Abstract
Background.
The prognostic significance of baseline contrast enhancing tumor prior to second- or third-line therapy in recurrent glioblastoma (GBM) for overall survival (OS) remains controversial, particularly in the context of repeated surgical resection and/or use of anti-angiogenic therapy. In the current study, we examined recurrent GBM patients from both single and multicenter clinical trials to test whether baseline enhancing tumor volume, including central necrosis, is a significant prognostic factor for OS in recurrent GBM.
Methods.
Included were 497 patients with recurrent GBM from 4 data sources: 2 single-center sites (University of Toronto, University of California Los Angeles) and 2 phase II multicenter trials (AVF3708G, Bevacizumab ± Irinotecan, NCT00345163; XL184-201, Cabozantinib, NCT00704288). T1 subtraction maps were used to define volume of contrast enhancing tumor, including central necrosis. Cox multivariable and univariate analyses were used to evaluate the relationship between tumor volume prior to second- or third-line therapy and OS.
Results.
Both continuous measures of baseline tumor volume and tumors dichotomized into large (≥15cc) and small (<15cc) tumors were significant predictors of OS (P<.0001), independently of age and treatment. Univariate analysis demonstrated significant OS differences (P<.05) between large (≥15cc) and small (<15cc) tumors in patients under all therapeutic scenarios. Only patients treated with cabozantinib who previously failed anti-angiogenic therapy did not show an OS dependence on baseline tumor volume.
Conclusions.
Baseline tumor volume is a significant prognostic factor in recurrent GBM. Clinical trial treatment arms must have a balanced distribution of tumor size, and tumor size should be considered when interpreting therapeutic efficacy.
Keywords: bevacizumab, cabozantinib, glioblastoma, recurrent, T1 subtraction, tumor volume
Importance of the Study
GBM is an incurable brain tumor characterized by a very short OS. Multiple studies have implicated baseline pretreatment (ie, prior to second- or third-line therapy) contrast enhancing tumor size as a significant prognostic factor for OS in recurrent GBM, which is often the patient population involved in early phase trials with new therapeutics. In the current study of data from both multicenter and single-center trials, we demonstrate that baseline pretreatment contrast enhancing tumor volume, including any central necrosis, is a strong prognostic factor in determining OS in recurrent GBM regardless of therapy, as long as patients had not already failed anti-angiogenic therapy. These results have important implications in clinical trial design, suggesting that steps should be taken to ensure balance among treatment arms in terms of distribution of tumor size and effects of pretreatment tumor size considered when interpreting therapeutic efficacy.
Contrast enhancement on CT or T1-weighted MRI is the standard for quantifying tumor burden in glioblastoma (GBM), as multiple studies have confirmed regions of enhancement to contain the most aggressive portions of the tumor.1–4 Although still controversial,5 maximum resection in newly diagnosed GBM appears to provide a significant survival advantage.6–13 Less certain is the importance of extent of resection in recurrent GBM,14 , 15 although data suggest that maximum resection at recurrence also prolongs survival.16–18 A secondary analysis examining the association between extent of resection and survival in recurrent GBM from the DIRECTOR trial,19 a prospective clinical trial examining temozolomide (TMZ) rechallenge using 2 different dose regimens, showed a tendency toward longer survival in patients with complete resection versus patients who did not undergo surgery, but these trends were not statistically significant, potentially due to a relatively small sample size. The study demonstrated that baseline residual enhancing tumor volume was a significant predictor of overall survival (OS) and time to second progression, presumably due to extent of resection being defined as the percentage of tumor removed, which is strongly influenced by both the initial tumor size as well as the amount of residual tumor remaining after surgery. The observation that pretreatment enhancing tumor volume or residual enhancing volume after surgical resection is a significant prognostic factor for recurrent GBM is consistent with other multicenter reports on recurrent GBM20 , 21 treated with bevacizumab (BV). Less clear is the role of BV therapy in recurrent GBM. Specifically, it is not clear whether these trends remain if patients previously failed BV or were never treated with BV during their clinical course.
In the current study we examined a large dataset of recurrent GBM patients from both single institutions as well as several multicenter clinical trials to test the hypothesis that baseline pretreatment enhancing tumor volume, including central necrosis, is a significant prognostic factor for OS in recurrent GBM. We hypothesize that large tumor volume at baseline is associated with shortened OS regardless of the type of therapy employed at recurrence (such as anti-angiogenic or antineoplastic) and whether or not patients previously failed anti-angiogenic therapies.
Methods
Patients
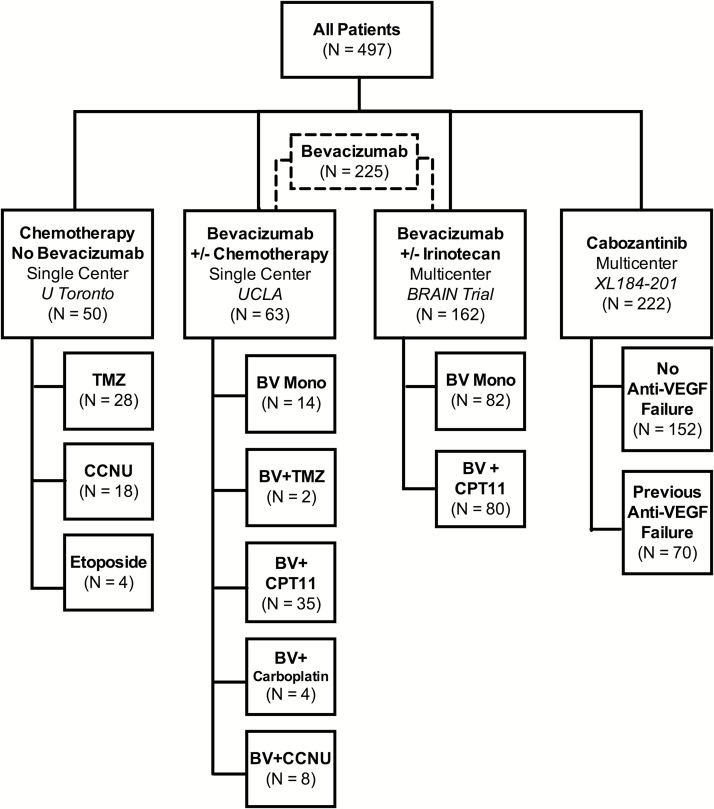
A total of 497 patients with pathologically confirmed GBM with recurrence based on MRI, clinical data, and/or histology from 4 data sources were included in this retrospective study. All patients included were limited to 1–2 prior recurrences before inclusion in the current study. Data were obtained from 2 single-center sites (University of Toronto, University of California Los Angeles) and 2 phase II multicenter trials (AVF3708G, Bevacizumab ± Irinotecan, ClinicalTrials.gov #NCT00345163; XL184-201, Cabozantinib Monotherapy, ClinicalTrials.gov #NCT00704288). Median age for the combined cohort was 55 years (mean age = 54 y) and median OS postrecurrence was 8.5 months (mean OS = 11.3±0.4 SEM). Data acquisition was performed in compliance with all applicable regulations of the Health Insurance Portability and Accountability Act. Details of patient groups are illustrated in Fig. 1.
Fig. 1.
Patient flow diagram. Imaging and patient information were obtained from 4 different data sources: recurrent GBM patients treated at the University of Toronto treated with various chemotherapies (N = 50); recurrent GBM patients treated at UCLA with bevacizumab with and without concurrent chemotherapies (N = 63); recurrent GBM patients treated with bevacizumab with and without concurrent chemotherapies in the BRAIN trial (N = 162); and recurrent GBM patients treated with cabozantinib monotherapy in XL184-201 (N = 222). Patients from UCLA and the BRAIN trial were combined to create a larger cohort of bevacizumab treated patients (N = 225).
Bevacizumab Naïve Recurrent GBM Treated with Chemotherapy (Single Center)
A cohort of 50 patients from the University of Toronto who were treated with chemotherapy met the following criteria: (i) pathological confirmed GBM with recurrence based on MRI, clinical data, and/or histology; (ii) never treated with BV (during any recurrences consistent with the available standard of care at the time) but instead were treated with either TMZ (N=28; N=6 were rechallenged with 5 days TMZ per 28-day cycle; N=22 were treated with continuous TMZ), lomustine (CCNU; N=18), or etoposide (N=4) (Fig. 1); (iii) had baseline anatomic MRIs prior to second- or third-line treatment available for analysis; and (iv) had treatment at least 3 months after completion of radiation therapy to reduce the probability of pseudoprogression and treatment-induced necrosis. The local ethics committee at the University of Toronto approved use of these data from a previous study.22
Recurrent GBM Treated with Bevacizumab with or without Chemotherapy (Single Center)
A cohort of 63 patients from the University of California Los Angeles (UCLA) treated with BV with or without concurrent chemotherapy met the following criteria: (i) had pathologically confirmed GBM with recurrence based on MRI, clinical data, and/or histology; (ii) were regularly treated every 2 weeks per cycle with BV (5 or 10mg/kg body weight) alone or in combination with chemotherapy (BV monotherapy, N=14; BV+TMZ, N=2; BV+CPT11, N=35; BV+carboplatin, N=4; BV+CCNU, N=8) at first or second recurrence (Fig. 1); (iii) had baseline (pre-BV treatment) anatomic MRIs available for analysis; and (iv) had treatment with BV at least 3 months after completion of radiation therapy to reduce the probability of pseudoprogression and treatment-induced necrosis. All UCLA patients in this study signed institutional review board–approved informed consent to have their data included in our research database for subsequent studies.
Recurrent GBM Treated with Bevacizumab with or without Irinotecan (Multicenter Phase II Trial)
The BRAIN trial (study sponsor identification, AVF3708g; ClinicalTrials.gov registration #NCT00345163) was an open-label, multicenter (11 sites) randomized, noncomparative phase II trial performed to assess the effectiveness of BV, a humanized monoclonal antibody for vascular endothelial growth factor (VEGF), at 10mg/kg or BV and irinotecan hydrochloride injection (CPT11) (Camptosar, Pfizer) at a dose of 340mg/m2 or 125mg/m2 (with or without concomitant enzyme-inducing anti-epileptic drugs) in patients with recurrent, histologically confirmed GBM. The study spanned July 2006 through September 2007. Specific inclusion and exclusion criteria for this clinical trial can be found at clinicaltrials.gov/ct2/ show/NCT00345163. A total of 162 patients with recurrent GBM with both pre- and postcontrast T1-weighted images at baseline were included in the current study. Of these 162 patients, 82 were treated with BV monotherapy, while 80 were treated with BV and irinotecan (Fig. 1). In all patients, initial standard radiation therapy and chemotherapy (concurrent radiation therapy and TMZ treatment) failed, and radiation therapy had been completed more than 8 weeks previously. Baseline images were obtained prior to treatment according to study guidelines. The current imaging analysis was performed retrospectively using data from the study sponsor (Genentech). All participants in the BRAIN trial signed an institutional review board–approved informed consent at their respective institutions prior to enrolling in the multicenter clinical trial.
Recurrent GBM Treated with Cabozantinib (XL184) Monotherapy (Multicenter Phase II Trial)
Patients (N=222) from XL184-201, a multicenter (8 sites), phase II, open-label, uncontrolled study of cabozantinib, a tyrosine kinase inhibitor with principal targets of methionine (MET), VEGF receptor 2, Axl, and Ret, at a dose of 140 or 100mg (free base equivalent weight, oral, daily) in patients with recurrent GBM at first or second relapse were also included in the current retrospective study. The study spanned June 2008 through July 2014. Specific inclusion and exclusion criteria for this trial can be found at clinicaltrials.gov/ct2/show/NCT00704288. Of these 222 patients, 152 did not previously fail anti-angiogenic agents (eg, bevacizumab, cediranib, pazopanib), while 70 patients previously failed an anti-angiogenic agent (Fig. 1). In all patients, initial standard radiation therapy and chemotherapy (concurrent radiation therapy and TMZ treatment) failed, and radiation therapy (or previous investigational drugs) had been completed more than 28 weeks previously. Baseline images were obtained within 14 days prior to treatment according to study guidelines. The current imaging analysis was performed retrospectively using data from the study sponsor (Exelixis). All participants in XL184-201 signed an institutional review board–approved informed consent at their respective institutions prior to enrolling in the multicenter clinical trial.
Magnetic Resonance Imaging
Anatomic MRIs were acquired for all patients in the current study with a 1.5T or 3T clinical MR scanner using pulse sequences supplied by their respective manufacturers and according to their local standard-of-care protocols. Standard anatomic images were obtained with the axial T1-weighted fast spin-echo sequence or magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence (repetition time [msec]/echo time [msec]/ inversion time [msec] = 400–3209/3.6– 21.9/0–1238; slice thickness = 1–6.5mm; intersection gap = 0–2.5mm; number of averages = 1–2; matrix size = 176–512×256–512; and field of view = 24–25.6cm). Additionally, T2-weighted fast spin-echo and fluid-attenuated inversion-recovery (FLAIR) sequences were obtained. In addition, parameter matched T1-weighted images enhanced with gadopentetate dimeglumine (Magnevist, Berlex), 0.1 mmol/kg, were acquired shortly after contrast material injection.
Contrast Enhanced T1-Weighted Digital Subtraction Maps
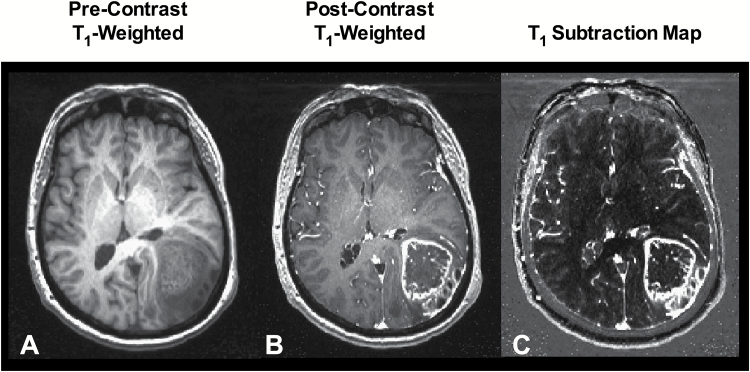
Contrast enhanced T1-weighted subtraction maps (Fig. 2) were created using techniques previously described.20 First, linear registration was performed between nonenhanced and contrast enhanced T1-weighted images by using a 12 degree-of-freedom transformation and a correlation coefficient cost function in FSL (FLIRT; FMRIB Software Library, Oxford, England; http:// www.fmrib.ox.ac.uk/fsl/). Additionally, T2-weighted and/or FLAIR were also registered to pre- and postcontrast T1-weighted images using a 12 degree-of-freedom transformation and a mutual information cost function. Next, “Gaussian normalization” of image intensity for both nonenhanced and contrast enhanced T1-weighted images was performed using custom c-code and bash scripts, courtesy of the National Institutes of Mental Health Magnetoencephalography 3Core Facility (3dNormalize; kurage.nimh.nih.gov/ meglab/Med/3dNormalize), which normalizes image intensity by dividing each voxel by the standard deviation of the image intensity from the whole brain [S Nor(x,y,z) = S (x,y,z)/σ WB], where S is raw image signal intensity, Nor is normalized, x,y,z are voxel coordinates, and WB is whole brain. Next, voxel-by-voxel subtraction between normalized nonenhanced and contrast enhanced T1-weighted images was performed. Image voxels with a positive (greater than zero) before-to-after change in normalized contrast enhancement signal intensity (ie, voxels increasing in MR signal after contrast agent administration) within T2-weighted FLAIR hyperintense regions were isolated to create the final T1 subtraction maps in order to exclude large vessels and other hyperintense regions outside the primary tumor area. Estimates of tumor volume included areas of contrast enhancement on T1 subtraction maps plus any areas of central necrosis. Central necrosis was defined as regions fully or partially enclosed by contrast enhancement deemed to be cystic or necrotic based on expert interpretation. Areas of questionable necrosis were included in the volume measurement. Initial segmentation was performed automatically, and final segmented volumes were edited by 2 independent observers with more than 18 years of combined experience to exclude large vessels and any obvious nontumor regions.
Fig 2.
Contrast-enhanced T1-weighted digital subtraction maps. In order to increase lesion conspicuity and increase automation in tumor segmentation, (A) pre- and (B) postcontrast T1-weighted images were intensity normalized, coregistered, and subtracted voxel-by-voxel, highlighting only areas of increased signal intensity following contrast administration. The resulting T1 subtraction maps (C) were used to quantify enhancing lesion volume, which also included areas of central necrosis.
Statistical Analysis
Log-rank analysis on Kaplan–Meier data and Cox proportional hazards regression models were used to understand the relationship between baseline pretreatment tumor volume and OS in a variety of subsets of patients (Fig. 1), including (i) all patients on all therapies (N=497); (ii) all patients treated with BV from both UCLA and the BRAIN trial (N=225); (iii) patients treated with BV monotherapy in the BRAIN trial (N=82); (iv) patients treated with BV+CPT11 in the BRAIN trial (N=80); (v) patients treated with BV and mixed chemotherapies from UCLA (N=63); (vi) all chemotherapy-only treated patients from the University of Toronto (N=50); (vii) TMZ-treated patients from the University of Toronto (N=28); (viii) CCNU-treated patients from the University of Toronto (N=18); (ix) all patients treated with cabozantinib (N=222); (x) patients treated with cabozantinib who did not previously fail BV (N=152); and (xi) patients treated with cabozantinib who previously failed BV (N=70). Log-linear regression (Model: log(Volume) = m·OS +β) and log-rank test for trends were used to explore trends between baseline tumor volume and OS. Covariates available for multivariable Cox regression analyses included age and treatment type (chemotherapy, BV, or cabozantinib). No adjustments for multiple comparisons were performed. All statistical tests were performed using GraphPad Prism v6.0h or Stata v12.
Results
Combined Cohort of Recurrent GBM Patients
Median volume of contrast enhancing tumor burden for the combined cohort of recurrent GBM patients was 15.3cc (interquartile range = 6.6cc and 30.4cc; mean volume = 20.8±0.9cc SEM) and median OS was 8.5 months (interquartile range = 5.1 mo and 15.4 mo). A Cox proportional hazards model of OS consisting of primary treatment type at recurrence (chemotherapy, bevacizumab, or cabozantinib), patient age, and baseline tumor volume (continuous) confirmed that baseline pretreatment contrast enhancing tumor volume was a significant prognostic factor for OS, when accounting for treatment type and age, in patients with recurrent GBM (Cox, P < .0001, hazard ratio [HR] = 1.0159, 95% CI = 1.0117–1.0201). Treatment type (Cox, P = .1720, HR = 1.0561, 95% CI = 0.9531–1.1702) and age (Cox, P = .2970, HR = 1.0058, 95% CI = 0.9975–1.0141; Table 1) did not significantly influence OS when accounting for the other independent variables in the model. A second Cox model dichotomizing tumors based on the median enhancing tumor volume (15cc) suggested that patients with large tumors had a significantly shorter OS compared with those with small tumors when accounting for age and treatment type (Cox, Volume, P < .0001, HR = 1.8051, 95% CI = 1.4883–2.1893; Table 2). (Note that interactions among age, treatment type, and volume were not shown to be significant and were therefore not included in the final model).
Table 1.
Multivariate Cox regression model results including age, treatment type (chemotherapy, bevacizumab, or cabozantinib), and continuous measures of baseline contrast enhancing lesion volume (enhancement plus central necrosis)
| Variable | Coefficient | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| Age | 0.0057 ± 0.0042 | 1.0058 | (0.9975–1.0141) | .2970 |
| Treatment(chemotherapy, bevacizumab, cabozantinib) | 0.0546 ± 0.0524 | 1.0561 | (0.9531–1.1702) | .1720 |
| Pretreatment Volume (continuous)(cubic centimeters) | 0.0158 ± 0.0021 | 1.0159 | (1.0117–1.0201) | <.0001 |
Table 2.
Multivariate Cox regression model results including age, treatment type (chemotherapy, bevacizumab, or cabozantinib), and dichotomized measures of baseline contrast enhancing lesion volume (enhancement plus central necrosis) into large (> 15cc) and small (< 15cc) categories.
| Variable | Coefficient | Hazard Ratio | 95% CI | P |
| Age | 0.0036 ± 0.0042 | 1.0036 | (0.9954–1.0119) | .3942 |
| Treatment(chemotherapy, bevacizumab, cabozantinib) | 0.0566 ± 0.0523 | 1.0582 | (0.9551–1.1724) | .2796 |
| Pretreatment Volume Large (>15cc) vs small (< 15cc) (cubic centimeters) | 0.5906 ± 0.0985 | 1.8051 | (1.4883–2.1893) | <.0001 |
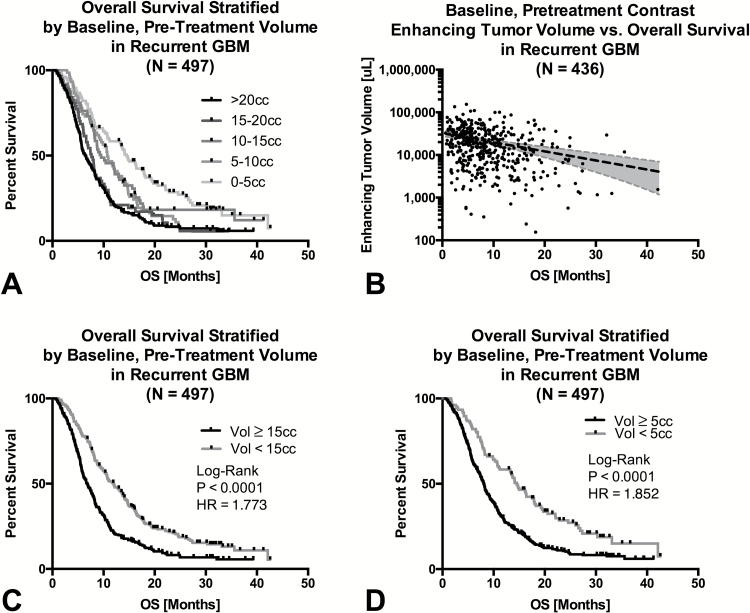
When patients were divided into several groups based on pretreatment tumor volume (0–5cc, 5–10cc, 10–15cc, 15–20cc, and >20cc), a significant trend was observed (Fig. 3A; log-rank test for trends, P < .0001) with patients exhibiting pretreatment tumor volumes of 0–5cc, 5–15cc, and >15cc exhibiting similar trends in outcome. Log-linear regression in patients who had expired (N=436 of 497) demonstrated a significant negative correlation between pretreatment baseline tumor volume and OS (Fig. 3B; P < .0001; Slope, m = −0.021, β = 4.511, y-intercept = 32cc), suggesting GBM patients with a baseline tumor volume >32cc are likely to have minimal appreciable survival in the recurrent setting. Consistent with multivariable analyses, univariate log-rank analyses on Kaplan–Meier data demonstrated a significant OS benefit in patients with pretreatment contrast enhancing tumor volume less than 15cc, or approximately 3cm in diameter (Fig. 3C; log-rank, P < .0001, HR = 1.773, 95% CI = 1.509–2.226). Similarly, patients with pretreatment tumor volume less than 5cc, or approximately 2cm in diameter, also have a significant survival advantage (Fig. 3D; log-rank, P < .0001, HR = 1.852, 95% CI = 1.407–2.131).
Fig. 3.
Influence of baseline contrast enhancing tumor volume on OS in a composite cohort of patients with recurrent GBM (N = 497). (A) Kaplan–Meier plots of OS in recurrent GBM grouped by baseline contrast enhancing tumor volume. Results demonstrate 3 distinct groupings of OS for tumors >15cc, between 5cc and 15cc, and less than 5cc. (B) In patients who expired at the time of evaluation (436 of 497 patients), results showed a significant log-linear trend indicating decreasing OS with increasing tumor volume. (C) Kaplan–Meier plots of OS in recurrent GBM patients stratified by large (≥15cc) and small (<15cc) tumor volume. (D) Kaplan–Meier plots of OS in recurrent GBM patients stratified by very small (<5cc) and larger (≥5cc) tumor volume.
Recurrent GBM Patients Treated with Bevacizumab with or without Concurrent Chemotherapy
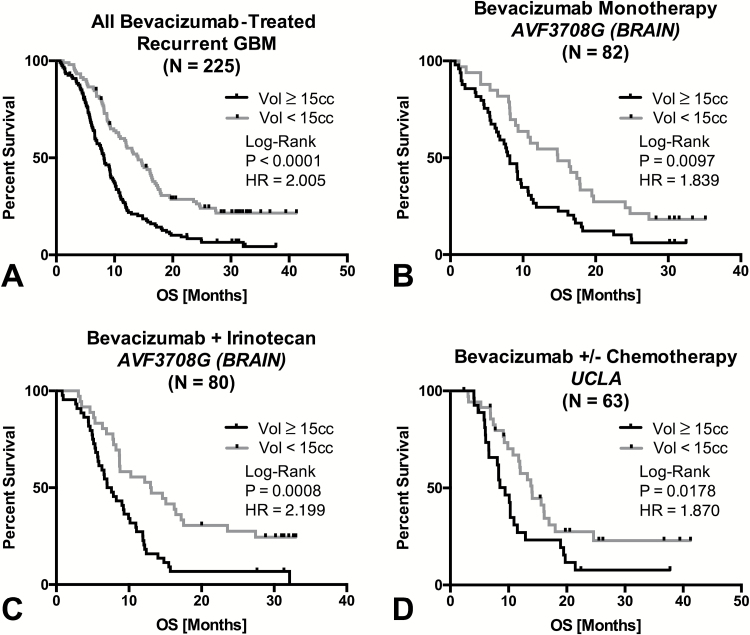
Consistent with trends in the entire patient cohort, all GBM patients (N=225) treated with BV at recurrence exhibiting pretreatment baseline tumor volume less than 15cc also had a significant survival advantage compared with patients demonstrating tumor volumes larger than 15cc (Fig. 4A; log-rank, P = .0001, HR = 2.005, 95% CI = 1.550–2.760). This trend was observed in BRAIN trial patients treated with BV monotherapy (Fig. 4B; log-rank, P = .0097, HR = 1.839, 95% CI = 1.168–2.926) as well as patients in the BRAIN trial treated with combination BV+CPT11 (Fig. 4C; log-rank, P = .0008, HR = 2.199, 95% CI = 1.444–3.763). Recurrent GBM patients with small tumors treated at UCLA under a larger variety of concurrent therapies including carboplatin, TMZ, and CCNU also exhibited a similar OS benefit, albeit with a slightly lower effect size (Fig. 4D; log-rank, P = .0178, HR = 1.870, 95% CI = 1.091–3.579).
Fig 4.
Influence of baseline contrast enhancing tumor volume on OS in recurrent GBM treated with bevacizumab. (A) Kaplan–Meier plots of OS in all recurrent GBM patients treated with bevacizumab (N = 225) stratified by large (≥15cc) and small (<15cc) tumor volume. (B) Kaplan–Meier plots of OS in recurrent GBM patients treated with bevacizumab monotherapy in the BRAIN trial (N = 82) stratified by large (≥15cc) and small (<15cc) tumor volume. (C) Kaplan–Meier plots of OS in recurrent GBM patients treated with concurrent bevacizumab and irinotecan in the BRAIN trial (N = 80) stratified by large (≥15cc) and small (<15cc) tumor volume. (D) Kaplan–Meier plots of OS in recurrent GBM patients treated with bevacizumab with or without chemotherapy at UCLA (N = 63) stratified by large (≥15cc) and small (<15cc) tumor volume.
Recurrent GBM Patients Treated with Chemotherapy and Never Treated with Bevacizumab
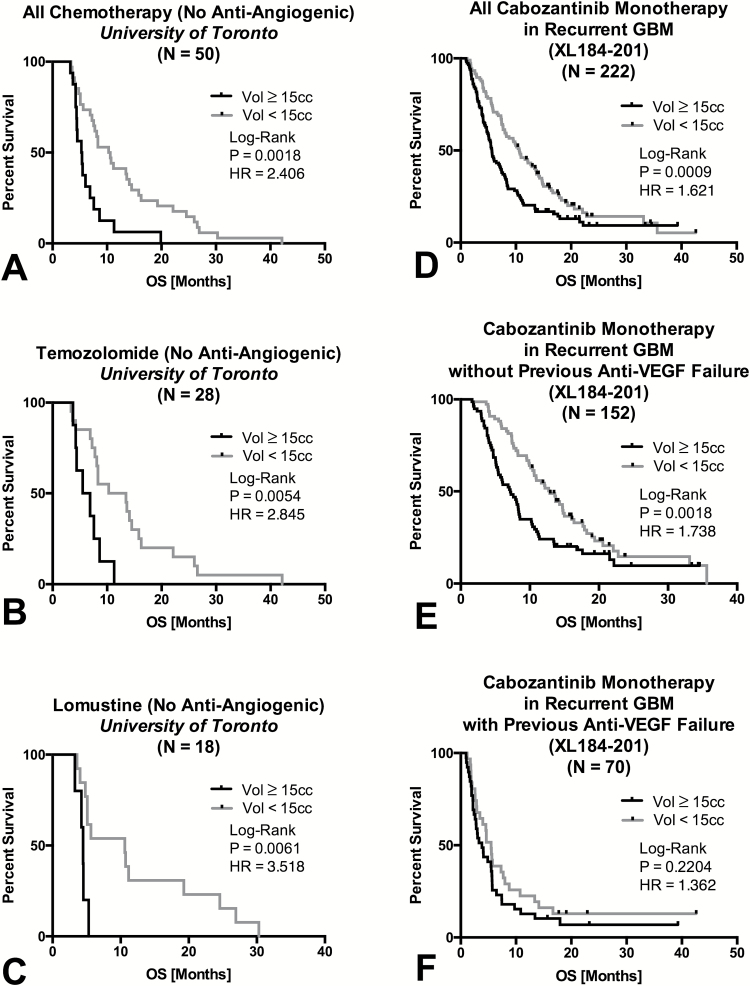
Examination of recurrent GBM patients treated with chemotherapy (TMZ, CCNU, or etoposide) who were never treated with BV during their clinical history (N=50) revealed a similar significant survival advantage for patients with small baseline enhancing tumor volume (Fig. 5A; log-rank, P = .0018, HR = 2.406, 95% CI = 1.646–7.367). This trend was significant for patients treated with TMZ (Fig. 5B; log-rank, P = .0054, HR = 2.845, 95% CI = 1.777–16.92) as well as CCNU (Fig. 5C; log-rank, P = .0061, HR = 3.518, 95% CI = 2.176–48.29), suggesting that baseline enhancing tumor volume may be prognostic independently of the cytotoxic chemotherapy used at the time of recurrence.
Fig 5.
Influence of baseline contrast enhancing tumor volume on OS in recurrent GBM treated with chemotherapy (never receiving anti-angiogenic therapy) or cabozantinib (XL184). (A) Kaplan–Meier plots of OS in recurrent GBM patients treated with a variety of chemotherapies at the University of Toronto, but never treated with anti-angiogenic therapy, stratified by large (≥15cc) and small (<15cc) tumor volume. (B) Kaplan–Meier plots of OS in recurrent GBM patients treated with TMZ stratified by large (≥15cc) and small (<15cc) tumor volume. (C) Kaplan–Meier plots of OS in recurrent GBM patients treated with CCNU stratified by large (≥15cc) and small (<15cc) tumor volume. (D) Kaplan–Meier plots of OS in recurrent GBM patients treated with cabozantinib monotherapy stratified by large (≥15cc) and small (<15cc) tumor volume. (E) Kaplan–Meier plots of OS in a subset of recurrent GBM patients treated with cabozantinib monotherapy who never previously failed anti-VEGF therapy, stratified by large (≥15cc) and small (<15cc) tumor volume. (F) Kaplan–Meier plots of OS in a subset of recurrent GBM patients treated with cabozantinib who previously failed anti-VEGF therapies, stratified by large (≥15cc) and small (<15cc) tumor volume. Note similar, uniformly poor OS in patients with large and small tumors who previously failed these therapies.
Recurrent GBM Treated with Cabozantinib Monotherapy
Lastly, we tested whether baseline enhancing tumor volume was prognostic for patients treated with cabozantinib monotherapy at recurrence as part of a multicenter clinical trial. Consistent with all other therapies examined, patients with baseline enhancing tumor volumes less than 15cc demonstrated a significant survival advantage compared with patients exhibiting lesions larger than 15cc (Fig. 5D; log-rank, P = .0009, HR = 1.621, 95% CI = 1.232–2.210). Interestingly, this trend was only apparent in patients who did not previously fail anti-VEGF therapy (Fig. 5E; log-rank, P = .0018, HR = 1.738, 95% CI = 1.247–2.583), as patients who previously failed anti-VEGF therapy did uniformly poor regardless of tumor volume (Fig. 5F; log-rank, P = .2204, HR = 1.362, 95% CI = 0.8327–2.236).
Discussion
Results from the current study support the hypothesis that baseline pretreatment contrast enhancing tumor volume is a significant prognostic characteristic for OS in recurrent GBM, regardless of treatment, in patients who did not previously fail anti-VEGF therapy. This conclusion is supported by analysis of multiple datasets including 2 multicenter phase II trials along with 2 single-institution datasets. This study represents the largest and most comprehensive set of evidence suggesting that baseline pretreatment tumor size is prognostic under a variety of therapeutic scenarios commonly employed in recurrent GBM, including both chemotherapies and anti-angiogenic agents.
The observation that baseline pretreatment tumor volume is prognostic for OS in recurrent GBM regardless of therapy has important implications for clinical trial design and interpretation. For example, randomized trials with 2 or more arms may need to employ methods to help ensure that patients are distributed across arms evenly with respect to median baseline tumor size, particularly for trials with smaller sample sizes. Additionally, single arm trials using historical OS rates or posttreatment OS as endpoints compared with historical OS rates may need to ensure similar median tumor size within these studies. Interestingly, the median baseline tumor volume observed in the current study (~15cc) appears consistent across the multiple data sources combined in the current trial along with data from other single-center sources23 and Radiation Therapy Oncology Group 0625, a phase II multicenter trial testing BV in recurrent GBM.21 At the very least, the current study suggests baseline tumor volume or size should be accounted for as a statistical covariate in the evaluation of therapeutic efficacy in recurrent GBM clinical trials.
We observed a significant survival advantage in recurrent GBM patients with enhancing tumor volume less than 5cc, or less than 2cm in diameter, compared with patients with larger tumor volume. Although not directly evaluated, these results appear consistent with the view that complete or near complete surgical resection prolongs survival in recurrent GBM patients, since patients with less than 5cc of enhancing tumor likely obtained surgery to confirm tumor progression prior to enrollment in the various trials. However, despite this interesting observation, future prospective randomized studies aimed at confirming whether patients with large tumors at recurrence specifically benefit from debulking surgery are still warranted.
Although the vast majority of patients with recurrent GBM showed decreased OS with increasing tumor volume, patients who previously failed anti-VEGF therapy did not appear to demonstrate a difference in OS based on tumor volume. The data appear to show a trend toward shorter survival in patients with larger tumors, which would likely be significant with more patients; however, patients who failed anti-VEGF therapy demonstrated uniformly poor OS (4–6 mo) compared with the cohort median OS (~9 mo). These results may suggest baseline enhancing tumor volume may not be a strong prognostic factor in patients who previously failed anti-VEGF therapies.
Study Limitations
A significant limitation to the current study was lack of uniform clinical, molecular, and genetic information (eg, O6-DNA methylguanine-methyltransferase, epidermal growth factor receptor, isocitrate dehydrogenase 1) on all patients pooled into the composite cohort. Lack of information including sex, racial demographics, previous treatments, performance status, steroid dose, molecular subtypes, core activated pathways, mutation status, and other factors may have significantly influenced our results. For example, a recent study by Burth et al24 noted that KPS is a significant prognostic factor in newly diagnosed GBM, so it is conceivable that KPS may also influence OS in recurrent GBM. However, most of the patients included in the current study had relatively high KPS at baseline, as these were often eligibility requirements for the respective multicenter trials. Despite this lack of information, trends observed in baseline tumor volume are consistent with previous studies that accounted for these factors.20 , 21 Another limitation was the lack of uniform imaging acquisition, which may have led to inaccuracies when segmenting the enhancing lesion due to differences in inherent image contrast. To account for these differences in image quality and contrast, we performed intensity normalization and digital subtraction and included a manual inspection of all cases to increase consistencies in quantitation. Despite these efforts, it is conceivable that errors remain, as differences in acquisition parameters inevitably change interpretation of tumor boundaries. Lastly, the current study was limited in the number of patients on particular therapies. Most notably, the current study had a low number of patients treated with chemotherapies that never crossed over to BV or another anti-VEGF therapy. Additionally, it is not clear whether baseline enhancing tumor volume is prognostic in other anti-angiogenic therapies, including cediranib, aflibercept, ramucirumab, sorafanib, sunitinib, vandetanib, and pazopanib. Thus, accounting for tumor size in recurrent GBM clinical trials is warranted to expand our understanding of the influence of lesion extent on patient survival, while ensuring that interpretation of therapeutic efficacy in new trials is not biased by strong factors including tumor size.
Funding
This work was supported by American Cancer Society (ACS) Research Scholar Grant (RSG-15-003-01-CCE) (B.M.E.); National Brain Tumor Society (NBTS) Research Grant (B.M.E., T.F.C.); Art of the Brain (T.F.C.); Ziering Family Foundation in memory of Sigi Ziering (T.F.C.); Singleton Family Foundation (T.F.C.).
Conflicts of interest statement. B.M.E., A.L., P.Y.W., and T.F.C. are paid consultants, members of the advisory board, and research grant recipients from Roche/Genentech. L.E.A is an employee of Roche/Genentech. D.T.A, G.M.S., and C.H. are paid employees and stockholders of Exelixis.
References
- 1. Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. [DOI] [PubMed] [Google Scholar]
- 2. Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 3. Barajas RF, Jr, Phillips JJ, Parvataneni R, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro Oncol. 2012;14(7):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Earnest Ft, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. [DOI] [PubMed] [Google Scholar]
- 5. Vogelbaum MA. Does extent of resection of a glioblastoma matter? Clin Neurosurg. 2012;59:79–81. [DOI] [PubMed] [Google Scholar]
- 6. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 7. Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–379. [DOI] [PubMed] [Google Scholar]
- 8. Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 9. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;2(4):753–764; discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 10. McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 11. Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055; discussion 1055–1046. [DOI] [PubMed] [Google Scholar]
- 12. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 13. Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 14. Vogelbaum MA. The benefit of surgical resection in recurrent glioblastoma. Neuro Oncol. 2016;18(4):462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hervey-Jumper SL, Berger MS. Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery. 2014;75(5):491–499; discussion 498–499. [DOI] [PubMed] [Google Scholar]
- 16. Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
- 17. Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 18. Coburger J, Wirtz CR, Konig RW. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field iMRI. J Neurosurg Sci. 2015. [DOI] [PubMed] [Google Scholar]
- 19. Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellingson BM, Kim E, Woodworth DC, et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 2015;46(5):1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellingson BM, Sahebjam S, Kim HJ, et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am J Neuroradiol. 2014;35(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang RY, Rahman R, Hamdan A, et al. Recurrent glioblastoma: volumetric assessment and stratification of patient survival with early posttreatment magnetic resonance imaging in patients treated with bevacizumab. Cancer. 2013;119(19):3479–3488. [DOI] [PubMed] [Google Scholar]
- 24. Burth S, Kickingereder P, Eidel O, et al. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]