longer a universally fatal disease; nevertheless, there are approximately 50,000 new reports of HIV infection per year in the United States. Despite a decrease in incidence of HIV-associated non-Hodgkin lymphoma (NHL) since the advent of cART, AIDS-related lymphomas remain the most common HIV-associated malignancy. While the incidence of AR-PCNSL has also declined since the 1990s,5 sporadic cases continue to represent a therapeutic challenge, particularly as these may occur in patients with limited access to health care and/or a history of medical non-adherence .6 , 7 At present, there is little information to guide clinical management for these patients.
The optimized integration of cART with cytotoxic chemotherapy has not yet been established for each of the AIDS-related malignancies. The simultaneous administration of antiretroviral constituents of cART, including HIV protease inhibitors during chemotherapy for aggressive lymphomas, has been somewhat controversial, particularly with infusional regimens in which pharmacokinetic parameters of chemotherapeutic agents may be disrupted by interactions with cART. Moreover, it has been demonstrated that despite concomitant cART, treatment of aggressive AIDS-related NHL with infusional chemotherapy results in statistically significant declines in the CD4 (helper T cell) and CD8 (cytotoxic T cell) populations in peripheral blood.8 , 9 Nevertheless, a recent retrospective overview of treatment factors associated with favorable outcomes in systemic HIV-associated NHL demonstrated that concurrent use of cART with chemotherapy was associated with improved rates of complete response and survival. However, this study excluded patients with AR-PCNSL.10 To date, there is limited data on the safety and/or efficacy of the integration of cART with chemotherapy for AR-PCNSL, and for these reasons, as of 2012, authorities in this field have proposed up-front WBRT plus cART as the standard care, with the presumption that AR-PCNSL remains essentially incurable.11
The past decade has witnessed significant evolution in the treatment of PCNSL among immunocompetent patients, with increased emphasis on high-dose methotrexate (HD-MTX)–based therapy and deferral or elimination of WBRT, widely recognized to be associated with irreversible cognitive dysfunction.12–15 Moreover, given the problem of HIV encephalopathy, patients with AR-PCNSL may be particularly susceptible to radiation-induced brain injury.16 Given that AIDS has become a chronic illness, survivorship and quality of life have emerged as key issues for HIV-infected patients.
Since 2001, our practice at the University of California San Francisco (UCSF) has been to combine cART with HD-MTX–based induction regimens for newly diagnosed AR-PCNSL, with the goals of immediate immune reconstitution and the avoidance of WBRT. Here we provide the survival outcomes of this strategy as well as the results of a multicenter validation analysis which confirms the feasibility and efficacy of this general approach. Given the current rare occurrence of this neoplasm, prospective clinical trial investigation to guide practice in AR-PCNSL may be impossible, particularly given the problems of access to health care and possible medical non-adherence that appear to be significant among those diagnosed in the current era. Our diverse, combined experience in the management of AR-PCNSL in urban, public hospitals as well as tertiary, comprehensive cancer centers may therefore be relevant to clinical decision making for a major fraction of patients with this disease.
Patients and Methods
Patients
The medical records of 93 patients with AR-PCNSL diagnosed at the University of California San Francisco (UCSF) between April 1988 and August 2012 were obtained from the San Francisco General Hospital (SFGH) Tumor Registry, electronic medical records, and physical charts. Inclusion criteria required biopsy-proven CNS lymphoma in the absence of preceding or concomitant systemic lymphoma. AR-PCNSL cases in the greater San Francisco Bay Area were identified using the Surveillance, Epidemiology, and End Results (SEER) site recoding scheme, using the third edition of the International Classification of Diseases for Oncology. Investigators at Memorial Sloan-Kettering Cancer Center, the University of California San Diego, the H. Lee Moffitt Cancer Center, and Cook County Hospitals reviewed clinical databases at these institutions and provided for this analysis all AR-PCNSL cases, since at least 2004, in which patients were treated with cART plus HD-MTX at diagnosis. All newly diagnosed AR-PCNSL patients who were identified to have been treated at diagnosis with cART plus HD-MTX were included in this analysis; no patients were excluded because of only partial receipt of therapy or because of lack of follow-up. This study was approved by the UCSF Committee on Human Research and is in accordance with the Declaration of Helsinki. Response criteria were assessed as described.17 Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0.
Immunohistochemical Analysis and In situ Hybridization
Diagnostic specimens from AR-PCNSL cases from UCSF were selected for histopathologic analysis based on availability of adequate fixed and paraffin-embedded tumor material. Sections were incubated with working dilution of each antibody raised against these antigens: B-cell lymphoma (BCL)-2 (clone 100/d5; Leica); BCL-6 (clone LN22; Leica); cluster of differentiation (CD)10 (clone 56C6; Leica); multiple myeloma oncogene 1 (MUM1) (MRQ-43; Dako); myelocytomatosis (MYC) (clone Y69, Abcam), and Ki67 (MIB-1, Dako). Antibody immunoreactivity was visualized using the biotin-streptavidin peroxidase method. Each specimen was evaluated independently by 2 pathologists for percentage of tumor cells staining and recorded in 10% increments. For each case, the core with the highest percentage of tumor cells stained was used for analysis. Tumors were subclassified according to their expression of germinal center B-cell-like (GCB) versus nongerminal center markers as per Hans et al.18 Positive expression of BCL-2 was based upon a cutpoint of ≥50%. Positive expression for MYC was defined by nuclear staining in ≥40% of tumor cells.19 , 20 In situ hybridization was performed on paraffin-embedded tissue using a fluorescein-conjugated oligonucleotide probe complementary to the small nuclear Epstein–Barr virus (EBV) encoded RNA1 (EBER; Leica) and visualized using the BOND system.
Results
Patient Characteristics During the Pre-cART and cART Eras
Given the transformative impact of cART on the incidence of AR-PCNSL, we asked whether there might be demographic differences in AR-PCNSL in the pre-cART era versus post cART eras (after 1996, when cART became widely available at UCSF and SFGH). All 93 patients had a pathologic diagnosis of B-cell lymphoma and were determined to have either diffuse large B-cell lymphoma or large cell lymphoma NOS on pathologic review. The median age at diagnosis (36 y) and median KPS (30) were similar in the pre-cART (75 cases) versus cART eras (18 cases). Ten patients diagnosed in the cART era previously had received antiretroviral therapy prior to diagnosis of AR-PCNSL, but none were adherent (Supplemental Table 1).
Notably, subsequent to increased availability of cART beginning in 1997, there was a 60% increase in the proportion of minority patients diagnosed with AR-PCNSL compared with the pre-cART era at our institution; chi-square test P= .051. Odds ratio=3.51 with 95% CI=0.94–13.21 (Supplemental Table 1). This increase was confirmed by a population-based analysis via SEER registry data within the greater San Francisco Bay Area (Supplemental Fig. 1). This trend, combined with the fact that AR-PCNSL patients diagnosed in the cART era had more opportunistic infections compared with pre-cART diagnoses (83% vs 55%), suggests that these patients experience significant problems in access to health care and/or in medical non-adherence, issues that would likely impede successful completion of medical interventions for an aggressive lymphoma as well.
Treatment Patterns of AR-PCNSL in the cART Era at UCSF/SFGH
Among the 18 AR-PCNSL patients diagnosed at UCSF/SFGH during the cART era, 14 received CNS lymphoma-directed therapy. Four received WBRT (without chemotherapy) plus concomitant cART. Ten received induction HD-MTX–based chemotherapy; 8 received cART in combination with HD-MTX. The clinical characteristics of the 8 AR-PCNSL patients who received HD-MTX plus cART (median age, 33; median KPS, 30; and median CD4 count, 31.5, range 1–386) were similar to those of patients who were treated with WBRT plus cART (median age, 42.5; median KPS, 35; median CD4 count, 43, range 23–50). Both cohorts received antiretroviral regimens that included a spectrum of nucleoside as well as non-nucleoside-based reverse transcriptase inhibitors and/or HIV protease inhibitors. The median dose of WBRT was 3100 cGy and the median dose of MTX administered was 8g/m2 (range 3–8) infused over 4 hours, for a median of 8 cycles, with leucovorin rescue21 (Tables 1 and 2). Two patients with a history of non-adherence to antiretroviral regimens (CD4 counts at diagnosis of 5 and 15 cells/mm3, respectively) received only HD-MTX (8g/m2/dose for 5 and 7 infusions, respectively) without cART.
Table 1.
Clinical characteristics of the 20 AR-PCNSL patients who received cART plus HD-MTX. Combination ART employs 3 or more antiretroviral drugs either taken individually or in fixed dose combinations. Abbreviations: Dx, diagnosis; F/U, follow-up; ND, nondetectable. NA, not available. Patients 1–8 were treated at UCSF; patients 9–20 were treated at Memorial Sloan-Kettering, UC San Diego, Moffitt Cancer Center, and Cook County Hospital.
| Pt. | Age/ Sex | Yrs HIV+ |
Prior Opportunistic Infections |
KPS | CD4 at Dx | Viral Load at Dx |
cART Regimen at Dx |
CD4 Last F/U |
Viral Load Last F/U |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33/F | 2 | MAC, HSV, Molluscum Contagiosum |
40 | 1 | 506,444 | Tenofovir/emtricitabine Etravine |
246 | 114 |
| 2 | 30/M | 1 | HPV, PJP | 30 | 21 | 2,809 | Darunavir/ritonavir Abacavir |
480 | ND |
| 3 | 26/M | 5 | MAC | 30 | 1 | 169,534 | Abacavir/lamuvudine, atazanavir/ritonavir | 635 | 3219 |
| 4 | 43/F | 7 | MAC Pneumonia |
30 | 5 | 128,306 | Abacavir/lamuvudine, lopinavir/ritonavir tenofovir/zidovudine | 202 | 676 |
| 5 | 42/M | 10 | PJP | 20 | 56 | 380,004 | Lopinavir/ritonavir Tenofovir/emtricitabine |
NA | NA |
| 6 | 33/M | 8 | None | 20 | 386 | 75 | Nevirapine/stavudine Lamuvudine |
184 | 857 |
| 7 | 39/M | 1 | None | 50 | 42 | 35,419 | Lamuvidine/ zidovudine Efavirenz |
253 | ND |
| 8 | 52/M | 10 | CMV Retinitis, Candidiasis, PJP |
40 | 86 | 663 | Lamivudine/ stavudine, nelfinavir | 415 | ND |
| 9 | 33/M | 1 | Coccidiomycosis | 30 | 192 | 35,000,000 | Abacavir/dolutegravir Lamivudine |
370 | ND |
| 10 | 62/M | 1 | Pulmonary Aspergillosis, CMV, KS |
40 | 24 | 585,427 | Abacavir/dolutegravir Lamivudine |
200 | ND |
| 11 | 43/M | 1 | MAC, PCP, Candidiasis |
20 | 70 | 246 | Lopinavir/ritonavir tenofovir/emtricitabine | 260 | ND |
| 12 | 33/M | 1 | PCP | 50 | 70 | 205,000 | Ritonavir/atazanavir Tenofovir/emtricitabine Nevirapine |
205 | ND |
| 13 | 36/M | 1 | None | 20 | 7 | 31,159 | Tenofovir/emtricitabine Raltegravir |
NA | NA |
| 14 | 66/F | 5 | None | 80 | NA | NA | Nevirapine/zidovudine | NA | NA |
| 15 | 45/F | NA | Syphilis | 80 | 84 | NA | Efavirenz/emtricitabine Tenofovir |
NA | NA |
| 16 | 40/M | 20 | KS, HSV | 50 | 276 | 26 | Tenofovir/emtricitabine atazanavir |
556 | 30 |
| 17 | 57/M | 27 | Tb | 80 | 530 | ND | Emtricitabine rilpivirine/ tenofovir |
NA | NA |
| 18 | 53/M | 1 | None | 50 | 471 | 4,249 | Efavirenz/emtricitabine tenofovir |
NA | NA |
| 19 | 65/M | 1 | Molluscum Contagiosum |
40 | 156 | 690 | Efavirenz/emtricitabine tenofovir |
401 | ND |
| 20 | 51/M | 10 | Syphilis | 40 | 345 | 68,000 | Efavirenz/emtricitabine tenofovir |
191 | ND |
Table 2.
Methotrexate doses, adjunctive agents, serious toxicities, responses, and outcomes among the 20 AR-PCNSL patients who received cART plus HD-MTX. Temozolomide was administered with HD-MTX as described.21 Procarbazine and vincristine were administered with HD-MTX as described. 40 Abbreviations: Dx, diagnosis; PFS, progression-free survival; EA, etoposide plus high-dose cytarabine; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; M-R, methotrexate plus rituximab; R-MBVP, rituximab, methotrexate, carmustine, etoposide, prednisone.
| Pt. | # Cycles HD-MTX |
Max Dose MTX (g/m2) |
Other Agents |
cART Regimen at Dx |
Toxicities (≥gr. 3) |
Best Response To Induction |
PFS (mo) |
2nd Line Intervention | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 3 | None | Etravine, Tenofovir/Emtricitabine |
None | Stable Disease |
2.9 | Lenalidomide | 24+ |
| 2 | 8 | 8 | Temozolomide Etoposide/ Ara-C (EA) |
Abacavir, darunavir/ Ritonavir |
Gr. 4 thrombocytopenia | CR | 42.9+ | 42.9+ | |
| 3 | 1 | 3 | None | Abacavir, lamuvudine, atazanavir/ritonavir | None | Not Assessed | 78+ with WBRT |
78+ | |
| 4 | 6 | 3.5 | None | Abacavir/lamuvudine, lopinavir/ ritonavir, Tenofovir, zidovudine |
None | CR | 88.4+ | 88.4+ | |
| 5 | 2 | 3 | None | Lopinavir/ritonavir, Tenofovir/ emtricitabine |
Gr. 5 sepsis (not neutropenic) |
Not Assessed | NA | 2.0 | |
| 6 | 11 | 8 | None | Lamuvudine, nevirapine, Stavudine |
None | CR | 16 | R-ICE | 24.9 |
| 7 | 8 | 8 | None | Efavirenz, Lamuvidine/Zidovudine |
None | CR | 103.5+ | 103.5+ | |
| 8 | 9 | 8 | Rituximab | Lamivudine/stavudine, Nelfinavir |
None | CR | 157.3+ | 157.3+ | |
| 9 | 5 | 3 | Rituximab | Abacavir/dolutegravir/ Lamivudine |
None | CR | 12+ | 12+ | |
| 10 | 4 | 3 | None | Abacavir/dolutegravir/ Lamivudine |
Gr. 3 neutropenia, Thrombocytopenia |
CR | 8+ | 8+ | |
| 11 | 2 | 3 | Temozolomide, Rituximab |
Lopinavir, ritonavir tenofovir/ emtricitabine | Gr. 3 febrile neutropenia Gr. 4 pancreatitis |
CR | 79+ | 79+ | |
| 12 | 6 | 3 | None | Ritonavir, atazanavir, nevirapine, Tenofovir/emtricitabine |
None | CR | 125+ | 125+ | |
| 13 | 1 | 3.5 | Procarbazine, vincristine | Raltegravir Tenofovir/emtricitabine |
Gr. 5 Sepsis (Neutropenic) |
Not Assessed | 1 | 1 | |
| 14 | 2 | 8 | None | Nevirapine/zidovudine | None | PD | 2 | WBRT | 3 |
| 15 | 2 | 8 | None | Efavirenz/emtricitabine/ Tenofovir |
None | PD | 1 | WBRT | 6 |
| 16 | 8 | 3.5 | Rituximab, procarbazine, vincristine, Ara-C | Tenofovir/emtricitabine Atazanavir |
Gr. 3 neutropenia | CR | 29+ | 29+ | |
| 17 | 7 | 3.5 | Rituximab, procarbazine, vincristine | Emtricitabine Rilpivirine/Tenofovir |
Gr. 3 Neutropenia | CR | 19+ | 19+ | |
| 18 | 7 | 3.5 | Ara-C | Efavirenz/emtricitabine Tenofovir |
Gr. 3 alt elevation | CR | 24 | M-R WBRT |
32 |
| 19 | 7 | 3.5 | Rituximab, procarbazine, vincristine, Ara-C | Efavirenz/emtricitabine Tenofovir |
Gr. 3 neutropenia, Zoster |
CR | 60+ | 60+ | |
| 20 | 8 | 8 | Rituximab, procarbazine, vincristine, Ara-C | Efavirenz/emtricitabine Tenofovir |
Gr. 3 neutropenia, Gr. 3 renal failure, Gr. 3 AST/ ALT elevation |
PR | 6 | R-MBVP WBRT |
8 |
During this period, because of advanced disease and poor performance status, 3 patients succumbed to AR-PCNSL before receipt of any intervention (including cART) and 1 received cART but died of brain tumor progression before initiation of either HD-MTX or WBRT.
Efficacy of cART plus HD-MTX in AR-PCNSL
Median survival for the 4 patients with newly diagnosed AR-PCNSL treated with the addition of cART to WBRT was similar to that achieved with WBRT alone in the pre-cART era: one month22 (Fig. 1). By contrast, the median progression-free and overall survival for the 8 patients who received cART plus HD-MTX–based therapy exceeds 60.45 months. Complete responses on MRI were attained in 5 patients; one obtained stable disease, and responses to HD-MTX in 2 patients were not assessed (Tables 1 and 2). While there was evidence for clinical efficacy of HD-MTX in the absence of cART, in that the 2 AR-PCNSL patients treated with HD-MTX monotherapy achieved complete responses on MRI, both succumbed to AR-PCNSL at 11.3 and 13.8 months, respectively.
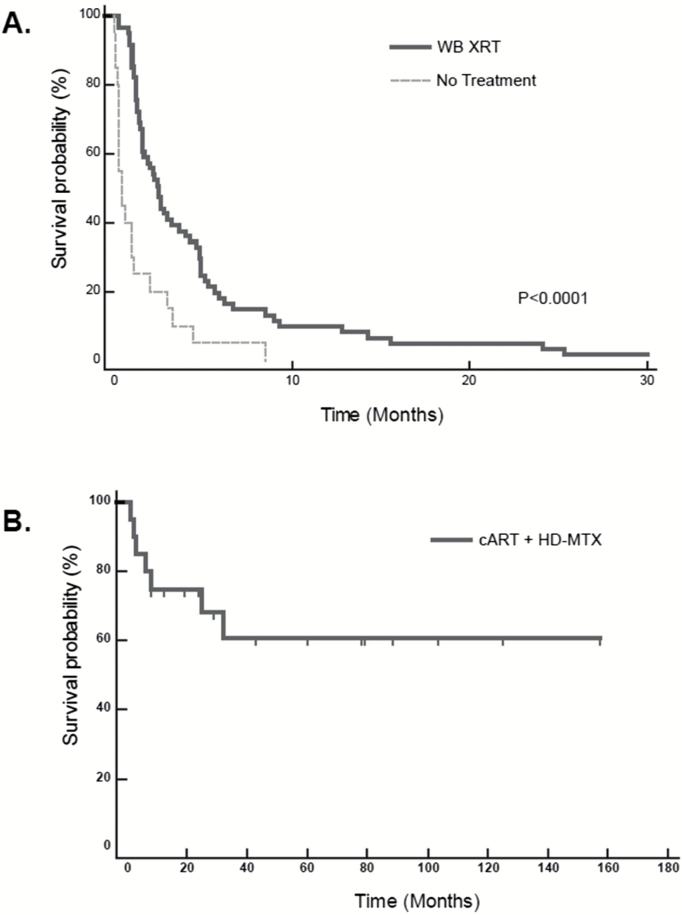
Fig. 1.
Long-term survival of AR-PCNSL patients treated with cART plus HD-MTX–based therapy and comparison to WBRT.
1A. Median survival for all 75 AR-PCNSL patients in the pre-cART era was 2 months and only slightly longer for the cohort of 57 patients who received WBRT, 2.5 months; P<.0001. During the pre-cART era at SFGH, there was only one AR-PCNSL patient who survived beyond 30 months (>191 mo, WBRT cohort).
1B. Median survival for the 20 patients who received cART plus HD-MTX–based therapy has not been reached, with median follow-up of 27 months (range 1–156.3 mo).
Multicenter Validation of cART plus HD-MTX–Based Therapy
Based upon the encouraging outcomes with HD-MTX administered in combination with cART in treatment of AR-PCNSL in this series, we evaluated the reproducibility of these results in a multicenter study involving regionally diverse institutions with expertise in AIDS-related malignancies: Memorial Sloan-Kettering Cancer Center, the University of California San Diego, the H. Lee Moffitt Cancer Center, and Rush University Medical College. We identified 12 patients (10 male, 2 female) treated for diagnoses of AR-PCNSL with cART plus HD-MTX–based regimens between 2005 and 2015, without WBRT. These had the following clinical characteristics: median age, 48; median KPS, 45; median CD4 count, 156, range 7–530. Ten had a pathologic diagnosis of PCNSL. Efficacy of cART plus HD-MTX–based therapy in this cohort was similar to results obtained at UCSF. Complete responses on MRI were observed in 8 patients, and each of the 3 patients with active leptomeningeal disease at diagnosis attained cytologic remission (Tables 1 and 2). Median progression-free survival for this cohort has not been reached.
Adjuncts to HD-MTX and Adverse Events with Coadministration of cART plus HD-MTX–Based Therapy in AR-PCNSL
Among the 20 AR-PCNSL patients who received cART plus HD-MTX–based therapies, 10 received only HD-MTX monotherapy (3–8g/m2) and 10 were treated with HD-MTX (3–8g/m2) in combination with adjunctive agents including CNS-penetrant alkylators: procarbazine (5 patients) or temozolomide (2 patients). Seven received adjunctive rituximab. Five patients received high-dose Ara-C (2g/m2) as monotherapy (four patients) or high-dose Ara-C as a component of etoposide and high-dose cytarabine consolidation. Because of adherence issues with respect to chemotherapy, one patient was treated with WBRT plus cART as consolidation after only one cycle of induction HD-MTX monotherapy plus cART (Table 2).
Integration of cART plus HD-MTX–based therapy was generally well tolerated, with the exception of 2 mortalities attributed to sepsis during induction, each in patients with a KPS of 20 (Table 2). Overall, neutropenic complications were the most common serious adverse event (≥grade 3) with induction HD-MTX–based therapy in this series and involved 7 patients, 4 of whom received adjunctive procarbazine plus vincristine and 1 who received temozolomide. There were no complications attributed to immune reconstitution inflammatory syndrome, and the rates of grade 3 or 4 hepatic or renal toxicities in this series were similar to studies of HD-MTX–based therapy among immunocompetent patients with PCNSL.21 There were no serious toxicities likely attributed to rituximab in patients who received anti-CD20 plus HD-MTX in combination with cART, and 5 of the 7 patients who received adjunctive rituximab for AR-PCNSL have experienced durable remission. Remarkably, 2 patients were successfully treated with cART plus HD-MTX–based therapy while receiving effective antifungal therapy for concurrent meningeal coccidioidomycosis and pulmonary aspergillosis, opportunistic infections that antedated the treatment of AR-PCNSL. Of note, among the 10 patients who experienced serious toxicities (≥grade 3) during induction with HD-MTX plus cART, 7 received concurrent alkylating agents. However, in most cases, these toxicities were successfully managed, and survival outcomes in these patients were favorable.
Antiretroviral Efficacy of cART During HD-MTX–Based Therapy
Given the remarkable efficacy of HD-MTX administered in combination with cART in treatment of AR-PCNSL in this series, we attempted to determine to what extent the antiretroviral efficacy of cART relates to long-term survival. Among the 20 AR-PCNSL patients who were treated with HD-MTX–based therapy plus cART, with a median follow-up of greater than 2 years, thus far there have been only 7 deaths: 2 from sepsis, 3 from disease progression during induction at between 1 and 6 months, and 2 from progression of disease after attainment of complete response at 16 and 24 months. Notably, one of these delayed relapses occurred in the setting of ineffective HIV control with a CD4 count decline from 438 at the completion of therapy to 112.
With a median follow-up of greater than 50 months, 13 AR-PCNSL patients in this cohort are free of lymphoma. Given that a CD4+ cell count <100 cells/microliter is an established risk factor for short overall survival in HIV-related lymphomas,11 it is noteworthy that among the 15 AR-PCNSL patients in this high-risk group in our study who were treated with HD-MTX–based therapy, the only to survive greater than 2 years were those in whom HIV viral load suppression and CD4 count improvement were documented during and/or immediately after HD-MTX, via concomitant cART (P<.005; Fisher’s exact test) (Fig. 2).
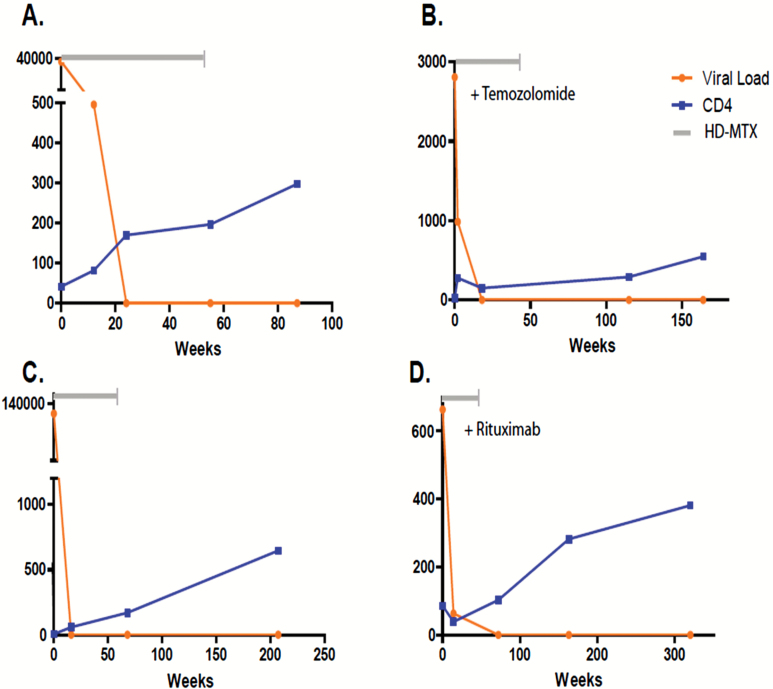
Fig. 2.
Control of HIV viral load and CD4+ cell reconstitution with cART instituted with HD-MTX during initial treatment of AR-PCNSL in 4 responding patients. Orange line: viral load (copies/mL); blue line: CD4 count (cells/mL). Grey line: duration of HD-MTX–based therapy. 2A. Patient 7 received high-dose methotrexate monotherapy (8 cycles) plus a combination of nonnucleoside and nucleoside analogue reverse transcriptase inhibitors: efavirenz, lamivudine plus zidovudine. 2B. Patient 2 received HD-MTX (8 cycles) plus temozolomide followed by consolidative chemotherapy with infusional etoposide plus high-dose cytarabine. During chemotherapy, this patient received an antiretroviral regimen containing a nucleoside reverse transcriptase inhibitor, abacavir, plus ritonavir-boosted darunavir. 2C. Patient 4 received high-dose methotrexate monotherapy (6 cycles) plus a regimen of nucleoside reverse transcriptase inhibitors abacavir, lamivudine, zidovudine, and tenofovir plus ritonavir-boosted loprinavir. 2D. Patient 8 received HD-MTX (9 cycles) plus rituximab in combination with a regimen consisting of 2 nucleoside analogue reverse transcriptase inhibitors, lamivudine plus stavudine, as well as nelfinavir, a protease inhibitor. Each of these AR-PCNSL patients achieved complete responses to induction HD-MTX plus cART regimens and none has progressed after a median follow-up of greater than 95 months.
While the addition of temozolomide had no impact on cART-induced HIV viral load suppression, its inclusion with the HD-MTX backbone was associated with a more blunted rate of CD4 cell count recovery compared with patients who received HD-MTX without an alkylator. Moreover, inclusion of procarbazine plus vincristine with HD-MTX led to reductions of CD4 counts at completion of therapy in the 2 patients who received concurrent cART. Similarly, while administration of WBRT to patient 3 after one cycle of HD-MTX plus cART did not negatively impact successful cART-mediated HIV viral load suppression, an attenuated rate of CD4 cell count recovery was detected compared with patients who received cART plus HD-MTX monotherapy, adjunctive rituximab, and/or lenalidomide after HD-MTX failure (Table 2).
AR-PCNSL Tumor Pathology and Immunophenotype in Pre- versus Post-cART Eras
Given the evidence that improved immune function associated with antiretroviral treatment might skew the biology of systemic AIDS-related large B-cell lymphomas toward a GCB phenotype, associated with improved prognosis,23 we tested the hypothesis that AR-PCNSL that arose in patients with prior use of antiretroviral regimens, including cART, might be biologically distinct from tumors that developed without prior antiretroviral exposure. From the 93 cases of AR-PCNSL analyzed in this study, we identified 42 diagnostic tumor specimens that were adequate for immunohistochemical testing (Table 3). We analyzed markers of lymphoma pathobiology in 36 AR-PCNSL specimens from the pre-cART cohort and compared these with 6 AR-PCNSL tumors from the cART era cohort, including 3 of the patients who received cART plus HD-MTX and were long-term survivors; 5 of these patients had prior exposure to antiretroviral regimens, but none had been adherent. All cases, pre- and post-CART, were of the nongerminal center immunophenotype, per the Hans classification. As expected, the majority of tumors from both eras were EBV+.24 , 25
Table 3.
Clinical characteristics and immunophenotypes of the diagnostic tumor specimens of AR-PCNSL from pre-cART vs cART eras. Both of these series had similarly distributed clinical and disease characteristics. Double-hit status, defined by coexpression of BCL-2 and MYC, was demonstrated in 26.8% of AR-PCNSL cases and without major differences between the pre-cART and cART eras. None of the tumors pre-cART scored positive for BCL-6, whereas 2 cases from patients previously treated with antiretroviral therapy were BCL-6+, consistent with the hypothesis that prior partial immune reconstitution may impact AIDS-related CNS lymphomagenesis. Nevertheless, we determined that overall, AR-PCNSL displays a remarkably uniform, nongerminal center immunophenotype that is distinct from the immunophenotype of PCNSL arising in the immunocompetent, a condition in which between 50% and 80% of cases coexpress MUM-1 and BCL-6,41 , 42 and in which 5%–15% are EBV+.43–45
| Characteristic | Pre-cART | cART Era |
|---|---|---|
| Total patients | 36 | 6 |
| Male | 35 (97%) | 5 (83%) |
| Median age (range) | 36 (27–50) | 36.5 (26–55) |
| Median KPS (range) | 30 (20–40) | 30 (20–50) |
| Median CD4+ cells/mm3 | 7 (0–157) | 5 (1–50) |
| Molecular markers | ||
| EBV+ | 35 (97%) | 6 (100%) |
| Median MIB-1+, no. ≥80% | 65, 8 | 65, 2 |
| BCL-2+ | 36 (100%) | 6 (100%) |
| MYC+ | 10 (28%) | 1 (17%) |
| CD10+ | 0 (0%) | 0 (0%) |
| BCL-6+ | 0 (0%) | 2 (33%) |
| MUM-1+ | 33 (92%) | 6 (100%) |
Discussion
To our knowledge this is likely the only cohort of AR-PCNSL patients analyzed to date that have received cART in combination with HD-MTX–based therapy. Our main finding is the evidence for reproducible feasibility and efficacy of combined cART plus HD-MTX in patients with AR-PCNSL. While retrospective, this multicenter clinical series nevertheless demonstrates that CD4+ cell reconstitution and HIV viral load reduction mediated by cART can be achieved in a cohort of patients with AR-PCNSL during HD-MTX chemotherapy, and correlates with long-term survival. Of the 12 AR-PCNSL patients in whom HIV control was achieved with cART administered with HD-MTX and/or lenalidomide, without WBRT, each has maintained apparent durable remissions of CNS lymphoma, lasting beyond the median of 50 months. Median KPS for this cohort was 90 at last follow-up.
This study has a number of limitations. As above, given its retrospective nature, it is possible that the electronic medical record dataset at each site was incomplete, with respect both to the number of AR-PCNSL patients diagnosed, as well as to details related to therapy, including treatment-related toxicities. In addition, it is remarkable that we could identify only 20 patients with AR-PCNSL who were treated with cART plus HD-MTX among the 5 participating institutions. Given that the median KPS of 40 and age of 42.5 for this cohort are similar overall to the patient characteristics in the pre-cART era at SFGH, the small number of patients identified is likely primarily a consequence of the dramatic decline in the incidence of AR-PCNSL since the emergence of cART.
Nevertheless, it is worth noting that outcomes achieved with cART administered in combination with HD-MTX in this multicenter analysis appear to be highly favorable compared with results described in 15 patients with AR-PCNSL who received HD-MTX without cART, in which median overall survival was 9.7 months (73 days for the 10 patients with biopsy-proven disease).26
These findings underscore the reality that AR-PCNSL is typically an end-stage manifestation of AIDS and that these patients urgently need immune reconstitution, to control both opportunistic infections as well as lymphoma. Given the dismal survival of AR-PCNSL patients treated with WBRT, even in the cART era, we hypothesize that local effects of WBRT on the CNS lymphoma microenvironment may be particularly disruptive to T-cell-mediated antitumoral immune surveillance mechanisms that would be potentiated by cART. Moreover, brain radiotherapy, alkylating agents, and glucocorticoids may also potentiate peripheral CD4 cell depletion, as demonstrated in studies of high-grade glioma patients treated with radiotherapy.27 , 28 Our observation that HD-MTX and/or lenalidomide does not appear to significantly attenuate CD4 cell recovery in AR-PCNSL patients who receive cART is novel and relevant in this regard and provides an explanation for the marked differences in outcome compared with patients treated with WBRT in this series. The role of lenalidomide in AR-PCNSL deserves further evaluation, particularly given its efficacy in the potentiation of T-cell responses.29–32 That peripheral CD4 counts can increase during HD-MTX–based therapy may also be relevant to the design of immunotherapy strategies that sequence the use of cytotoxics with agents that potentiate T-cell function.
While racial and/or ethnic disparities in the receipt or adherence to antiretroviral medications have been established in HIV,33–35 we believe a change in the racial/ethnic demographics of AR-PCNSL since availability of cART has not been described. An important lesson from this study is that AIDS patients who initially are non-adherent to antiretroviral therapy may ultimately be successfully treated with complex interventions that require inpatient chemotherapy plus cART.
Finally, while evidence for modest survival prolongation with cART in AR-PCNSL has been noted,16 , 36 , 37 the magnitude of long-term progression-free survival observed in the major proportion of patients within this cohort has not been previously demonstrated in analyses of survival outcomes with WBRT and/or chemotherapy in the absence of cART for AR-PCNSL. This is particularly striking given the high rate of antecedent and concurrent opportunistic infections that impacted 75% of the patients in this cohort. Another unique aspect of this study is the determination of the immunohistochemical expression profiles of diagnostic tumor specimens of AR-PCNSL in the context of clinical outcomes in patients. Our analysis of tumor biology, pre- and post-CART, demonstrates a near uniform, EBV+, BCL-2+ nongerminal center immunophenotype, even among patients who received prior antiretroviral therapy. These observations support our hypothesis that early immune reconstitution during chemotherapy for AR-PCNSL is an important clinical variable that correlates with long-term survival.
Given these observations as well as the established long-term toxicities of WBRT in PCNSL, we recommend that integration of cART plus HD-MTX be considered as a first-line intervention for AR-PCNSL. There was was no evidence for untoward toxicity with rituximab in this series, suggesting that coadministration of rituximab should be considered in AR-PCNSL, particularly in patients with a baseline CD4+ cell count >50.38 , 39 We did not detect evidence of improved outcomes with the addition of alkylating agents, vincristine, and cytarabine to the HD-MTX backbone, and these agents were associated with an increased rate of neutropenic complications and a potentially more attenuated rate of CD4 recovery with cART. We demonstrate that long-term disease-free survival can reproducibly be achieved in AR-PCNSL, with HD-MTX and without dose-intensive genotoxic or EBV-directed viral therapy, if early CD4 count recovery is achieved. While our experience suggests that AR-PCNSL remains a highly aggressive neoplasm during the cART era, durable clinical responses are feasible in AR-PCNSL patients who are treated with cART plus HD-MTX, even among those with a history of medical non-adherence and significant opportunistic infections.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
Supported by the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763, 1R21 CA184694-01), NIH R01CA139-83-01A1, and by the Leukemia & Lymphoma Society (JLR).
Conflict of interest disclosure. Dr Rubenstein receives research funding from Genentech and Celgene.
Supplementary Material
Acknowledgments
We are grateful to the myriad dedicated physicians and nurses who have cared for patients with AIDS-related PCNSL since the beginning of the HIV epidemic. We are grateful to Walter Finkbeiner, MD, PhD, Department of Pathology, SFGH, for assistance in obtaining diagnostic tumor specimens from AR-PCNSL patients. We are thankful to Joseph McGuire for assistance with the Cancer Center Registry Data.
References
- 1. Levine AM. Acquired immunodeficiency syndrome-related lymphoma. Blood. 1992;80(1):8–20. [PubMed] [Google Scholar]
- 2. Forsyth PA, DeAngelis LM. Biology and management of AIDS-associated primary CNS lymphomas. Hematol Oncol Clin North Am. 1996;10(5):1125–34. [DOI] [PubMed] [Google Scholar]
- 3. Raez LE, Patel P, Feun L. Natural history and prognostic factors for survival in patients with acquired immune deficiency syndrome (AIDS)-related primary central nervous system lymphoma (PCNSL). Crit Rev Oncog. 1998;9(3–4):199–208. [PubMed] [Google Scholar]
- 4. Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med. 1993;119(11):1093–104. [DOI] [PubMed] [Google Scholar]
- 5. Corn BW, Marcus SM, Topham A. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer. 1997;79(12):2409–13. [PubMed] [Google Scholar]
- 6. Katz IT, Ryu AE, Onuegbu AG. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moss AR, Hahn JA, Perry S. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004;39(8):1190–8. [DOI] [PubMed] [Google Scholar]
- 8. Bower M, Stebbing J, Tuthill M. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood. 2008;111(8):3986–90. [DOI] [PubMed] [Google Scholar]
- 9. Little RF. AIDS, T cells, chemotherapy: HAART-breaking? Blood. 2008;111(8):3921–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barta SK, Xue X, Wang D. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun A, Bae K, Gore EM. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16(3):859–63. [DOI] [PubMed] [Google Scholar]
- 14. Monje ML, Mizumatsu S, Fike JR. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–62. [DOI] [PubMed] [Google Scholar]
- 15. Thiel E, Korfel A, Martus P. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–47. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann C, Tabrizian S, Wolf E. Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART-induced immune recovery. AIDS. 2001;15(16):2119–27. [DOI] [PubMed] [Google Scholar]
- 17. Abrey LE, Batchelor TT, Ferreri AJ. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–43. [DOI] [PubMed] [Google Scholar]
- 18. Hans CP, Weisenburger DD, Greiner TC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 19. Kluk MJ, Chapuy B, Sinha P. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7(4):e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson NA, Slack GW, Savage KJ. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubenstein JL, Hsi ED, Johnson JL. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chamberlain MC. Long survival in patients with acquired immune deficiency syndrome-related primary central nervous system lymphoma. Cancer. 1994;73(6):1728–30. [DOI] [PubMed] [Google Scholar]
- 23. Little RF, Pittaluga S, Grant N. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101(12):4653–9. [DOI] [PubMed] [Google Scholar]
- 24. Ambinder RF. Epstein-Barr virus associated lymphoproliferations in the AIDS setting. Eur J Cancer. 2001;37(10):1209–16. [DOI] [PubMed] [Google Scholar]
- 25. MacMahon EM, Glass JD, Hayward SD. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338(8773):969–73. [DOI] [PubMed] [Google Scholar]
- 26. Jacomet C, Girard PM, Lebrette MG. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS. 1997;11(14):1725–30. [DOI] [PubMed] [Google Scholar]
- 27. Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62(5):1423–6. [DOI] [PubMed] [Google Scholar]
- 28. Grossman SA, Ye X, Lesser G. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsay AG, Clear AJ, Kelly G. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114(21):4713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gandhi AK, Kang J, Havens CG. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schafer PH, Gandhi AK, Loveland MA. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther. 2003;305(3):1222–32. [DOI] [PubMed] [Google Scholar]
- 32. Corral LG, Haslett PA, Muller GW. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 33. Silverberg MJ, Leyden W, Quesenberry CP., Jr Horberg MA: Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009;24(9):1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saha S, Jacobs EA, Moore RD. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS. 2010;24(7):415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dale SK, Bogart LM, Wagner GJ. Medical mistrust is related to lower longitudinal medication adherence among African-American males with HIV. J Health Psychol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17(12):1787–93. [DOI] [PubMed] [Google Scholar]
- 37. Uldrick TS, Pipkin S, Scheer S. Factors associated with survival among patients with AIDS-related primary central nervous system lymphoma. AIDS. 2014;28(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noy A, Lee JY, Cesarman E. modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood. 2015;126(2):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sparano JA, Lee JY, Kaplan LD. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115(15):3008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morris PG, Correa DD, Yahalom J. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Camilleri-Broet S, Criniere E, Broet P. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–6. [DOI] [PubMed] [Google Scholar]
- 42. Braaten KM, Betensky RA, de Leval L. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063–9. [PubMed] [Google Scholar]
- 43. Kitai R, Matsuda K, Adachi E. Epstein-Barr virus-associated primary central nervous system lymphoma in the Japanese population. Neurol Med Chir (Tokyo). 2010;50(2):114–8. [DOI] [PubMed] [Google Scholar]
- 44. Krogh-Jensen M, Johansen P, D’Amore F. Primary central nervous system lymphomas in immunocompetent individuals: histology, Epstein-Barr virus genome, Ki-67 proliferation index, p53 and bcl-2 gene expression. Leuk Lymphoma. 1998;30(1–2):131–42. [DOI] [PubMed] [Google Scholar]
- 45. Geddes JF, Bhattacharjee MB, Savage K. Primary cerebral lymphoma: a study of 47 cases probed for Epstein-Barr virus genome. J Clin Pathol. 1992;45(7):587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.