We read with great interest the recent article by Ni et al.1 on the role of the tyrosine receptor kinase B (TrkB) as a drug target for the treatment of astrocytomas. Among central nervous system tumors, astrocytomas are the most frequent type of glioma, and grade IV astrocytoma (also called glioblastoma) is the most aggressive type of primary brain cancer. As pointed out by the authors, signaling triggered by TrkB, which is activated by its endogenous ligand, the neurotrophin brain-derived neurotrophic factor (BDNF), plays a crucial role in normal development and plasticity of the central nervous system,2 but increasing evidence also indicates a role for BDNF/TrkB upregulation in different types of cancer.3 , 4 By systematically screening a library of human tyrosine kinases for their oncogenic potential in astrocytoma formation and then characterizing functional features and molecular mechanisms associated with TrkB-induced oncogenesis, Ni et al. have provided compelling findings indicating that TrkB can play a role in astrocytoma formation.1
It is worth pointing out that previous studies not cited by Ni et al.1 had already identified an increase in TrkB expression in both low-grade astrocytoma and glioblastoma,5 , 6 and a role for TrkB signaling in the growth of gliomas and other types of brain tumors or peripheral tumors of possible neural origin has been previously proposed. For example, BDNF-induced activation of TrkB increases the viability of brain-tumor stem cells (BTSCs) isolated from gliomas through activation of the extracellular-regulated kinase (ERK) and Akt pathways, whereas TrkB knockdown or pharmacological inhibition reduces BTSC growth and BDNF-dependent ERK activation.7 Another recent study has suggested that glioma growth may be regulated by TrkB expressed in exosomes.8 We have recently provided evidence for a potential role of TrkB inhibition as a strategy to reduce cell proliferation and potentiate the effects of chemotherapy in medulloblastoma9 and Ewing sarcoma.10
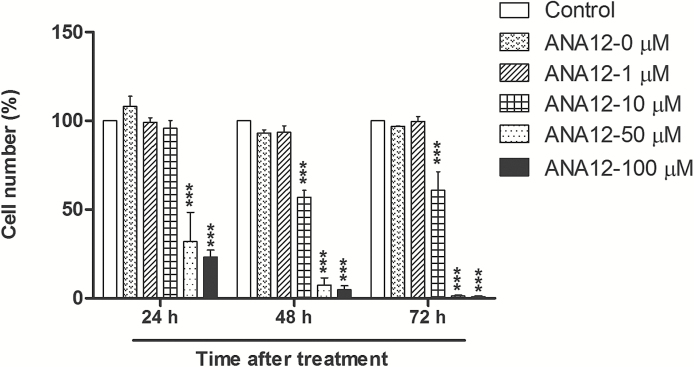
In order to investigate the antitumor effects of pharmacological inhibition of TrkB-associated signaling, Ni et al. used the compounds AZD1480, a janus kinase (JAK) inhibitor, and RXDX-101, which nonselectively inhibit different members of the Trk receptor family (TrkA, TrkB, and TrkC), C-ros oncogene 1 (ROS1), and the anaplastic lymphoma kinase (ALK).1 Thus, they did not assess the effects of selective TrkB blockade, and the effects they observed could be influenced by inhibition of other types of Trk receptors or signaling molecules downstream of Trk. We have recently started a series of experiments aiming to verify whether specific TrkB inhibition reduces glioma cell proliferation using ANA-12, a small-molecule selective TrkB antagonist. Our first results showed that ANA-12 effectively and dose-dependently reduces the viability of a human glioblastoma cell line with almost complete disappearance of cultured cells 72 hours after treatment (Fig. 1). Therefore, selective TrkB inhibition might prove to be an effective experimental therapeutic strategy, possibly with fewer off-target toxicities compared with multitarget drugs in patients with astrocytomas harboring oncogenic TrkB.
Fig. 1.
Selective tyrosine receptor kinase B (TrkB) inhibition reduces the viability of human glioma cells. A172 cells obtained from the American Type Culture Collection (Rockville, Maryland) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) low glucose supplemented with 10 % (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin. Cells were incubated in a humidified atmosphere of 5 % CO2 at 37 °C, were seeded at a density of 5 x 103 cells/well in 96-well plates and allowed to grow for 24 hours. The medium was replaced for treatments with increasing concentrations of ANA-12 (1, 10, 50, or 100 µM). Control cells were exposed to vehicle (dimethylsulfoxide, [DMSO], 1.6 %) alone, and cells in the ANA-12 0 µM group were not exposed to either vehicle or drug as an additional control showing that the vehicle had no effect by itself. Cell viability was assessed by trypan blue cell counting 24, 48, and 72 hours after treatment. Data normalized to control cells are presented as means ± SEM and represent 2 independent experiments performed in quadruplicate; *** P < .001 compared with control cells exposed to vehicle alone (2-way ANOVA followed by Bonferroni’s post hoc tests). All experimental procedures were approved by the institutional research ethics committee (GPPG HCPA number 1406–22).
Funding
Supported by the National Council for Scientific and Technological Development (CNPq; grant numbers 484185/2012–8 and 303276/2013–4 to R.R., and 309430/2012–7 to G.S.); Coordination for the Improvement of Higher Education Personnel (CAPES); the South American Office for Anticancer Drug Development; the Children’s Cancer Institute (ICI); and the Clinical Hospital institutional research fund (FIPE/HCPA).
References
- 1. Ni J, Xie S, Ramkissoon SH, et al. Tyrosine receptor kinase B is a drug target in astrocytomas. Neuro Oncol. 2016; doi: 10.1093/neuonc/now139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. [DOI] [PubMed] [Google Scholar]
- 3. Thiele CJ, Li Z, McKee AE. On Trk–the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15(19):5962–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roesler R, de Farias CB, Abujamra AL, Brunetto AL, Schwartsmann G. BDNF/TrkB signaling as an anti-tumor target. Expert Rev Anticancer Ther. 2011;11(10):1473–1475. [DOI] [PubMed] [Google Scholar]
- 5. Wadhwa S, Nag TC, Jindal A, et al. Expression of the neurotrophin receptors Trk A and Trk B in adult human astrocytoma and glioblastoma. J Biosci. 2003;28(2):181–188. [DOI] [PubMed] [Google Scholar]
- 6. Assimakopoulou M, Kondyli M, Gatzounis G, Maraziotis T, Varakis J. Neurotrophin receptors expression and JNK pathway activation in human astrocytomas. BMC Cancer. 2007;7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawn S, Krishna N, Pisklakova A, et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem. 2015;290(6):3814–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinet S, Bessette B, Vedrenne N, et al. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget. 2016; doi: 10.18632/oncotarget.10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomaz A, Jaeger M, Buendia M, et al. BDNF/TrkB signaling as a potential novel target in pediatric brain tumors: anticancer activity of selective TrkB inhibition in medulloblastoma cells. J Mol Neurosci. 2016;59(3):326–333. [DOI] [PubMed] [Google Scholar]
- 10. Heinen TE, Dos Santos RP, da Rocha A, et al. Trk inhibition reduces cell proliferation and potentiates the effects of chemotherapeutic agents in Ewing sarcoma. Oncotarget. 2016;7(23):34860–34880. [DOI] [PMC free article] [PubMed] [Google Scholar]