Abstract
Brain metastases (BM) occur frequently in many cancers, particularly non–small cell lung cancer (NSCLC), breast cancer, and melanoma. The development of BM is associated with poor prognosis and has an adverse impact on survival and quality of life. Commonly used therapies for BM such as surgery or radiotherapy are associated with only modest benefits. However, recent advances in systemic therapy of many cancers have generated considerable interest in exploration of those therapies for treatment of intracranial metastases.
This review discusses the epidemiology of BM from the aforementioned primary tumors and the challenges of using systemic therapies for metastatic disease located within the central nervous system. Cumulative data from several retrospective and small prospective studies suggest that molecularly targeted systemic therapies may be an effective option for the treatment of BM from NSCLC, breast cancer, and melanoma, either as monotherapy or in conjunction with other therapies. Larger prospective studies are warranted to further characterize the efficacy and safety profiles of these targeted agents for the treatment of BM.
Keywords: Brain metastases, breast cancer, blood-brain barrier, melanoma, non–small cell lung cancer
Involvement of the central nervous system (CNS) is a complication of many cancers. Brain metastases (BM) from systemic malignancies account for the majority of intracranial cancers, with an estimated incidence rate of 8.3 to 11.0 per 100000 as compared with an incidence rate of 6.6 per 100000 for all primary malignant CNS tumors.1 The development of BM is usually associated with poor prognosis and significant adverse effects on survival and quality of life. Overall, BM are associated with a low 2-year survival rate (8%) and a high burden of neurologic symptoms including headaches, nausea and vomiting, focal motor deficits, cognitive decline, delirium, and seizures.2
BM affect 8%–10% of all cancer patients1 , 3 and 40% of patients with metastatic cancer.4 , 5 The majority of BM originate from lung cancer (40%–50%), breast cancer (15%–25%), and melanoma (5%–20%).1 , 3 BM predominate in the cerebral hemispheres (80%) followed by the cerebellum (15%) and brainstem (5%), a pattern of distribution reflective of proportional blood flow to these respective regions.6 The incidence of BM is believed to be increasing, likely resulting from longer patient survival due to more effective systemic therapies for the primary cancer and the increased use of neuroimaging in neurologically asymptomatic patients.7 , 8
Treatment options for BM are limited and suboptimal. Historically, the mainstay of therapy has been local treatments such as surgery or radiation therapy (RT) (ie, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), or stereotactic radiation therapy (SRT). The choice of local therapy is generally guided by the number and location of BM, the extent and prognosis of systemic disease, and the performance status of the patient. Patients with minimal systemic disease, good performance status, and solitary brain metastasis in a noneloquent location of the brain are often treated with surgical resection followed by RT.9 Patients with minimal systemic disease, good performance, and oligometastatic disease in the brain are generally treated with SRT and deferred WBRT.10 In contrast, patients with greater metastatic burden are usually treated with WBRT despite a lack of randomized trials showing effectiveness of WBRT compared with best standard care; there is an emerging perspective, however, that these patients can be treated with SRS, especially when effective systemic therapy may be available.11 , 12 In a select group of patients (<15% of all patients with BM), aggressive local treatment can prolong survival to ≥12 months.13 The evolving classification of specific molecular subtypes within most cancers (eg, hormone receptor status in breast cancer or anaplastic lymphoma kinase [ALK] rearrangement in non–small cell lung cancer [NSCLC]) will likely alter treatment of BM based on improved prognostication linked to specific tumor subtypes.
Historically, the role of systemic therapy in the treatment of BM has been limited by concerns regarding limited penetration across the blood-brain barrier (BBB), rapid efflux from brain, or intrinsic chemotherapy resistance resulting from multiple prior lines of therapy.14 Furthermore, patients with symptomatic or uncontrolled BM have generally been excluded from randomized controlled trials of systemic pharmacotherapies. Additionally, CNS outcome measures were often not reported separately from systemic efficacy outcomes. However, novel targeted therapies have substantially improved systemic disease control and survival in molecularly defined cancer populations, which have generated considerable interest in the investigation of these therapies to complement or even replace local therapies for treatment of BM. Although the constraints on parenchymal brain drug delivery also apply to targeted therapies, these agents are increasingly being selected for further study in patients with BM based, in part, on their CNS penetration.
The objective of this review is to summarize the efficacy of emerging systemic therapies, especially targeted therapies and immunotherapies, for the treatment of BM, focusing on the 3 cancers (NSCLC, breast cancer, and melanoma) that most often metastasize to the CNS.
Targeting Brain Metastases: Challenges
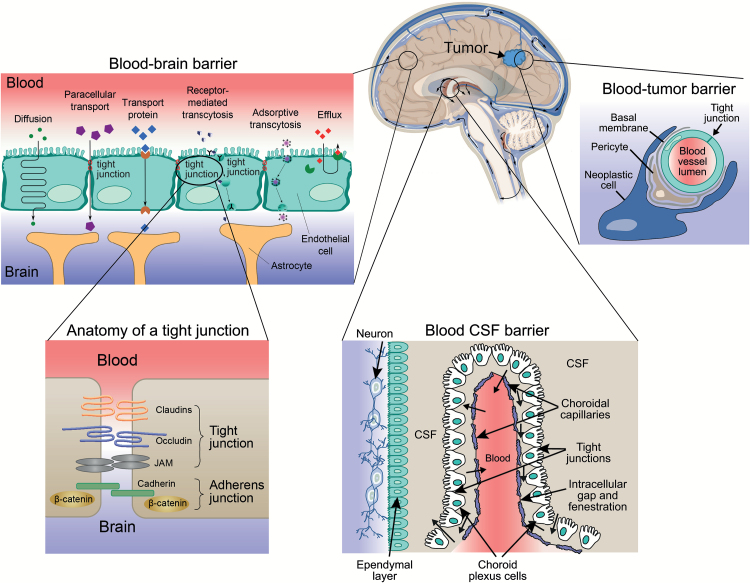
When employed in treating BM, a systemic therapy must traverse the BBB, the blood-cerebrospinal fluid (CSF) barrier, and the blood-tumor barrier in sufficient concentration to enable its therapeutic effect (Fig. 1). These various barriers render the brain and CSF inaccessible to most chemotherapeutic drugs because of large size (>150kDa), ionization, hydrophilicity, and/or protein-binding.7 , 14 Additionally, many of the small lipophilic molecules that cross the BBB and blood-tumor barriers can be exported from the brain by highly regulated transmembrane efflux pumps located in the endothelial vasculature of the CNS.7 , 14 Although BM may increase permeability to systemic drugs by disrupting the structural integrity of the BBB and blood-tumor barrier, this is usually not sufficient to achieve therapeutic levels of most drugs in the CNS.7 , 14 Dose escalation of systemically administered drugs (eg, high-dose methotrexate) is generally limited by systemic toxicity. Furthermore, subtherapeutic concentrations of anticancer drugs achieved in the brain may potentially contribute to acquired treatment resistance.15
Fig. 1.
Pathways across the blood-brain barrier, blood-cerebrospinal fluid barrier, and blood-tumor barrier.
The blood-brain barrier (BBB) is a continuous layer of epithelial cells joined by high-resistance tight junctions surrounded by pericytes and sealed by astrocytic perivascular endfeet.7 , 14 The blood-cerebrospinal fluid (CSF) barrier demarcates the space between the choroid plexus and CSF. Unlike the BBB capillaries, the functional unit of the choroid plexus is fenestrated, has no tight junctions, and thus facilitates molecular transport such as CSF bulk flow, metabolic inactivation, and transcapillary exchange. The blood-tumor barrier refers to a tumor’s vasculature that is a variously permeable barrier. Similar to the BBB, it is a major factor limiting the access of many therapeutic agents to brain tumors.182 The blood-tumor barrier consists of continuous, nonfenestrated capillaries (like those of normal brain), fenestrated capillaries, and interendothelial gaps.182 Expression of efflux transporters located at the blood-tumor barrier represents an additional mechanism that prevents intracellular penetration of anticancer drugs.183 (Brain with tumor adapted with permission under a Creative Commons Attribution License from OpenStax College, http://legacy.cnx.org/content/m45981/1.4/; blood-brain barrier reused with permission under a Creative Commons Attribution License from Kübelbeck A, https://commons.wikimedia.org/wiki/File:Blood-brain_barrier_transport_en.png; anatomy of tight junction adapted with permission under a Creative Commons Attribution License from Polakis P. J Cell Biol. 2008;183(3):371–373; blood CSF barrier adapted with permission under a Creative Commons Attribution License from Bhaskar S, et al. Part Fibre Toxicol. 2010;7:3; blood-tumor barrier adapted from Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res Brain Res Rev. 2001;35(2):161–204, with permission from Elsevier.)
Brain Metastases from Non–small Cell Lung Cancer
Lung cancer, the most common cancer overall, has the highest incidence of BM among all cancers. Approximately 40%−50% of all CNS metastases arise from lung cancer (Table 1), and approximately half of all patients with NSCLC develop BM during the course of their disease. The median overall survival (OS) for patients with BM from NSCLC is 7 months per the Grade Prognostic Assessment (GPA) index.16 Fortunately, emerging systemic therapies may improve outcomes for this challenging subgroup of patients.
Table 1.
Prevalence of brain metastases by subtype of lung, breast, and skin cancers
| Primary Tumor Site/Type | Frequency/Prevalence of Brain Metastases |
|---|---|
| Lung | 40%−50% of all BM1 , 3 |
| NSCLC | ≈50% of NSCLC cases185 |
| Somatic EGFR mutant | 44% of NSCLC BM cases (despite a genotype prevalence of only 10% in nonsquamous NSCLC)31 |
| ALK positive | 35%−50% of NSCLC BM cases26 , 32 , 64 |
| SCLC | 40%−50% of SCLC cases (10% of cases at diagnosis)186 |
| Breast | 15%−25% of all BM1 , 3 |
| Triple negative | 25%−46% of TNBC cases89 , 126 |
| HER2 positive | 38% of HER2-positive breast cancer cases187 |
| Luminal | 5% of luminal breast cancer cases128 |
| Melanoma | 5%−20% of all BM1 , 3 |
| BRAF WT | ≤50% of melanoma cases148 , 188 , 189 ; 55%−75% of melanoma cases based on autopsy report1 , 148 , 149 |
| BRAF mutant | Similar frequency of BM as BRAF-WT melanoma190 |
Abbreviations: ALK, anaplastic lymphoma kinase; BM, brain metastases; BRAF, v-Raf murine sarcoma viral oncogene homolog B; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; TNBC, triple-negative breast cancer; WT, wild-type.
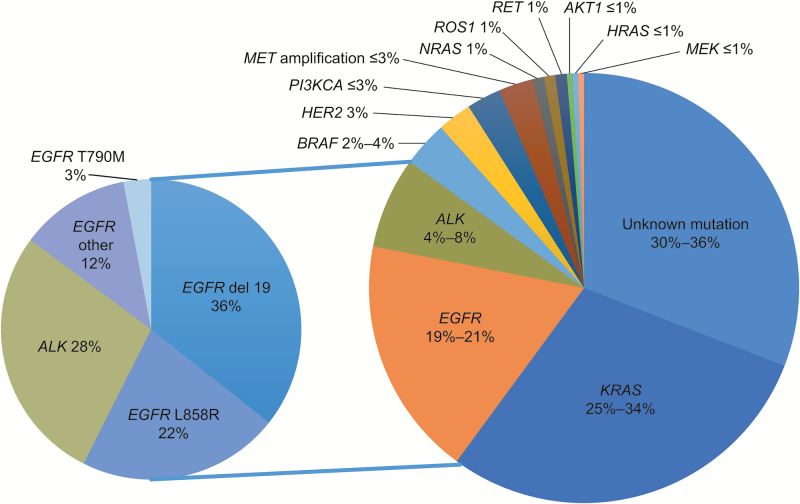
Non–small cell lung cancer is heterogeneous and composed of several molecular subtypes associated with specific driver oncogenes (Fig. 2)17 , 18; these molecular subtypes are characterized by different prognoses and responses to therapy. Recent advances in the treatment of NSCLC can be credited to improved understanding of the pathogenesis of these molecular subtypes. For example, 10%–35% of NSCLC tumors harbor a somatic activating mutation in the epidermal growth factor receptor (EGFR) gene.19 Patients with activating mutations in EGFR have overall response rates up to 85% and progression-free survival (PFS) as long as 13 months with the EGFR tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib, or afatinib compared with cytotoxic chemotherapy (up to 38% and 7 mo, respectively).20–25 Rearrangement of ALK is observed in approximately 5% of the adenocarcinoma population and predicts a good overall response rate to ALK TKIs as with crizotinib (65%) versus cytotoxic chemotherapy (20%).26 Immune checkpoint inhibitors have also led to impressive durable responses in a subgroup of patients.27–30 All of these emerging therapies are increasingly proving to be effective in treating patients with BM.
Fig. 2.
Non–small cell lung cancer mutations.
Although more than one-third of driver mutations in non–small cell lung cancer (NSCLC) are still unknown, approximately one-half of primary single mutations have been identified. The approval of targeted therapies for epidermal growth factor receptor (EGFR)-mutated and anaplastic lymphoma kinase (ALK)-rearranged lung adenocarcinomas has led to a better understanding of individual driver and resistance mutations upon tumor progression.17 , 18 (Large pie chart adapted with permission. © 2016 American Society of Clinical Oncology. All rights reserved.17 , 18)
Targeted Therapy with EGFR Tyrosine Kinase Inhibitors
Patients with EGFR-mutant NSCLC often develop multiple small BM with little peritumoral edema.31 , 32 Efficacy of EGFR TKIs in patients with EGFR-mutant NSCLC is well established; however, efficacy in patients with BM is not as clear because patients with symptomatic or uncontrolled BM were excluded from pivotal, randomized controlled trials. Consequently, data on the efficacy of TKI therapy in the CNS have been gleaned mostly from retrospective studies of those trials that enrolled patients with BM.
While the low molecular weight and nonpolar nature of TKIs permit passive diffusion across the BBB, many TKIs are substrates for efflux transporter proteins (Table 2). Nevertheless, CSF concentrations of erlotinib, gefitinib, and afatinib exceed those required to inhibit growth of cells harboring EGFR mutations in vitro (Table 2). An early prospective study of 41 patients with unselected NSCLC BM treated with gefitinib resulted in 4 (10%) intracranial partial responses with a median duration of response of 13.5 months (Table 3).33 , 34 In another study of unselected patients, a 70% CNS response was observed with first-line erlotinib or gefitinib.35 These patients were mostly Asian, female never-smokers who had a high incidence of EGFR mutations; thus, the prevalence of mutation in this study was likely higher than the general NSCLC population.19
Table 2.
Physicochemical and pharmacokinetic properties of new targeted therapies that may be useful for treatment of brain metastases in non–small cell lung cancer, breast cancer, and melanoma
| Therapeutic Agent | MW, Daa | Log Pb | BCRP and P-gp | Median Inhibitory Concentration, ng/ml | CSF Concentration in Patients with BM, ng/ml | CSF Penetration Rate in Patients with BM, %c | Drug Efflux Transporters Restricted CNS Penetration, BCRP/P-gpd | Clinical Development Status | |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | Inhibitor | ||||||||
| Erlotinib | 429.90 | 2.7 | Yes183 | Yes191 | 7.9 (EGFR-WT NSCLC)192 | 24−54 (N = 25)192–194 | 2.8−5.1192–194 | Yes183 , 191 | FDA approved for locally advanced or metastatic EGFR-mutant NSCLC and locally advanced, unresectable or metastatic pancreatic cancer, in combination with gemcitabine |
| Gefitinib | 446.9 | 3.2 | Yes195 | Yes196 | 0.13 (EGFR-WT NSCLC)197 | 3.7−6.2 (N = 30)194 ,198 | 1.1−1.4194 ,198 | Yes195 | FDA approved for locally advanced or metastatic EGFR-mutant NSCLC |
| Afatinib | 485.9 | 3.7 | Yes199 | Yes199 | 0.5200 | 0.5 (N = 1) 200 | <1 200 | Unknown | FDA approved for metastatic EGFR- mutant NSCLC |
| Crizotinib | 450.3 | 1.8 | Yes201 | Yes198 | 108 (EML4-ALK E13;A20 translocation; NCI- H3122)202 ; 48 (H228)63 | 0.62 (N = 1) 202 | 0.26 202 | Yes201 | FDA approved for locally advanced or metastatic ALK-positive NSCLC |
| Ceritinib | 558.1 | 5.0 | Unknown | Unknown | 11 (Ba/F3 NPM-ALK WT) 203 ; 2 (H228); 0.08 (ALK enzymatic assay)63 | Unknown | Unknown | Yes204 | FDA approved for patients with ALK- positive NSCLC who have progressed on or are intolerant to crizotinib |
| Alectinib | 482.6 | 5.5 | Noe,205 | Unknown | 0.92 (ALK cell-free assay)67 | 1.367 | 8667 | No205 | FDA breakthrough therapy designation for patients with ALK-positive NSCLC who have progressed on crizotinib |
| Nivolumab | 146000 | N/A | N/A | N/A | Not expected to cross intact BBB. Mechanism of action is in the periphery on T cells, which then cross the BBB | FDA approved for: (1) advanced squamous NSCLC after platinum- based chemotherapy; and (2) unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF- V600-mutation positive, a BRAF inhibitor | |||
| Trastuzumab | 145000 | N/A | N/A | N/A | 43.5 (p185HER2 extra-cellular domain)206 | Not expected to cross intact BBB | FDA approved for HER2-overexpressing breast cancer, and HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma | ||
| Lapatinib | 580.5 | 5.1207 | Yes207 | Yes207 | 6 (EGFR and HER2)207 | 1.3−4.5 (N = 2)208 | 0.9−1.3208 | Yes207 | FDA approved as part of combination treatment for advanced or metastatic (with an anthracycline, a taxane, and trastuzumab) and postmenopausal (with letrozole) HER2- overexpressing breast cancer |
| Vemurafenib | 489.9 | 5.1 | Yes209 , 210 | Yes210 | 470 (N = 6)211 | 0.98211 | Yes209 , 212 | FDA approved for unresectable or metastatic melanoma with BRAF- V600E mutation | |
| Dabrafenib | 615.7 | 5.4 | Yes162 , 213 | Unknown | Unknown | Yes162 | FDA approved for unresectable or metastatic melanoma with BRAF- V600E mutation and in combination with trametinib for BRAF- V600E and BRAF-V600K mutations | ||
| Ipilimumab | 148000 | N/A | N/A | N/A | <148 (CTLA-4)214 | Not expected to cross intact BBB. Mechanism of action is in the periphery on T cells, which then cross the BBB | FDA approved for unresectable or metastatic melanoma | ||
| Pembrolizumab | 149000 | N/A | N/A | N/A | Not expected to cross intact BBB. Mechanism of action is in the periphery on T cells, which then cross the BBB | FDA approved for unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF-V600 mutation positive, a BRAF inhibitor | |||
Abbreviations: ALK, anaplastic lymphoma kinase; BBB, blood-brain barrier; BCRP, breast cancer resistance protein; BM, brain metastases; BRAF, v-Raf murine sarcoma viral oncogene homolog B; CNS, central nervous system; CSF, cerebrospinal fluid; CTLA-4, cytotoxic T-lymphocyte antigen; EGFR, epidermal growth factor receptor; EML4, echinoderm microtubule associated protein like 4; FDA, U.S. Food and Drug Administration; HER2, human epidermal growth factor receptor 2; MW, molecular weight; N/A, not applicable; NCI, National Cancer Institute; NPM, nucleophosmin; NSCLC, non–small cell lung cancer; P-gp, P-glycoprotein; WT, wild type.
aObtained from the PubChem Compound Database (http://www.ncbi.nlm.nih.gov/pccompound).
bObtained from the Drugbank database (http://www.drugbank.ca/) and Chemspider database (http://www.chemspider.com).
cAssessed using human blood and CSF samples.
dBased on nonclinical studies using mouse models.
eAlectinib was not transported by P-gp in cell transport assay, suggesting it is a poor or non-P-gp substrate.
Table 3.
Efficacy of whole brain radiation therapy and targeted therapies for brain metastases from non–small cell lung cancer, stratified by driver mutation status
| Publication and Study Design | No. and Type of Patients | Any Prior Therapy for BM | Targeted Therapy Dosage Regimen | Intracranial ORR, % (CR + PR) | Intracranial Disease Control, % (CR + PR + SD) | Median Time to CNS Progression, mo | PFS, mo | OS, Incidence or Median Duration |
|---|---|---|---|---|---|---|---|---|
| Studies of unselected patients with NSCLC | ||||||||
| Ceresoli et al.,33 prospective, noncomparative | 41 consecutive (80% platinum pretreated) | WBRT, 44% | Gefitinib 250mg/d | 10 | 27 | NR | 3 | 27% at 11 mo |
| Wu et al.,34 phase 2 | 40 chemotherapy pretreated | Gefitinib 250mg/d | 32 (0+32) | 77 | NR | 9 | 15 mo | |
| Kim et al.,35 prospective, noncomparative | 23 Korean never- smokers; chemotherapy- naïve | No | Gefitinib 250mg/d or Erlotinib 150mg/d | 70 (0+70) | 74 | NR | 7.1 | 18.8 mo |
| Pesce et al.,58 multicenter, randomized phase 2 | 59 | Only prior chemotherapy allowed | WBRT + temozolomide 75mg/m2 × 21/28 d (N = 43) | NR | NR | 1.8 | 1.8 | 4.9 mo |
| WBRT + gefitinib 250mg/d (N = 16) | 2.0 | 1.8 | 6.3 mo | |||||
| Sperduto et al.,57 multicenter, randomized phase 3 | 125 with 1–3 BM | Prior brain resection allowed | WBRT + SRS (N = 44) | NR | NR | 8.1 | NR | 13.4 mo |
| WBRT + SRS + temozolomide 75mg/m2 × 21 d (N = 40) | 4.6 | 6.3 mo | ||||||
| WBRT + SRS + erlotinib 150mg/d (N = 41) | 4.8 | 6.1 mo | ||||||
| Studies in EGFR-mutant NSCLC | ||||||||
| Gow et al.,37 retrospective | 63 Taiwanese with BM from adenocarcinomas (73% mutant) | No | WBRT | All, 46a | NR | NR | NR | 14.7 mo |
| EGFR WT, 24a | ||||||||
| EGFR mutant, 54a,* | ||||||||
| WBRT + TKI | All, 67a | |||||||
| EGFR mutant, 84a | ||||||||
| Eichler et al.,31 retrospective | 93 (44% mutant) | 83% WBRT (alone,53%; + craniotomy,22%; + SRS, 8%);5% erlotinib | Treatment with TKI after BM diagnosis (EGFR WT, 19%; EGFR mutant, 78%) | NR | NR | EGFR WT, 8.4 | NR | EGFR WT, 7.6 mo |
| EGFR mutant, 14.5 mo | ||||||||
| EGFR mutant, 12.4 | ||||||||
| All patients, TKI vs no TKI: 19.1 vs 7.3 mo (P = .001) | ||||||||
| EGFR mutant, TKI vs no TKI: 17.5 vs 9.6 (P = .03) | ||||||||
| Lee et al.,36 retrospective | 43 (70% mutant) | No | WBRT (31% of EGFR-WT 50% of EGFR-mutant patients also received a TKI) | All, 70 (12+58) | 84 | 18 | NR | 15 mo |
| EGFR WT, 46 | 12 | 11 mo | ||||||
| EGFR mutant, 80* | 21** | 15 mo* | ||||||
| Porta et al.,38 retrospective | 69 (25% mutant) | 80% WBRT | Erlotinib 150mg/db | All, 26 (15+11) | NR | 2.9 | NR | 4.3 mo |
| EGFR mutant, 82 (47+35)*** | 11.7 | 12.9 mo | ||||||
| Park et al.,39 open- label, single- center phase 2 | 28 Korean with mutant EGFR | No | Gefitinib 250mg/d (N = 22) or erlotinib 150mg/d (N = 6) | NR | 93 | 6.6 | NR | 15.9 mo |
| Wu et al.,40 open- label, multicenter phase 2 | 48 Chinese with asymptomatic BM; chemotherapy pretreated (17% mutant EGFR) | No | Erlotinib 150mg/d | All, 58 (4+54) | NR | All, 10.1 | NR | All, 18.9 |
| EGFR WT, 33 (0+33) | EGFR WT, 4.4d | EGFR WT, 18.4 mo | ||||||
| EGFR mutant, 75 (12+62) | EGFR mutant, 15.2d,* | EGFR mutant, 37.5 mo | ||||||
| Studies in ALK-positive NSCLC | ||||||||
| Costa et al.,61 retrospective | 275 asymptomatic with BM | 60% WBRT | Crizotinib 250mg BID | 33e | 62f | 13.2 | 6.0 | 74% at 6 mo |
| 40% Untreated | 18e | 56f | 7.0 | 5.9 | 77% at 6 mo | |||
| Shaw et al.,215 phase 1 | 124 (74 with BM) | 79% ALK inhibitor | Ceritinib 750mg/d | All, 35 | NR | NR | 8.3 | NR |
| Pretreated, 29 | 7.0 | |||||||
| Treatment- naïve, 60 | NE | |||||||
| Mok et al.,216 open- label multicenter phase 2 | 20g | Chemotherapy and crizotinib | Ceritinib 750mg/d | NR | 80 | NR | 5.4 | NR |
| Felip et al.,217 open- label multicenter phase 2 | 10 ALK inhibitor naïveg | Prior chemotherapy allowed | Ceritinib 750mg/d | NR | 80 | NR | NR | NR |
| Gadgeel et al.,67 open-label multicenter phase 1/2 | 21 with resistance or intolerance to crizotinib | 81% WBRT; 95% chemotherapy | Alectinib 300−900mg BID | 52 (29+24) | 62 | NR | NR | NR |
| Gandhi et al.,68 open- label multicenter phase 2 | 16 with BM after crizotinibg | Prior chemotherapy allowed | Alectinib 600mg BID | 69 (13+56) | 100 | NR | NR | NR |
| Ou et al.,69 open- label multicenter phase 2 | 34 with BM after crizotinibg | Prior chemotherapy allowed | Alectinib 600mg BID | 56 (15+41) | NR | NR | NR | NR |
| Camidge et al.,218 open-label multicenter phase 1/2 | 12 | All other therapies allowed | Brigatinib 30−300mg/d | 50 | NR | 22 | NR | NR |
| Bauer et al.,74 and Shaw et al.,75 phase 1/2 | 17 with BM after ≥1 TKI | Treatment naïve or disease progression after previous ALK inhibitor | PF-06463922 10−200mg/d | 29 | NR | NR | NR | NR |
| Gettinger et al.,219 multicenter phase 2 | 10 | No | AP26113 180mg/d | 40 | 60 | NR | NR | NR |
Abbreviations: ALK, anaplastic lymphoma kinase; BID, twice daily; BM, brain metastases; CNS, central nervous system; CR, complete response; d, day; EGFR, epidermal growth factor receptor; mo, month; NE, not estimable; NR, not reported; NSCLC, non–small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiotherapy; WT, wild type.
aA combination of major and good responses.
bEight of 17 patients with brain metastases from EGFR-mutant NSCLC received erlotinib as sole therapy.
cThe remaining patients underwent radiosurgery or SRS with or without WBRT, or no localized therapy.
dPFS definition was a composite of occurrence of symptomatic BM, confirmed morphologically proven intracranial progressive disease, and extracranial progressive disease.
eBased on confirmed CNS responses.
fAt week 12.
gMeasurable BM at baseline.
*P < .05 vs EGFR WT; **P < .01 vs EGFR WT; ***P < .001 vs unselected control patients.
Data from other retrospective studies also suggest that patients with BM from EGFR-mutant NSCLC have better outcomes with either WBRT or TKI therapy than patients with BM from EGFR–wild-type NSCLC (Table 3).31 , 36 , 37 A retrospective analysis of 69 cases previously treated with erlotinib reported time to progression within the brain of 11.7 months for patients with EGFR mutations compared with 5.8 months for those with EGFR–wild-type or unassessed tumors, despite the fact that only 16% of patients with EGFR mutations had received WBRT versus 85% of those without the mutation (Table 3).38 Subsequent prospective phase 2 studies of patients with EGFR-mutant NSCLC indicated that TKI therapy provided intracranial responses of ≥75%.39 , 40
Despite an initial favorable intracranial response, patients often have CNS progression while maintaining systemic disease control on TKI therapy.41 , 42 A linear correlation is seen between between plasma and CSF concentrations of EGFR TKIs, suggesting that a higher dose may lead to higher CSF concentration and thereby potentially improve CNS disease response.41 , 43 For instance, in case reports and early phase clinical trials, a response as high as 81% has been reported with an erlotinib twice weekly pulse dose level of 600−1350mg.44 However, an independent, retrospective study of 10 patients showed minimal efficacy with this strategy.45 Similarly equivocal data have been reported regarding use of high-dose afatinib.46 , 47 A novel TKI in development, AZD3759, shows significant CNS penetration in preclinical models and promising early clinical efficacy in EGFR-mutant NSCLC patients with BM.48
Later-generation EGFR inhibitors osimertinib and rociletinib specifically target the T790M resistance mutation in EGFR and have demonstrated efficacy (objective response rates, 61% and 59%, and PFS, 9.6 and 13.1 mo, respectively) in patients with the T790M resistance mutation who have progressed on prior TKI therapy.49 , 50 Osimertinib was recently approved for this patient population.51 Patients with stable treated BM were included in studies for both drugs, with reports of systemic and possible CNS responses in these studies indicating that these novel agents may be active in the CNS.52 , 53
The use of EGFR inhibitors concurrently with RT has been proposed to improve intracranial efficacy based on preclinical studies in murine models.54 , 55 A retrospective analysis of 63 patients reported improved intracranial response rates in patients who received an EGFR TKI during WBRT, especially patients with EGFR mutations (Table 3).37 In a phase 2 trial of erlotinib in combination with WBRT in 40 patients with BM, overall response rate was 86%, and median survival time was 11.8 months.56 Despite the fact that the Radiation Therapy Oncology Group (RTOG) phase 3 trial of WBRT plus SRS with either temozolomide or erlotinib in unselected NSCLC patients with 1–3 BM did not meet its accrual goals and closed prematurely, there was a trend suggesting that adjunctive temozolomide or erlotinib may increase toxicity with a deleterious effect on time to progression and survival.57 Similarly, no significant benefit was observed by the addition of gefitinib to WBRT in a phase 2 randomized trial in unselected patients with BM.58 Data are currently inadequate to indicate whether TKI therapy administered concurrently with CNS-directed RT in patients with EGFR mutations is beneficial. Given the possible safety concerns raised in the RTOG study, concurrent TKI therapy is not routinely recommended in patients receiving WBRT.
Targeted Therapy with ALK Tyrosine Kinase Inhibitors
Although NSCLC with ALK rearrangement comprises a small subset of all NSCLC patients (4%–8%), this is an important subpopulation with distinct epidemiology and biology. Patients with ALK rearrangement (ALK-positive disease) are younger and usually have no or light smoking history. ALK-positive tumors are sensitive to ALK TKIs, with excellent systemic disease control. Initial findings from clinical trials of patients with ALK-positive NSCLC treated with ALK TKIs have shown promising CNS responses (Table 3). Crizotinib was the first ALK inhibitor approved for treatment of patients with metastatic ALK-positive NSCLC. While this drug has demonstrated clinically meaningful disease control, the brain is the most common or only site for disease progression.59 , 60 This observation can likely be attributed to subtherapeutic crizotinib concentrations in the brain (Table 2). Pooled analysis of a phase 3 randomized trial (PROFILE 1007) with a single-arm phase 2 trial (PROFILE 1005) reported a 12-week intracranial disease control rate with crizotinib of 56% among 109 patients with untreated asymptomatic BM compared with 62% in 166 patients with previously treated BM.61 In the small subset of patients with CNS target lesions at baseline, the confirmed intracranial response rate was 18% in patients who did not receive brain RT and 33% in patients with previously treated BM. Thus, CNS disease control may be achievable with crizotinib initially but is not durable.
Second-generation ALK TKIs ceritinib, alectinib, and brigatinib have shown efficacy in crizotinib-resistant patients including activity against BM, in part because of improved BBB penetration (Tables 2 and 3). Ceritinib was approved for treatment of patients with metastatic ALK-positive NSCLC whose disease progressed while taking crizotinib or who are intolerant to crizotinib.62 Ceritinib is selective for ALK at low concentrations in vitro and exhibits activity against crizotinib-resistant tumors in ALK-positive NSCLC xenograft models.63 In a phase 1 trial of patients with ALK-positive advanced solid tumors (ASCEND-1), approximately half had BM at baseline, and single-agent ceritinib produced an objective response rate of 59% with responses observed in both crizotinib-pretreated and crizotinib-naïve patients.64 Median PFS was 6.9 months in crizotinib-pretreated patients and 10.4 months in crizotinib-naïve patients.64 In a 1-year follow-up, objective response rates were 56.4% (92/163) and 72% (60/83), respectively, with median duration of response of 8.3 and 17.0 months, respectively.65 Half of patients had BM, and the intracranial disease control rate was 65.3% (median time to intracranial response, 6.1wk) in crizotinib-pretreated patients and 79% (median time to intracranial response, 9.9wk) in crizotinib-naïve patients. An ongoing phase 2 study (NCT02336451) is specifically looking at the activity of ceritinib as first-line therapy in crizotinib-treated and crizotinib-naïve patients with untreated, asymptomatic and measureable BM and leptomeningeal disease.66
Results from dose-finding phase 1 and phase 2 studies indicate that alectinib has promising antitumor activity in patients with ALK-rearranged NSCLC after progression on crizotinib, including those with CNS metastases (Table 3).67–69 Intracranial response rates >60% have been reported with alectinib.67–69 Based on these data, alectinib was recently approved for treatment of patients with ALK-rearranged NSCLC who had progressed post-crizotinib.70 In recent phase 2 studies of alectinib in crizotinib-refractory, ALK-rearranged NSCLC, 51%–61% of patients had CNS metastases.71 , 72 Among patients with BM at baseline, the disease control rate was 83%, with a duration of response of 10.3 months. Alectinib is also being investigated specifically in patients with BM in several studies (NCT02075840, NCT02521051, and NCT02604342).66
Brigatinib is another second-generation ALK inhibitor. In a phase 1 study of 79 patients with ALK-positive NSCLC, the response rate and disease control rate were 53% and 87%, respectively, in the 15 patients with measurable BM CNS disease.73 Lorlatinib (PF-06463922) has demonstrated clinical activity in patients with ALK-rearranged and ROS proto-oncogene 1, receptor tyrosine kinase (ROS1)-rearranged NSCLC with promising CNS efficacy.74 , 75
Recent studies have demonstrated a survival benefit with the combination of TKI and RT in patients with BM from ALK-rearranged NSCLC. In a retrospective, multi-institution study examining OS and intracranial PFS in 90 patients, treatment with SRS or WBRT and TKIs prolonged survival.76
Other potential molecular drivers and signaling transduction pathways—such as ROS1, RET proto-oncogene (RET), mesenchymal-epithelial transition factor receptor tyrosine kinase gene (MET), v-Raf murine sarcoma viral oncogene homolog B (BRAF), and tyrosine kinase receptor (TRK)/tyrosine kinase receptor B (TRKB)—are being explored in early-phase clinical trials as therapeutic targets in NSCLC and other cancers. The ability of these agents to penetrate the CNS and elicit intracranial responses will be a major factor in developing these novel targeted therapies and improving the survival of patients with NSCLC.
Immunotherapy
Immune checkpoint inhibitors, particularly those targeting the programmed death 1 (PD-1) pathway, result in impressive disease control in a subset of patients with NSCLC. Tumors evade the immune system using multiple mechanisms including the expression of PD-1 ligands (PD-L1 or PD-L2) by cells in the tumor microenvironment; binding of PD-L1 or PD-L2 to PD-1 receptors leads to inhibition of cytotoxic T cells. Pharmacologic inhibition of the PD-1 receptor/ligand interaction reverses such immune evasion and restores T-cell immunity against the tumor.77 , 78 Notably, PD-1 inhibitors lead to systemic activation of T cells, which can cross the BBB.79 , 80 Two monoclonal antibodies to PD-1, nivolumab and pembrolizumab, are approved for the treatment of NSCLC. Although the objective response rate is generally low with these agents, responses are impressively durable, and treatment leads to a meaningful survival improvement in responders while preserving quality of life.
Phase 3 studies confirming the efficacy of nivolumab as second-line therapy for NSCLC included patients with treated stable BM27 , 28; there was no indication of increased neurological complications or toxicities in these patients. A large phase 1 study has demonstrated the efficacy of pembrolizumab in the treatment of advanced NSCLC (objective response rate, 19%; median duration of response, 12.5 mo); however, only 10% of patients included in the study had BM.29 Preliminary results from an ongoing phase 2 trial of pembrolizumab showed an intracranial response rate of 45% in 11 patients with untreated BM; there were no serious neurologic complications.30
These early data show that immune checkpoint inhibitors may be an effective treatment for patients with BM, although whether the responses are durable remains to be determined. There are ongoing trials specifically looking at responses of BM from NSCLC and/or melanoma to nivolumab (NCT02621515, NCT02374242, and NCT02320058) and pembrolizumab (NCT02085070).66
Chemotherapy
In patients who are refractory to targeted TKI treatments or refractory to or not candidates for immunotherapy, cytotoxic therapy remains an option for suitable patients with NSCLC and will continue to play a role in providing modest systemic responses and improving survival. Intracranial responses with cytotoxic chemotherapy usually correlate with systemic responses in patients with NSCLC. Intracranial response rates as high as 68% have been reported with chemotherapy in asymptomatic patients81; however, PFS is usually limited to several months, and OS ranges from 5 to 16 months (Table 4). The best intracranial outcomes were achieved using regimens containing cisplatin and pemetrexed82 , 83 or bevacizumab, carboplatin, and paclitaxel.81
Table 4.
Efficacy of cytotoxic chemotherapies for brain metastases from non–small cell lung cancer
| Publication and Study Design | No. and Type of Patients | Any Prior Local Therapy for BM | Dosage Regimen | ORR, % (CR + PR) | Intracranial ORR, % (CR + PR) | Intracranial Disease Control, % (CR + PR + SD) | Median Overall PFS, mo | Median Time to CNS Progression, mo |
Median OS,
mo |
|---|---|---|---|---|---|---|---|---|---|
| Robinet et al.,220 multicenter, randomized phase 3 | 176 inoperable single BM | No | Cisplatin 100mg/m2 on d 1 and vinorelbine 30mg/m2 on d 1, 8, 15, 22 Q4W + WBRT 30 Gy/10 fx/12 on progression (arm A) or on d 1 (arm B) | Arm A: 21 | Arm A, 27 (1+26) | NR | Arm A, 4 | NR | Arm A, 6 |
| Arm B: 20 | Arm B, 33 (8+25) | NR | Arm B, 3 | NR | Arm B, 5 | ||||
| Barlesi et al.,82 multicenter phase 2 | 43 asymptomatic inoperable BM | No | Cisplatin 75mg/m2 + pemetrexed 500mg/m2 Q3W for 4 cyclesa | 35 | 42 (2+40) | 84 | 4.0 | NR | 7.4 |
| Galetta et al.,221 multicenter phase 2 | 25 asymptomatic inoperable BM | No | 2 cycles of fotemustine 80 mg/m2 d 1, 8 and cisplatin 80mg/m2 d 1, Q3W | 12 (0+12) | NR | 60 | 2.6 | NR | 4.7 |
| Dinglin et al.,83 single-center phase 2 | 41 newly diagnosed inoperable BM | No | Cisplatin 75mg/m2 + pemetrexed 500mg/m2
Q3W for 6 cycles + WBRT 30 Gy/10 fx/12 d on d 1–12 of cycle 1 |
37 (0+37) | 68 (2+66) | 97.5 | 8.9 | 10.6 | 12.6 |
| Besse et al.,81 multicenter phase 2 | 67 asymptomatic BM | No | Bevacizumab 15mg/kg + carboplatin AUC × 6 + paclitaxel 200mg/m2 Q3W | 63 | 61 | NR | 6.7 | NR | 16.0 |
| Brosnan et al.,222 retrospective | 8 progressive BM | WBRT + SRS | Bevacizumab + irinotecan | NR | >50% | NR | 4.0 | NR | 5.2 |
Abbreviations: AUC, area under the concentration-time curve; BM, brain metastases; CNS, central nervous system; CR, complete response; d, day; NR, not reported; NSCLC, non–small cell lung cancer; mo, month; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; Q3W, every 3 weeks; Q4W, every 4 weeks; SD, stable disease; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
aResponding patients were eligible for 2 additional cycles.
Brain Metastases from Breast Cancer
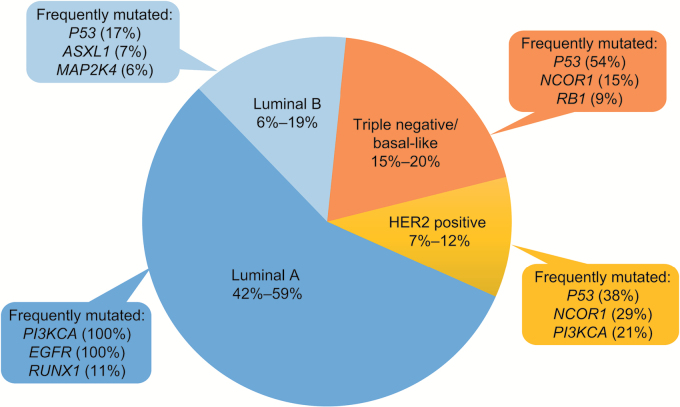
Autopsy reports indicate that the incidence of BM in women with metastatic breast cancer is as high as 30%.84–86 There is also evidence of an increasing incidence of BM from breast cancer.8 Notably, according to the GPA, prognostic index patients with BM from breast cancer have the longest median OS (13.8 mo).16 Several oncogenes have been identified as drivers of breast cancer (Fig. 3),87 , 88 and the propensity for BM from breast cancer is dependent on tumor subtype, with the highest frequency of BM observed in patients with triple-negative breast cancer (TNBC), followed by human epidermal growth factor receptor 2 (HER2)-positive and luminal breast cancers (Table 1).89 Patients with HER2-positive metastatic breast cancer are 2–4 times more likely to develop BM than patients with HER2-negative disease.90–92 The standard of care for this common complication of breast cancer includes WBRT, SRS, and surgery, which yield a median OS of 1–2 years in patients with HER2-positive breast cancer and ≤7 months in patients with TNBC.89 , 93 , 94
Fig. 3.
Breast cancer mutations.
Breast cancer can be categorized into 4 types based on histology and gene expression. The majority of breast cancers are categorized as luminal A, with triple-negative breast cancer the next most common.87 , 88 According to the Catalogue of Somatic Mutations in Cancer (COSMIC) database, certain genes are frequently mutated in each subtype (as indicated next to each subtype).87 , 88 (Adapted with permission under Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License from Kumar R et al. J Pharm Bioallied Sci. 2012.87 , 88)
Therapies for Brain Metastases from HER2-positive Breast Cancer
Overview
Until recently, development of targeted treatments in patients with BM and breast cancer was a low priority as BM presents at an advanced stage with little appreciable effect on OS. Currently, the only nonendocrine therapeutic target in breast cancer is HER2, which is overexpressed in 25%–30% of patients.95 The development of HER2-targeting therapies has been associated with an improvement in OS.96 Consequently, controlling or preventing BM in patients with HER2-positive breast cancer has increasingly become an important treatment consideration.
In patients with HER2-positive metastatic breast cancer, the recommended first-line systemic treatment is a combination of pertuzumab, trastuzumab, and taxane.97–99 Patients with active BM from HER2-positive breast cancer were excluded from participation in the pivotal clinical trials supporting this combination, and treatment regimens containing trastuzumab or lapatinib failed to prevent CNS relapse (MA.31 trial: lapatinib arm 18%, trastuzumab arm 24%; CLEOPATRA: 13% in both treatment arms; CEREBEL: lapatinib arm 3%, trastuzumab arm 5%).100–102 In a phase 3 randomized study of capecitabine plus lapatinib versus capecitabine alone for advanced-stage trastuzumab-refractory breast cancer, fewer patients in the combination arm had symptomatic CNS progression as part of the first progression event compared with those not receiving lapatinib.103 There is evidence that treatment regimens containing capecitabine may afford greater protective efficacy against BM from HER2-positive breast cancer,104 and capecitabine alone is known to have activity within the CNS, even in the absence of concurrent HER2-directed therapy.105 , 106 While fewer BM tend to occur in treatment arms containing small molecules (ie, lapatinib and/or capecitabine) than in those containing large biologics such as trastuzumab, pertuzumab, or ado-trastuzumab emtansine (T-DM1), OS in patients with BM is substantially improved in patients randomized to better-performing treatment arms.104 , 107 Finally, neratinib, an irreversible HER2-targeting small molecule TKI, plus paclitaxel may be more effective than trastuzumab plus paclitaxel in reducing CNS progression from HER2-positive metastatic breast cancer.108
Targeted Therapies
The failure of trastuzumab to prevent CNS relapse among patients with HER2-positive breast cancer can be ascribed to a lack of BBB penetration due to its large size (Table 2). Although retrospective data associated trastuzumab with extending OS in patients with BM from HER2-positive breast cancer, it is likely that this effect resulted from improved systemic disease control rather than any direct intracranial effect.109–111 Retrospective analysis of the EMILIA trial revealed that the more potent antibody-cytotoxic chemotherapy conjugate T-DM1 was associated with a protective efficacy similar to that of lapatinib plus capecitabine against BM from HER2-positive breast cancer: CNS progression in those without CNS metastases at baseline occurred in 2.0% of patients who received T-DM1 and 0.7% who received lapatinib plus capecitabine.104 In patients with asymptomatic BM at baseline, T-DM1 appeared to increase duration of OS compared with lapatinib plus capecitabine (Table 5).104
Table 5.
Efficacy of targeted therapies for brain metastases from HER2-amplified breast cancer
| Publication and Study Design | No. and Type of Patients | Any Prior Therapy for BM | Targeted Therapy Dosage Regimen | Intracranial ORR, % (CR + PR) | Intracranial Disease Control, % (CR + PR + SD) | Median Time to CNS Progression, mo | PFS, mo | OS, Incidence or Median Duration |
|---|---|---|---|---|---|---|---|---|
| Bartsch et al.,109 case- control retrospective | 17 | WBRT | Trastuzumab 8mg/kg STAT then 6mg/kg Q3W | NR | NR | 6 | NR | 7 mo |
| Park et al.,110 retrospective | 78 with symptomatic BM | WBRT, SRS, intrathecal chemotherapy | Trastuzumab before BM | 42 | 68 | 3.9 | NR | 4.0 mo |
| Trastuzumab after BM | 44 | 72 | 7.8 | 13.6 mo | ||||
| No trastuzumab | 2.9 | 5.5 mo | ||||||
| Church et al.,111 retrospective | 26 chemotherapy pretreated | Neurosurgery, SRS, WBRT | Trastuzumab | NR | NR | NR | NR | HER2 amplified, 11.9 mo* |
| Not HER2 amplified, 3.8 mo | ||||||||
| Krop et al.,104 phase 3 subgroup analysis | 95 with BM after trastuzumab and a taxane | 69% WBRT and/or local treatment | T-DM1 (N = 45) | NR | NR | NR | 5.9 | 26.8 mo* |
| 70% WBRT and/or local treatment | Capecitabine-lapatinib (n = 50) | 5.7 | 12.9 mo | |||||
| Lin et al.,112 phase 2 | 39 with BM after trastuzumab | 95% WBRT or SRS or both | Lapatinib 750mg BID | 2.6 (0+2.6) | 15.4a | NR | 3.0 | |
| Lin et al.,113 open-label, multicenter phase 3 | 242 with BM after trastuzumab | 95% WBRT; 26% SRS | Lapatinib 750mg BID | 6 (0+6)b | 43 | 2.4 | 6.4 mo | |
| 50 phase 2 completers | Lapatinib + capecitabine | 20 (0+20) | NR | 3.7 | ||||
| Freedman et al.,115 open- label, multicenter phase 2 | 40 with BM after CNS- directed therapy | 78% WBRT | Neratinib 240mg QD | 8 (0+8) | NR | NR | 1.9 | 8.7 mo |
| Bartsch et al.,118 retrospective | 43 with KPS >70 | Local therapy | Trastuzumab ± chemotherapy (N = 28) | NR | NR | NR | NR | 13 mo |
| Trastuzumab ± lapatinib (N = 15) | NYR | |||||||
| Bachelot et al.,121 open- label, multicenter phase 2 | 44 with untreated BM | No | Lapatinib 1250mg/d + capecitabine (2g/m2 d –14) × 21 d | 66 (0+66) | NR | 5.5 | 5.5 | 17.0 |
Abbreviations: BID, twice daily; BM, brain metastases; CNS, central nervous system; CR, complete response; d, day; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky Performance Score; mo, month; NR, not reported; NYR, not yet reached; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; Q3W, every 3 weeks; QD, once daily; SD, stable disease; SRS, stereotactic radiosurgery; STAT, loading dose; T-DM1, ado-trastuzumab emtansine; WBRT, whole brain radiotherapy.
aIn both CNS and non-CNS sites.
bCompared with an ORR of 15% in the 130 patients with measurable extra-CNS disease at baseline.
*P < .01 vs comparator arm.
Lapatinib has low-level antitumor activity as a single agent for HER2-positive breast cancer patients with progressive BM or BM refractory to trastuzumab and cranial radiotherapy.112 , 113 Positron emission tomography scanning shows that lapatinib distributes into HER2-positive breast cancer BM but not within normal brain tissue. This finding is consistent with clinical data indicating that lapatinib is suitable for treating rather than preventing BM in this population.114 Neratinib was also tested as a single agent in a phase 2 trial of women with HER2-positive BM from breast cancer who had CNS progression after resection, WBRT, or SRS.115 In this trial, the CNS objective response rate (all partial) was 8%, and the median PFS was 1.9 months.
Combinational Therapies
There is also interest in a combination of trastuzumab and nanoparticle albumin-bound paclitaxel following impressive objective response rates and PFS as first-line treatment for metastatic breast cancer (excluding BM).116 , 117 Case-controlled data indicate that median OS was improved in patients with BM from HER2-positive breast cancer when lapatinib was added to trastuzumab (not yet reached) compared with trastuzumab alone (13 mo).118
The efficacy of lapatinib alone in the treatment of active BM from HER2-positive breast cancer was increased when coadministered with capecitabine (objective response rate, 38% with lapatinib plus capecitabine vs 0% with lapatinib plus topotecan).113 , 119 Furthermore, the addition of lapatinib to capecitabine significantly prolonged time to tumor progression relative to capecitabine alone (8.4 vs 4.4 mo) in patients with metastatic HER2-postitive breast cancer, although progressive BM was an exclusion criterion.120 Subsequent findings from the phase 2 LANDSCAPE trial established primary systemic therapy with lapatinib plus capecitabine as an effective and safe alternative to WBRT in patients with asymptomatic to oligosymptomatic BM from HER2-positive breast cancer. The intracranial response rate was 66% in this selected population, thereby delaying the need for WBRT by 8.3 months.121 A prospective study showed significant uptake of both lapatinib and capecitabine into BM following surgical resection in patients with metastatic breast cancer.122
Dual anti-HER2 therapy with trastuzumab and lapatinib plus capecitabine was an effective and well-tolerated regimen in patients with metastatic HER2-positive breast cancer.123 One case report describes how trastuzumab and lapatinib plus capecitabine as second-line therapy after T-DM1 resulted in partial remission of BM without systemic disease progression and a >14-month delay in time to WBRT.124
Finally, in a case series, ONT-380, a HER2-targeting covalently binding TKI, demonstrated promising activity against BM from HER2-positive breast cancer in combination with other systemic agents.125
Therapies for Brain Metastases from Other Breast Cancer Subtypes
Targeted Therapies and Hormonal Manipulation
Up to 40% of patients with TNBC develop symptomatic BM during the course of their disease,126 yet no targeted therapies for this disease subtype have been developed. Notably, the high expression of PD-L1 in TNBC suggests that this pathway is a potential therapeutic target. In preliminary results of a phase 1 study (KEYNOTE 012) of pembrolizumab, patients with TNBC (13% with BM) had an overall response rate of 19%.127 Furthermore, several ongoing clinical trials are investigating pembrolizumab in TNBC (NCT02447003, NCT02555657, NCT02622074, NCT02513472, and NCT02648477).66
About 5% of patients with metastatic luminal-type, estrogen-receptor–overexpressing breast cancer develop BM during their course of disease.128 Of note, estrogen-receptor expression changes from initial overexpression in the primary tumor to absence of expression in the corresponding BM in up to 50% of cases.129 , 130 Moreover, in cases where the estrogen receptor continues to be expressed, the estrogen receptor 1 gene (ESR1) often contains mutations that result in a constitutively active protein.131 Concentrations of the estrogen receptor antagonist tamoxifen and its metabolites have been shown to be up to 46-fold higher in brain tissue and BM than in serum.132 Furthermore, case reports of patients with BM from breast cancer have demonstrated prolonged survival and prolonged remissions with tamoxifen endocrine therapy, the aromatase inhibitor letrozole, and the progestin megestrol acetate.133–135 With more clinically approved drugs for estrogen-receptor–expressing breast cancer, including the mechanistic target of rapamycin (mTOR) inhibitor everolimus, it will be of interest to see if a further extension of survival is possible for TNBC BM. Despite an ability to cross the BBB, palbociclib, an inhibitor of cyclin-dependent kinase (CDK)4/6 recently approved for first- and subsequent-line management of hormone-receptor–positive metastatic breast cancer, is unlikely to be effective against BM from breast cancer as it is a substrate for CNS drug efflux pumps.136
Chemotherapy
When treating BM from breast cancer with cytotoxic chemotherapy, agents that exert antitumor activity against breast cancer in the extraneural compartment are selected as opposed to agents with extensive penetration of the BBB but limited systemic activity (eg, temozolomide).137–140 In patients naïve to cyclophosphamide and anthracyclines, FEC (5-FU, epirubicin, cyclophosphamide) and CMF (cyclophophsamide, methotrexate, 5-FU) have purported activity in patients with BM.141 Of the few cytotoxic chemotherapeutic agents that can penetrate the BBB when disrupted by BM or radiation, cisplatin demonstrated clinical activity in patients with BM from breast cancer, particularly TNBC, as a single agent and in combination with other chemotherapies or with vinorelbine plus WBRT.142–145 Phase 2 trials have also been completed evaluating capecitabine monotherapy in patients with CNS progression after WBRT alone or with SRS and no prior systemic therapy for BM (NCT01077726, NCT00977379, and NCT00570908).66
The novel cytotoxic agent sagopilone, a microtubule stabilizer that penetrates the BBB and is not a substrate for CNS efflux transporters, has been evaluated in a single-arm, phase 2 study of 15 breast cancer patients with BM. A CNS partial response was seen in 13% of patients, with a median PFS and OS of 1.4 and 5.3 months, respectively.146 Additionally, a peptide-facilitated, brain-penetrating formulation of paclitaxel (GRN1005) was well tolerated and decreased tumor size in heavily pretreated patients with advanced solid tumors, including those who had BM and/or failed prior taxane therapy.147
Brain Metastases from Melanoma
An estimated 50% of patients with stage IV melanoma develop BM, but the prevalence may be as high as 75% based on autopsy reports.1 , 148 , 149 According to the GPA for BM, patients with BM from melanoma have a median OS of 6.74 months.16 Patients with multiple BM and extensive extracranial disease have extremely poor survival outcomes (as short as 1–2 mo in neurologically symptomatic patients).150 , 151 The prognosis may be somewhat better for patients with brain involvement at initial diagnosis of stage IV melanoma than for those who develop BM later.152 Some patients with solitary BM without known extracranial disease may survive for several years after local treatment.152 , 153
Melanoma is generally not considered as sensitive to RT or traditional cytotoxic chemotherapy as many other primary malignancies, and these treatment shortcomings are accentuated when a patient develops BM. While surgery and RT may lead to prolonged survival and symptom palliation in patients with oligometastatic CNS involvement, these therapies do not protect against development of new BM.154 Systemic therapy, although underinvestigated in melanoma patients with CNS involvement, represents a more viable treatment approach for what is essentially a systemic disease with subclinical metastases. Temozolomide and fotemustine were considered promising treatments for BM because of their CNS penetration; however, response rates with these agents were poor, and responses were transient. Fortunately, recent advances in systemic therapy for melanoma, both in molecularly targeted therapy and immunotherapy, render new hope for effective use of systemic agents for BM.
Targeted Therapy with BRAF and/or MEK Inhibitors
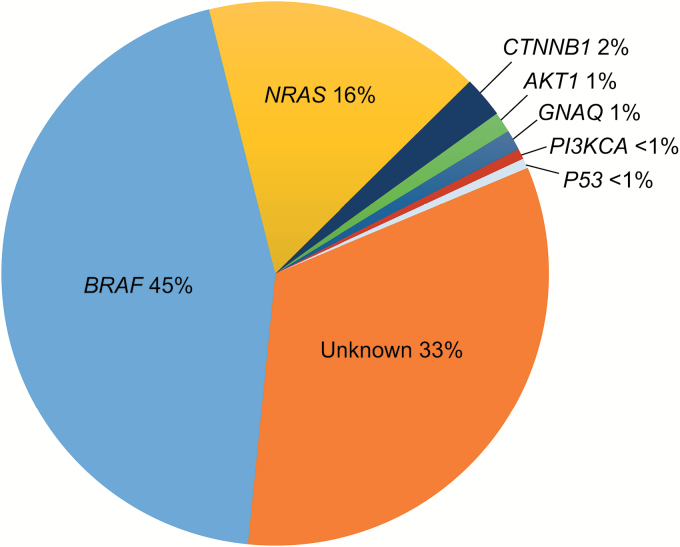
Recent discoveries have identified numerous driver genetic mutations in melanoma, particularly in BRAF and neuroblastoma RAS viral (v-Ras) oncogene homolog (NRAS; Fig. 4). Approximately 40%–60% of melanoma patients harbor a BRAF driver mutation, which results in the substitution of valine at codon 600 of the BRAF serine-threonine kinase (BRAF V600). The small molecule BRAF inhibitors dabrafenib and vemurafenib target the RAF/MEK/ERK (MAPK) pathway,155 , 156 and are associated with high response rates and improved survival in metastatic melanoma patients with BRAF V600-mutant tumors.157 , 158 The efficacy is improved further when BRAF inhibitors are used in combination with MEK inhibitor therapy to counter reactivation of the MAPK pathway.159–161 BRAF inhibitors are associated with quick-onset regressions in the vast majority of patients and represent a rational option for control and palliation of BM from melanoma.
Fig. 4.
Melanoma mutations.
Mutations in v-Raf murine sarcoma viral oncogene homolog B (BRAF) account for almost half of the driving mutations in melanoma, with another 16% driven by mutations in neuroblastoma RAS viral (v-Ras) oncogene homolog (NRAS).184 Therapies designed to target these mutations have improved outcomes in patients with melanoma.
BRAF inhibitors have been associated with intracranial responses despite limited intracranial bioavailability (Table 2).162 Dabrafenib therapy led to regression of BM in a phase 1 trial of patients with untreated BM from BRAF V600-mutant melanoma.163 In a phase 2 trial of patients with BRAF V600E-mutant melanoma, dabrafenib resulted in comparable intracranial and overall response rates (39% and 38%, respectively) in those with previously untreated BM and similar response rates (31% and 31%, respectively) in those with progressive BM despite prior treatment, indicating that the central and peripheral activity of this agent is concordant (Table 6).164 Duration of response in this study ranged from 20 to 28 months. Similarly, in an open-label trial of patients with BRAF V600-mutant melanoma and symptomatic BM, vemurafenib demonstrated antitumor activity at both intracranial and extracranial sites, with a duration of response in the brain of 4.4 months (Table 6).165 MEK inhibition with MEK162 was also promising in a phase 2 study that included patients with treated and stable BM harboring NRAS or BRAF mutations.166 Dual BRAF and MEK inhibition (eg, with dabrafenib plus trametinib or vemurafenib plus cobimetinib) is now established as the standard of care for appropriately selected patients with advanced melanoma and tumors that harbor a BRAF V600 mutation, although the efficacy in patients with BM has not yet been determined.159 , 161
Table 6.
Efficacy of targeted therapies for brain metastases from metastatic melanoma
| Publication and Study Design | No. and Type of Patients | Any Prior Local Therapy for BM | Targeted Therapy Dosage Regimen |
ORR, % (CR + PR) |
Intracranial ORR, % (CR + PR) |
Disease Control, % (CR + PR + SD) | Intracranial Disease Control, % (CR + PR + SD) | Median Duration of Intracranial Response, mo | Median Overall PFS, mo | Median OS, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Long et al.,164 multicenter phase 2 |
BRAF-V600E mutant BM (N = 139) |
47% | Dabrafenib 150mg BID | 37.8 | 39.2 | 79.7 | 81.1 | 4.6 | 3.7 | 7.6 |
| 30.8 | 30.8 | 83.1 | 89.2 | 6.5 | 3.8 | 7.2 | ||||
|
BRAF-V600K mutant BM (n = 33) |
54% | 0 | 6.7 | 46.7 | 33.3 | 2.9 | 1.9 | 3.7 | ||
| 27.8 | 22.2 | 50.0 | 50.0 | 3.8 | 3.7 | 5.0 | ||||
| Dummer et al.,165 pilot study | BRAF-V600 mutation with symptomatic BM (N = 24) | Yes | Vemurafenib 960mg BID | 42 (0+42) | 16 (0+16)a | 80 | 84a | 4.4b | 3.9 | 5.3 |
| Margolin et al.,168
multicenter phase 2 |
Asymptomatic BM (N = 51) | 41% | Ipilimumab 10mg/kg | 10 (0+10) | 16 (0+16) | 18 | 24 | NR | 1.4c | 7.0 |
| Symptomatic BM (N = 21) | 48% | Ipilimumab 10mg/kg + corticosteroids | 5 (0+5) | 5 (5+0) | 5 | 10 | 1.2d | 3.7 |
Abbreviations: BID, twice daily; BM, brain metastases; BRAF, v-Raf murine sarcoma viral oncogene homolog B; CR, complete response; mo, month; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease.
aAmong 19 patients with measurable intracranial disease at baseline.
bCompared with 3.8 months at extracranial sites.
cCompared with a median of 1.5 months for disease progression in the brain.
dCompared with a median of 1.2 months for disease progression in the brain.
Immunotherapy
Another major drug development in melanoma has been the approval of immune checkpoint inhibitors targeting the PD-1 and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The anti-CTLA-4 monoclonal antibody ipilimumab was approved after demonstration of improved OS in previously treated patients with metastatic melanoma.167 Ipilimumab was investigated in a phase 2 trial of patients with advanced melanoma and BM.168 In the cohort of patients with neurologically asymptomatic disease who were not receiving corticosteroid treatment at study entry (N = 51), ipilimumab elicited a CNS response of 16% and CNS disease control rate of 24%, with intracranial responses generally concordant with extracranial responses. The 2-year survival rate in this cohort was 26%, suggesting the possibility of long-term survival in a sizable proportion of patients with an otherwise poor prognosis.
Nivolumab and pembrolizumab have also been approved for treatment of advanced melanoma.169 , 170 Nivolumab was associated with significant improvements in OS and PFS relative to dacarbazine in previously untreated patients without a BRAF mutation.171 Pembrolizumab was associated with prolonged PFS and OS and a more favorable toxicity profile than ipilimumab in patients with advanced melanoma who had not received previous therapy with immune checkpoint inhibitors.172 Additionally, pembrolizumab resulted in a high rate of sustained tumor regression among patients with advanced melanoma, including those with disease progression despite receiving ipilimumab.173
To date, there are no published data on the safety and efficacy of pembrolizumab or nivolumab for BM from melanoma because patients with active BM were excluded from entering these studies.171–173 However, several trials of nivolumab and pembrolizumab are specifically investigating response in BM from melanoma (NCT02621515, NCT02374242, NCT02320058, and NCT02085070).66 An interim analysis of the ongoing clinical trial of pembrolizumab (NCT02085070) reported durable partial responses in melanoma patients with untreated BM.174 Another ongoing trial is investigating the safety and efficacy of the combination of nivolumab plus ipilimumab in this setting (NCT02320058).66
Chemotherapy
Cytotoxic therapies have had only modest activity against melanoma in general and therefore have limited utility in the treatment of BM from melanoma. Dacarbazine, the long-standing standard of care for metastatic melanoma, has not reliably demonstrated response in CNS metastasis across numerous trials.175 , 176 Although the oral dacarbazine analog temozolomide has improved capacity to cross the BBB, most studies report response rates of <10% in patients with BM.176 , 177 Fotemustine, a nitrosourea that crosses the BBB, showed promise in early trials that included patients with BM, but intracranial response rates were low (6%) in later trials.178 , 179
Local and Systemic Combinatorial Approaches
The synergistic potential of RT in facilitating an immune response from immune checkpoint blockade is under evaluation. In a single-institution study of patients with BM from melanoma, univariate analysis revealed that SRS before or during treatment with ipilimumab was associated with higher rates of OS at 1 year than SRS after ipilimumab (65% or 56% vs 40%) and fewer instances of regional brain recurrences at 1 year (64% or 69% vs 92%).180 There was a trend toward improved local control in those who received SRS concomitantly with ipilimumab (1-y local recurrence, 0%) compared with those who received SRS before (13%) or after (11%) ipilimumab.
Outcomes in patients with BM from BRAF V600E-mutant melanoma when combining SRS or WBRT prior to or concomitantly with vemurafenib were also positive in a retrospective analysis.181 Most evaluable patients had an improvement in neurological symptoms (7/11; 64%) and a radiographic response of index lesions (36/48; 75%), of which 23 (48%) were complete responses and 13 (27%) were partial responses. The CNS local control rate, freedom from new BM, and OS at 6 months were 75%, 57%, and 92%, respectively.
Discussion
Systemic therapy of advanced cancer has been revolutionized by the advent of novel targeted therapeutics, which are associated with prolongation of survival and improvement in quality of life. The application of these therapies to patients with BM requires an understanding of their clinical pharmacology, efficacy, and safety as it relates to the CNS. Unfortunately, patients with active BM have generally been excluded from clinical trials of novel targeted therapies because of the concern for unexpected toxicities and the likelihood that the poor survival of patients with BM will reduce the effect size between comparator agents. Although knowledge about the efficacy and safety of targeted therapies for BM had previously been limited to retrospective observations and small prospective studies, that trend appears to be changing with increasing investigation of these novel therapies in prospective studies specifically for patients with BM.
The few single-arm phase 2 studies that have focused on targeted therapy in patients with BM from NSCLC, breast cancer, or melanoma highlight the potential of targeted systemic therapy to address intracranial disease. There are encouraging findings regarding the utility of the EGFR TKIs gefitinib and erlotinib in BM from EGFR-mutant tumors and the second-generation ALK TKIs ceritinib, alectinib, and brigatinib in BM from ALK-positive disease. Similar findings are evident with use of anti-HER2 targeted therapies trastuzumab, lapatinib, and T-DM1 for BM from HER2-overexpressing breast cancer, although efficacy is improved when capecitabine is added to either trastuzumab or lapatinib. The BRAF and MEK inhibitors, either alone or in combination, appear active in patients with BRAF-mutant melanoma. Various studies have reported intracranial response rates ranging from 31% (with dabrafenib in BRAF V600E-mutant melanoma) to as high as 75% (with erlotinib in EGFR-mutant NSCLC).35 , 40 , 67 , 121 , 164 Unfortunately intracranial responses with many of these drugs may not be as robust as extracranial responses, perhaps reflecting insufficient drug concentrations in brain tissue. Responses with these therapies are generally of quick onset but may not always be durable. Monotherapy with a TKI and close monitoring for progressive disease is a reasonable strategy for patients with small volume, asymptomatic BM associated with appropriate molecular subgroups of these diseases.
Emerging data suggest that immunotherapy with monoclonal antibodies that target the PD-1 or CTLA-4 pathways may lead to substantial and durable intracranial responses concordant with their systemic activity. This may be because immune checkpoint inhibitors do not require direct access to brain parenchyma, as their effects are mediated by proxy on peripheral T cells, which in turn penetrate into the CNS. The hallmark of successful immunotherapy is the potential for durable responses and long-term survival in responding patients, which appears to be preserved in patients with BM. Immunotherapy often has a delayed onset of response and may lead to inflammatory treatment effects, which are highly relevant to the patients with BM at risk of neurologic complications due to increased mass effects.
Given the aforementioned advances, there is increasing recognition of the need to include patients with untreated BM in clinical trials and to perform trials of systemic therapy specifically in patients with BM. This strategy is not without its challenges because such trials are intensive, expensive, and come with a risk of neurologic complications. However, such studies are required to determine the optimal combination of targeted treatments and traditional therapies used to treat patients with BM.
In conclusion, the emerging evidence for the potential of novel targeted therapies that successfully target BM from NSCLC, breast cancer, and melanoma represents a paradigm shift in the management of BM. Patients with BM increasingly have a realistic hope that current targeted therapies and further research into new targeted therapies will permit an improvement in both their survival and quality of life.
Funding
Writing and editorial assistance for this manuscript, detailed in the acknowledgments, was provided by QXV, a UDG Healthcare Company, and funded by Novartis.
Conflict of interest statement. CB has received honoraria for advisory board participation from Novartis and Clovis, and works as a co-investigator on clinical trials in the Division of Medical Oncology of the University of Washington, which receives grant funding on an ongoing basis from Clovis, Merck Novartis, Pfizer, Genentech, Inc., and AstraZeneca. VG has received honoraria for advisory board participation from Genentech and works as a principal or co-investigator on clinical trials in the Breast Program in the Division of Medical Oncology at the University of Washington, which receives grant funding on an ongoing basis from Novartis, Pfizer, Genentech, and AstraZeneca. LC has received honoraria for advisory board participation from Novartis, Merck, and Seattle Genetics, for participating as the steering committee chair of a clinical trial from Novartis, and for consulting work from Amgen. LC also works on clinical trials at the University of Washington, which receives grant funding on an ongoing basis from Novartis, Merck, Pfizer, Genentech, and Astra Zeneca/Medimmune. SB works at the University of Washington, which receives grant funding from Bristol-Myers-Squibb, EMD-Serono, Merck, Immune Design, Oncosec, NantKwest, and Amgen. MC has no conflicts of interest.
Acknowledgments
Writing and editorial assistance for this manuscript were provided by Nathan Yardley, PhD, and Shannon Davis of QXV, a UDG Healthcare Company, and funded by Novartis.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol. 2000;17(4):279–286. [DOI] [PubMed] [Google Scholar]
- 3. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. [DOI] [PubMed] [Google Scholar]
- 4. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 5. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 6. Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741–744. [DOI] [PubMed] [Google Scholar]
- 7. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frisk G, Svensson T, Bäcklund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012;106(11):1850–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patchell RA, Tibbs PA, Walsh JW. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 10. Brown PD, Asher AL, Ballman KV. A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol. 2015;33 suppl):abstr LBA4. [Google Scholar]
- 11. Yamamoto M, Serizawa T, Higuchi Y. A multi-institutional prospective observational study of stereotactic radiosurgery (SRS) for patients with multiple brain metastases (BMs): updated results of the JLGK0901 study—long-term results of irradiation-related complications and neurocognitive function (NCF). J Clin Oncol. 2015;33 (suppl):abstr 2020. [Google Scholar]
- 12. Tsao MN, Sultanem K, Chiu D. Supportive care management of brain metastases: what is known and what we need to know. Conference proceedings of the National Cancer Institute of Canada (NCIC) Workshop on Symptom Control in Radiation Oncology. Clin Oncol (R Coll Radiol). 2003;15(7):429–434. [DOI] [PubMed] [Google Scholar]
- 13. Mehta MP, Rodrigus P, Terhaard CH. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536. [DOI] [PubMed] [Google Scholar]
- 14. Fortin D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets. 2012;12(3):247–259. [DOI] [PubMed] [Google Scholar]
- 15. Fu F, Nowak MA, Bonhoeffer S. Spatial heterogeneity in drug concentrations can facilitate the emergence of resistance to cancer therapy. PLoS Comput Biol. 2015;11(3):e1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sperduto PW, Kased N, Roberge D. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kris MG, Johnson BE, Berry LD. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Awad MM, Oxnard GR, Jackman DM. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–730. [DOI] [PubMed] [Google Scholar]
- 19. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han JY, Park K, Kim SW. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. [DOI] [PubMed] [Google Scholar]
- 21. Mitsudomi T, Morita S, Yatabe Y. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. [DOI] [PubMed] [Google Scholar]
- 22. Maemondo M, Inoue A, Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. [DOI] [PubMed] [Google Scholar]
- 23. Zhou C, Wu YL, Chen G. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 24. Rosell R, Carcereny E, Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 25. Sequist LV, Yang JC, Yamamoto N. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. [DOI] [PubMed] [Google Scholar]
- 26. Shaw AT, Kim DW, Nakagawa K. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 27. Brahmer J, Reckamp KL, Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borghaei H, Paz-Ares L, Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garon EB, Rizvi NA, Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 30. Goldberg SB, Gettinger SN, Mahajan A. Activity and safety of pembrolizumab in patients with metastatic non-small cell lung cancer with untreated brain metastases. J Clin Oncol. 2015;33 (suppl):abstr 8035. [Google Scholar]
- 31. Eichler AF, Kahle KT, Wang DL. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8(12):1570–1573. [DOI] [PubMed] [Google Scholar]
- 33. Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crinò L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15(7):1042–1047. [DOI] [PubMed] [Google Scholar]
- 34. Wu C, Li YL, Wang ZM, Li Z, Zhang TX, Wei Z. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer. 2007;57(3):359–364. [DOI] [PubMed] [Google Scholar]
- 35. Kim JE, Lee DH, Choi Y. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351–354. [DOI] [PubMed] [Google Scholar]
- 36. Lee HL, Chung TS, Ting LL. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol. 2012;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gow CH, Chien CR, Chang YL. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res. 2008;14(1):162–168. [DOI] [PubMed] [Google Scholar]
- 38. Porta R, Sánchez-Torres JM, Paz-Ares L. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–631. [DOI] [PubMed] [Google Scholar]
- 39. Park SJ, Kim HT, Lee DH. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–560. [DOI] [PubMed] [Google Scholar]
- 40. Wu YL, Zhou C, Cheng Y. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 2013;24(4):993–999. [DOI] [PubMed] [Google Scholar]
- 41. Jackman DM, Holmes AJ, Lindeman N. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24(27):4517–4520. [DOI] [PubMed] [Google Scholar]
- 42. Katayama T, Shimizu J, Suda K. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4(11):1415–1419. [DOI] [PubMed] [Google Scholar]
- 43. Togashi Y, Masago K, Fukudo M. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol. 2011;68(4):1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu HA, Sima CS, Reales D. A phase I study of twice weekly pulse dose and daily low dose erlotinib as initial treatment for patients (pts) with EGFR-mutant lung cancers. J Clin Oncol. 2015;33 (suppl):abstr 8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackman DM, Mach SL, Heng JC. Pulsed dosing of erlotinib for central nervous system progression in EGFR-mutant non-small cell lung cancer [abstr 8116]. J Clin Oncol. 2013;31(suppl). [Google Scholar]
- 46. Yap TA, Vidal L, Adam J. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28(25):3965–3972. [DOI] [PubMed] [Google Scholar]
- 47. Bordi P, Tiseo M, Bortesi B, Naldi N, Buti S, Ardizzoni A. Overcoming T790M-driven acquired resistance to EGFR-TKIs in NSCLC with afatinib: a case report. Tumori. 2014;100(1):e20–e23. [DOI] [PubMed] [Google Scholar]
- 48. Kim D-W, Yang JC-H, Chen K. AZD3759, an EGFR inhibitor with blood brain barrier (BBB) penetration for the treatment of non-small cell lung cancer (NSCLC) with brain metastasis (BM): Preclinical evidence and clinical cases. J Clin Oncol. 2015;33(suppl):abstr 8016. [Google Scholar]
- 49. Jänne PA, Yang JC, Kim DW. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. [DOI] [PubMed] [Google Scholar]
- 50. Sequist LV, Soria JC, Goldman JW. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372(18):1700–1709. [DOI] [PubMed] [Google Scholar]
- 51. Tagrisso (osimertinib) tablet, for oral use Available at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo Accessed January 11, 2016.
- 52. Varga A, Camidge DR, Sequist LV. Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement. Eur J Cancer. 2015;51(supp 3):S598. [Google Scholar]
- 53. Ahn MJ, Tsai CM, Yang JCH. AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from phase II studies. Eur J Cancer. 2015;51(supp 3):S625. [Google Scholar]
- 54. Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5(10):2884–2890. [PubMed] [Google Scholar]
- 55. Chinnaiyan P, Huang S, Vallabhaneni G. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328–3335. [DOI] [PubMed] [Google Scholar]
- 56. Welsh JW, Komaki R, Amini A. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sperduto PW, Wang M, Robins HI. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pesce GA, Klingbiel D, Ribi K. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer. 2012;48(3):377–384. [DOI] [PubMed] [Google Scholar]
- 59. Camidge DR, Bang YJ, Kwak EL. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weickhardt AJ, Scheier B, Burke JM. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Costa DB, Shaw AT, Ou SH. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zykadia (ceritinib) capsules for oral use. Prescribing Information Available at http://www.pharma.us.novartis.com/product/pi/pdf/zykadia.pdf Accessed August 31, 2015.
- 63. Friboulet L, Li N, Katayama R. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shaw AT, Kim DW, Mehra R. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim DW, Mehra R, Tan DS. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. ClinicalTrials.gov Available at https://clinicaltrials.gov/ Accessed May 28, 2016.
- 67. Gadgeel SM, Gandhi L, Riely GJ. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–1128. [DOI] [PubMed] [Google Scholar]
- 68. Gandhi L, Shaw A, Gadgeel SM. A phase II, open-label, multicenter study of the ALK inhibitor alectinib in an ALK+ non-small-cell lung cancer (NSCLC) U.S./Canadian population who had progressed on crizotinib (NP28761). J Clin Oncol. 2015;33(suppl):abstr 8019. [Google Scholar]
- 69. Ou S-HI, Ahn JS, Petris LD. Efficacy and safety of the ALK inhibitor alectinib in ALK+ non-small-cell lung cancer (NSCLC) patients who have failed prior crizotinib: an open-label, single-arm, global phase 2 study (NP28673). J Clin Oncol. 2015;33(suppl):abstr 8008. [Google Scholar]
- 70. Alecensa (alectinib) capsules, for oral use. Prescribing Information Available at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo Accessed January 8, 2016.
- 71. Ou S-HI, Shaw A, Gandhi L. BMET-18 assessing central nervous system (CNS) response to alectinib in two phase II studies of pre-treated ALK+ non-small cell lung cancer (NSCLC): RECIST versus RANO criteria. Neuro Oncol. 2015;17(suppl 5):v48–v49. [Google Scholar]
- 72. Ou SH, Ahn JS, De Petris L. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661–668. [DOI] [PubMed] [Google Scholar]
- 73. Gettinger SN, Bazhenova L, Salgia R. Brigatinib (AP26113) efficacy and safety in ALK+ NSCLC: phase 1/2 trial results. Presented at 16th World Conference on Lung Cancer; September 9, 2015; Denver, CO. [Google Scholar]
- 74. Bauer TM, Shaw AT, Solomon B. Phase I/II study of PF-06463922, an ALK/ROS1 tyrosine kinase inhibitor, in patients with advanced non-small-cell lung cancer harboring specific molecular alterations. J Clin Oncol. 2015;33(suppl):abstr TPS2620. [Google Scholar]
- 75. Shaw AT, Bauer TM, Felip E. Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS1+ NSCLC. J Clin Oncol. 2015;33(suppl):abstr 8018. [Google Scholar]
- 76. Johung KL, Yeh N, Desai NB. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Freeman GJ, Long AJ, Iwai Y. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. [DOI] [PubMed] [Google Scholar]
- 80. Platten M, Ochs K, Lemke D, Opitz C, Wick W. Microenvironmental clues for glioma immunotherapy. Curr Neurol Neurosci Rep. 2014;14(4):440. [DOI] [PubMed] [Google Scholar]
- 81. Besse B, Le Moulec S, Mazières J. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, phase II study. Clin Cancer Res. 2015;21(8):1896–1903. [DOI] [PubMed] [Google Scholar]
- 82. Barlesi F, Gervais R, Lena H. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22(11):2466–2470. [DOI] [PubMed] [Google Scholar]
- 83. Dinglin XX, Huang Y, Liu H, Zeng YD, Hou X, Chen LK. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol. 2013;112(3):461–466. [DOI] [PubMed] [Google Scholar]
- 84. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. [DOI] [PubMed] [Google Scholar]
- 85. Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–180. [DOI] [PubMed] [Google Scholar]
- 86. Cho SY, Choi HY. Causes of death and metastatic patterns in patients with mammary cancer. Ten-year autopsy study. Am J Clin Pathol. 1980;73(2):232–234. [DOI] [PubMed] [Google Scholar]
- 87. Kumar R, Sharma A, Tiwari RK. Application of microarray in breast cancer: an overview. J Pharm Bioallied Sci. 2012;4(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. COSMIC, catalogue of somatic mutations in cancer Available at http://cancer.sanger.ac.uk/cosmic/browse/tissue#sn=breast&ss=NS&hn=carcinoma Accessed January 15, 2016.
- 89. Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19(23):6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–5286. [DOI] [PubMed] [Google Scholar]
- 91. Kaal EC, Vecht CJ. CNS complications of breast cancer: current and emerging treatment options. CNS Drugs. 2007;21(7):559–579. [DOI] [PubMed] [Google Scholar]
- 92. Gabos Z, Sinha R, Hanson J. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24(36):5658–5663. [DOI] [PubMed] [Google Scholar]
- 93. Cho E, Rubinstein L, Stevenson P. The use of stereotactic radiosurgery for brain metastases from breast cancer: who benefits most? Breast Cancer Res Treat. 2015;149(3):743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21(5):942–948. [DOI] [PubMed] [Google Scholar]
- 95. Slamon DJ, Leyland-Jones B, Shak S. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 96. Lin NU. Weighing the options for human epidermal growth factor receptor 2-directed therapy in metastatic breast cancer. J Clin Oncol. 2015;33(14):1530–1533. [DOI] [PubMed] [Google Scholar]
- 97. Gradishar WJ, Anderson BO, Blair SL. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12(4):542–590. [DOI] [PubMed] [Google Scholar]
- 98. Giordano SH, Temin S, Kirshner JJ. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cardoso F, Costa A, Norton L. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489–502. [DOI] [PubMed] [Google Scholar]
- 100. Swain SM, Kim SB, Cortés J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Swain SM, Baselga J, Kim SB. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pivot X, Manikhas A, Żurawski B. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. [DOI] [PubMed] [Google Scholar]
- 103. Cameron D, Casey M, Press M. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–543. [DOI] [PubMed] [Google Scholar]
- 104. Krop IE, Lin NU, Blackwell K. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL. Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am J Clin Oncol. 2001;24(4):421–424. [DOI] [PubMed] [Google Scholar]
- 106. Siegelmann-Danieli N, Stein M, Bar-Ziv J. Complete response of brain metastases originating in breast cancer to capecitabine therapy. Isr Med Assoc J. 2003;5(11):833–834. [PubMed] [Google Scholar]
- 107. Swain SM, Baselga J, Miles D. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Awada A, Colomer R, Bondarenko I. Efficacy and CNS progression analysis from the randomized phase 2 trial of neratinib + paclitaxel vs trastuzumab + paclitaxel as first-line treatment for HER2+ metastatic breast cancer (NEfERTT). J Clin Oncol. 2015;33(suppl):abstr 610. [Google Scholar]
- 109. Bartsch R, Rottenfusser A, Wenzel C. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85(3):311–317. [DOI] [PubMed] [Google Scholar]
- 110. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56–62. [DOI] [PubMed] [Google Scholar]
- 111. Church DN, Modgil R, Guglani S. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol. 2008;31(3):250–254. [DOI] [PubMed] [Google Scholar]
- 112. Lin NU, Carey LA, Liu MC. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lin NU, Diéras V, Paul D. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 114. Saleem A, Searle GE, Kenny LM. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Freedman RA, Gelman RS, Wefel JS. Translational breast cancer research consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2016;34(9):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mirtsching B, Cosgriff T, Harker G, Keaton M, Chidiac T, Min M. A phase II study of weekly nanoparticle albumin-bound paclitaxel with or without trastuzumab in metastatic breast cancer. Clin Breast Cancer. 2011;11(2):121–128. [DOI] [PubMed] [Google Scholar]
- 117. Conlin AK, Seidman AD, Bach A. Phase II trial of weekly nanoparticle albumin-bound paclitaxel with carboplatin and trastuzumab as first-line therapy for women with HER2-overexpressing metastatic breast cancer. Clin Breast Cancer. 2010;10(4):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bartsch R, Berghoff A, Pluschnig U. Impact of anti-HER2 therapy on overall survival in HER2-overexpressing breast cancer patients with brain metastases. Br J Cancer. 2012;106(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lin NU, Eierman W, Greil R. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105(3):613–620. [DOI] [PubMed] [Google Scholar]
- 120. Geyer CE, Forster J, Lindquist D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. [DOI] [PubMed] [Google Scholar]
- 121. Bachelot T, Romieu G, Campone M. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 122. Morikawa A, Peereboom DM, Thorsheim HR. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Alés-Martínez JE, Filipovich E, Llano JLG, Viro JC, Sánchez-Escribano R. Dual anti-HER2 therapy (lapatinib and trastuzumab) plus capecitabine is a very effective and well-tolerated regimen (CLT) in metastatic HER2-positive breast cancer patients. J Clin Oncol. 2014;32(suppl):abstr e11513. [Google Scholar]
- 124. Bergen ES, Berghoff AS, Rudas M, Preusser M, Bartsch R. Breast cancer brain metastases responding to lapatinib plus capecitabine as second-line primary systemic therapy. Anticancer Drugs. 2015;26(5):579–581. [DOI] [PubMed] [Google Scholar]
- 125. Ferrario C, Welch S, Chaves JM. ONT-380 in the treatment of HER2+ breast cancer central nervous system (CNS) metastases (mets). J Clin Oncol. 2015;33(suppl):abstr 612. [Google Scholar]
- 126. Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167(4):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nanda R, Chow LQ, Dees EC. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer [abstract S1-09] Available at https://www.sabcs.org/About/Past-Symposia Accessed March 11, 2016. [DOI] [PMC free article] [PubMed]
- 128. Arvold ND, Oh KS, Niemierko A. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–160. [DOI] [PubMed] [Google Scholar]
- 129. Duchnowska R, Dziadziuszko R, Trojanowski T. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. van Kruchten M, de Vries EG, Brown M. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14(11):e465–e475. [DOI] [PubMed] [Google Scholar]
- 131. Jeselsohn R, Yelensky R, Buchwalter G. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20(7):1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lien EA, Wester K, Lønning PE, Solheim E, Ueland PM. Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer. 1991;63(4):641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Salvati M, Cervoni L, Innocenzi G, Bardella L. Prolonged stabilization of multiple and single brain metastases from breast cancer with tamoxifen. Report of three cases. Tumori. 1993;79(5):359–362. [DOI] [PubMed] [Google Scholar]
- 134. Stewart DJ, Dahrouge S. Response of brain metastases from breast cancer to megestrol acetate: a case report. J Neurooncol. 1995;24(3):299–301. [DOI] [PubMed] [Google Scholar]
- 135. Madhup R, Kirti S, Bhatt ML, Srivastava PK, Srivastava M, Kumar S. Letrozole for brain and scalp metastases from breast cancer–a case report. Breast. 2006;15(3):440–442. [DOI] [PubMed] [Google Scholar]
- 136. Raub TJ, Wishart GN, Kulanthaivel P. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43(9):1360–1371. [DOI] [PubMed] [Google Scholar]
- 137. Christodoulou C, Bafaloukos D, Linardou H. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. J Neurooncol. 2005;71(1):61–65. [DOI] [PubMed] [Google Scholar]
- 138. Kouvaris JR, Miliadou A, Kouloulias VE. Phase II study of temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases from solid tumors. Onkologie. 2007;30(7):361–366. [DOI] [PubMed] [Google Scholar]
- 139. Addeo R, De Rosa C, Faiola V. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113(9):2524–2531. [DOI] [PubMed] [Google Scholar]
- 140. Siena S, Crinò L, Danova M. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol. 2010;21(3):655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Boogerd W, Groenveld F, Linn S, Baars JW, Brandsma D, van Tinteren H. Chemotherapy as primary treatment for brain metastases from breast cancer: analysis of 115 one-year survivors. J Cancer Res Clin Oncol. 2012;138(8):1395–1403. [DOI] [PubMed] [Google Scholar]
- 142. Kolarić K, Roth A, Jelicic I, Matković A. Phase II clinical trial of cis dichlorodiammine platinum (Cis DDP) in metastatic brain tumors. J Cancer Res Clin Oncol. 1982;104(3):287–293. [DOI] [PubMed] [Google Scholar]
- 143. Viñolas N, Graus F, Mellado B, Caralt L, Estapé J. Phase II trial of cisplatinum and etoposide in brain metastases of solid tumors. J Neurooncol. 1997;35(2):145–148. [DOI] [PubMed] [Google Scholar]
- 144. Franciosi V, Cocconi G, Michiara M. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85(7):1599–1605. [PubMed] [Google Scholar]
- 145. Cassier PA, Ray-Coquard I, Sunyach MP. A phase 2 trial of whole-brain radiotherapy combined with intravenous chemotherapy in patients with brain metastases from breast cancer. Cancer. 2008;113(9):2532–2538. [DOI] [PubMed] [Google Scholar]
- 146. Freedman RA, Bullitt E, Sun L. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer. 2011;11(6):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kurzrock R, Gabrail N, Chandhasin C. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther. 2012;11(2):308–316. [DOI] [PubMed] [Google Scholar]
- 148. Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42(2):660–668. [DOI] [PubMed] [Google Scholar]
- 149. Budman DR, Camacho E, Wittes RE. The current causes of death in patients with malignant melanoma. Eur J Cancer. 1978;14(4):327–330. [DOI] [PubMed] [Google Scholar]
- 150. Gupta G, Robertson AG, MacKie RM. Cerebral metastases of cutaneous melanoma. Br J Cancer. 1997;76(2):256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fife KM, Colman MH, Stevens GN. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. [DOI] [PubMed] [Google Scholar]
- 152. Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. [DOI] [PubMed] [Google Scholar]
- 153. Zacest AC, Besser M, Stevens G, Thompson JF, McCarthy WH, Culjak G. Surgical management of cerebral metastases from melanoma: outcome in 147 patients treated at a single institution over two decades. J Neurosurg. 2002;96(3):552–558. [DOI] [PubMed] [Google Scholar]
- 154. Gorantla V, Kirkwood JM, Tawbi HA. Melanoma brain metastases: an unmet challenge in the era of active therapy. Curr Oncol Rep. 2013;15(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Davies H, Bignell GR, Cox C. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 156. Flaherty KT, Infante JR, Daud A. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chapman PB, Hauschild A, Robert C. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hauschild A, Grob JJ, Demidov LV. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 159. Larkin J, Ascierto PA, Dréno B. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 160. Robert C, Karaszewska B, Schachter J. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 161. Long GV, Stroyakovskiy D, Gogas H. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 162. Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Falchook GS, Long GV, Kurzrock R. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Long GV, Trefzer U, Davies MA. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 165. Dummer R, Goldinger SM, Turtschi CP. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–621. [DOI] [PubMed] [Google Scholar]
- 166. Ascierto PA, Schadendorf D, Berking C. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249–256. [DOI] [PubMed] [Google Scholar]
- 167. Hodi FS, O’Day SJ, McDermott DF. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Margolin K, Ernstoff MS, Hamid O. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 169. Weber JS, D’Angelo SP, Minor D. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 170. Topalian SL, Sznol M, McDermott DF. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Robert C, Long GV, Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 172. Robert C, Schachter J, Long GV. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 173. Hamid O, Robert C, Daud A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kluger HM, Goldberg SB, Sznol M. Safety and activity of pembrolizumab in melanoma patients with untreated brain metastases. J Clin Oncol. 2015;33(suppl):abstr 9009. [Google Scholar]
- 175. Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16(3):248–255. [DOI] [PubMed] [Google Scholar]
- 176. Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book. 2013:393–398. [DOI] [PubMed] [Google Scholar]
- 177. Agarwala SS, Kirkwood JM, Gore M. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101–2107. [DOI] [PubMed] [Google Scholar]
- 178. Jacquillat C, Khayat D, Banzet P. Final report of the French multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer. 1990;66(9):1873–1878. [DOI] [PubMed] [Google Scholar]
- 179. Avril MF, Aamdal S, Grob JJ. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118–1125. [DOI] [PubMed] [Google Scholar]
- 180. Kiess AP, Wolchok JD, Barker CA. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Narayana A, Mathew M, Tam M. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol. 2013;113(3):411–416. [DOI] [PubMed] [Google Scholar]
- 182. Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res. 2011;17(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Beadling C, Heinrich MC, Warrick A. Multiplex mutation screening by mass spectrometry evaluation of 820 cases from a personalized cancer medicine registry. J Mol Diagn. 2011;13(5):504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–1480. [DOI] [PubMed] [Google Scholar]
- 186. Quan AL, Videtic GM, Suh JH. Brain metastases in small cell lung cancer. Oncology. 2004;18(8):961–972; discussion 974, 979–980, 987. [PubMed] [Google Scholar]
- 187. Brufsky AM, Mayer M, Rugo HS. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 188. Davies MA, Liu P, McIntyre S. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 189. Peuvrel L, Saint-Jean M, Quéreux G. Incidence and characteristics of melanoma brain metastases developing during treatment with vemurafenib. J Neurooncol. 2014;120(1):147–154. [DOI] [PubMed] [Google Scholar]
- 190. Gummadi T, Zhang BY, Valpione S. Impact of BRAF mutation and BRAF inhibition on melanoma brain metastases. Melanoma Res. 2015;25(1):75–79. [DOI] [PubMed] [Google Scholar]
- 191. de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs. 2012;30(2):443–449. [DOI] [PubMed] [Google Scholar]
- 192. Deng Y, Feng W, Wu J. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014;2(1):116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Togashi Y, Masago K, Fukudo M. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5(7):950–955. [DOI] [PubMed] [Google Scholar]
- 194. Togashi Y, Masago K, Masuda S. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405. [DOI] [PubMed] [Google Scholar]
- 195. Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Leggas M, Panetta JC, Zhuang Y. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66(9):4802–4807. [DOI] [PubMed] [Google Scholar]
- 197. Coldren CD, Helfrich BA, Witta SE. Baseline gene expression predicts sensitivity to gefitinib in non-small cell lung cancer cell lines. Mol Cancer Res. 2006;4(8):521–528. [DOI] [PubMed] [Google Scholar]
- 198. Zhao J, Chen M, Zhong W. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 2013;14(2):188–193. [DOI] [PubMed] [Google Scholar]
- 199. Wind S, Giessmann T, Jungnik A. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig. 2014;34(3):173–182. [DOI] [PubMed] [Google Scholar]
- 200. Hoffknecht P, Tufman A, Wehler T. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Tang SC, Nguyen LN, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134:1484–1494. [DOI] [PubMed] [Google Scholar]
- 202. Costa DB, Kobayashi S, Pandya SS. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. [DOI] [PubMed] [Google Scholar]
- 203. Fontana D, Ceccon M, Gambacorti-Passerini C, Mologni L. Activity of second-generation ALK inhibitors against crizotinib-resistant mutants in an NPM-ALK model compared to EML4-ALK. Cancer Med. 2015;4(7):953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kort A, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol Res. 2015;102:200–207. [DOI] [PubMed] [Google Scholar]
- 205. Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351(2):215–221. [DOI] [PubMed] [Google Scholar]
- 206. Carter P, Presta L, Gorman CM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89(10):4285–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Polli JW, Humphreys JE, Harmon KA. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36(4):695–701. [DOI] [PubMed] [Google Scholar]
- 208. Gori S, Lunardi G, Inno A. Lapatinib concentration in cerebrospinal fluid in two patients with HER2-positive metastatic breast cancer and brain metastases. Ann Oncol. 2014;25(4):912–913. [DOI] [PubMed] [Google Scholar]
- 209. Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032). J Pharmacol Exp Ther. 2012;342(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zelboraf (vemurafenib) [US package insert]. South San Francisco, CA: Genentech;2014. [Google Scholar]
- 211. Sakji-Dupré L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25(4):302–305. [DOI] [PubMed] [Google Scholar]
- 212. Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm. 2012;9(11):3236–3245. [DOI] [PubMed] [Google Scholar]
- 213. Tafinlar (dabrafenib) [US package insert]. Research Triangle Park, NC: GlaxoSmithKline;2014. [Google Scholar]
- 214. O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110(12):2614–2627. [DOI] [PubMed] [Google Scholar]
- 215. Shaw AT, Mehra R, Tan DSW. Evaluation of ceritinib-treated patients with anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 study [abstract 1293P]. Presented at the European Society For Medical Oncology Annual Meeting; September 26–30, 2014; Madrid, Spain. [Google Scholar]
- 216. Mok T, Spigel D, Felip E. ASCEND-2: a single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). J Clin Oncol. 2015;33(suppl):abstr 8059. [Google Scholar]
- 217. Felip E, Orlov S, Park K. ASCEND-3: a single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl):abstr 8060. [Google Scholar]
- 218. Camidge DR, Bazhenova L, Salgia R. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl):abstr 8062. [Google Scholar]
- 219. Gettinger SN, Bazhenova L, Salgia R. Updated efficacy and safety of the ALK inhibitor AP26113 in patients (pts) with advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32 suppl):abstr 8047. [Google Scholar]
- 220. Robinet G, Thomas P, Breton JL. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):59–67. [DOI] [PubMed] [Google Scholar]
- 221. Galetta D, Gebbia V, Silvestris N. Cisplatin, fotemustine and whole-brain radiotherapy in non-small cell lung cancer patients with asymptomatic brain metastases: a multicenter phase II study of the Gruppo Oncologico Italia Meridionale (GOIM 2603). Lung Cancer. 2011;72(1):59–63. [DOI] [PubMed] [Google Scholar]
- 222. Brosnan EM, Fadul CE, Dragnev KH, Davis MC, Shah KA, Eskey CJ. Outcome of combined bevacizumab and irinotecan for patients with progressive brain metastases from non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl):abstr e19059. [Google Scholar]