ABSTRACT
Anesthesia and consciousness represent 2 mysteries not only for biology but also for physics and philosophy. Although anesthesia was introduced to medicine more than 160 y ago, our understanding of how it works still remains a mystery. The most prevalent view is that the human brain and its neurons are necessary to impose the effects of anesthetics. However, the fact is that all life can be anesthesized. Numerous theories have been generated trying to explain the major impact of anesthetics on our human-specific consciousness; switching it off so rapidly, but no single theory resolves this enduring mystery. The speed of anesthetic actions precludes any direct involvement of genes. Lipid bilayers, cellular membranes, and critical proteins emerge as the most probable primary targets of anesthetics. Recent findings suggest, rather surprisingly, that physical forces underlie both the anesthetic actions on living organisms as well as on consciousness in general.
KEYWORDS: anesthesia, anesthetics, consciousness, ether, ethylene, genes, lipids, membranes, plants, xenon
Introduction
The discovery of human anesthesia in 1846, by William T. Morton, especially its fast and reversible induction by ether; marks new era in our understanding of life.1-3 This was a unique discovery which rapidly revolutionized medicine, but turned out to be difficult to explain.1,2 Despite numerous theories proposed our understanding of anesthesia remains obscure.2,3 Among the many theories trying to explain anesthesia, 3 stand-out as the most influential. Firstly, Claude Bernard and his theory colloidal-coagulation of protoplasm,2,4 followed by Hans Horst Meyer and Charles Ernest Overton with their “Meyer-Overton rule,” based on lipid solubility of anesthetics, have dominated the field since the late 1960s.2 In the 1980s, the protein/receptor theory became dominant in explaining anesthesia via proteins acting as specific receptors for anesthetics.5 However, inconsistencies with this theory called for a further updates.3,6-10
All life can be anesthesized
The father of experimental biology, Claude Bernard, performed numerous experimental studies which allowed him to conclude “that all life is defined by the susceptibility to anesthesia.”2,11,12 Claude Bernard's paradigm is still valid today as all organisms, even prokaryotic bacteria, are sensitive to anesthetics.8,9,12,13-15 Sub-cellular organelles such as mitochondria and chloroplasts are sensitive to anesthetics as well,16-21 which is in line with the prokaryotic sensitivity of both membranes and proteins recorded in bacteria.14,22-24 Finally, several sub-cellular processes based on the cytoskeleton, such as cytoplasmic streaming and phagocytosis, are inhibited by exposure to diverse anesthetics.6,25-30
Early evolutionary origins of anesthesia: Endogenous anesthetics help to cope with stress
As discussed by James Sonner, the variability in response to anesthetics is extremely small in comparison to other drugs.8,31 Moreover, the wide molecular diversity of compounds acting as anesthetics is very large, and additionally, the mystery of universal sensitivity of all living organisms to these compounds remains. All this suggests, in line with the Claude Bernard thesis, that the ability to respond to anesthetics is essential for life.8 One possibility proposed recently by James Sonner and Robert Cantor is the existence of endogenous anesthetics which modulate organismal consciousness.9 In fact, there are several metabolites that induce loss of consciousness if available in sufficiently large amounts. Sonner and Cantor discussed some, for example, ammonia,32 acetone, β-hydroxybutyric acid,33 and propionic acid.34 Importantly, protective actions of endogenous anesthetics are active at the lipid bilayer of membranes,8,9,35 which explains why even bacteria are sensitive to anesthetics. In addition, the protective actions of anesthetics include also cardioprotection,36-38 protection from retinal damages,39 and immunoprotection.40 Finally, due to their actions also on bacteria, anesthetics also have antimicrobial effects.23,41
Microbes, algae and plants release large amounts of volatile anesthetics
It is not generally appreciated that algae and plants release abundantly volatiles including well-known anesthetics such as chloroform, divinyl ether, ethylene, and methyl halides,42-48 as well as n-alkanols which also have anesthetic actions.49,50 Plant volatiles are released in such large amounts that they have a strong impact on the Earth's biosphere and atmosphere.43,47 Importantly, algae and plants release these substances especially if under stress.44,46,48 In addition, the anesthetic nitrous oxide is released into atmosphere in large amounts from soils and oceans.1-53 Unfortunately, the authors of these papers fail to mention and discuss the fact that many of these stress-released algal, microbila and plant volatiles are also anesthetics. For example, nitrous oxide is almost exclusively regarded as a greenhouse gas, whereas divinyl ether is discussed typically as oxylipin54,55 and ethylene as plant stress hormone.56,57 However, the possibility that stressed organisms produce anesthetics to cope better with their stress should be considered. Besides classical anesthetics, stressed plant synthesize and produce many pain-relieving compounds,58-62 including ethanol which is produced in plants via the synthetic activity of alcohol dehydrogenase, which acts in reverse in plants.63,64 In stressed roots, for example, all 3 endogenous anesthetics (ethylene, divinyl ether, ethanol) are co-produced, which suggests delicate control of putative, still hypothetical, plant anesthesia. Relevant in this respect is the fact that all these 3 endogenous anesthetics are co-produced also in plant fruits, which flowering plants have evolved to be eaten alive by diverse animals. Moreover, ethylene, ethanol and other anesthetics also act to break the dormancy in plant seeds.65
Surprising status of ethylene: Ancient endogenous anesthetic essential for life?
Ethylene, a hydrocarbon and the simplest alkene, is a colourless flammable gas, which is widely used in the chemical industry. In biology, it is most famously known as a plant stress hormone. Less known is that ethylene is a very potent general anesthetic, which was used in human surgery as it has minimum side-effects and recovery from anesthesia is very rapid.66-68 Ethylene has similar physical and lipid solubility properties to xenon,66 and both these anesthetics have the least side effects. Importantly, not only plants, but also bacteria, fungi, algae and lichens are known to produce ethylene when under stress.69-80 This suggests that ethylene is a molecule with fundamental relevance for life. On the basis of the anesthetic properties of ethylene and ether, Chauncey Leake synthesized divinyl ether, and showed that it had excellent anesthetic properties.81,82 Divinyl ether maintains all the positive properties of ethylene, but is also more potent.83 Intriguingly, stressed plants produce endogenously both ethylene and divinyl ether.84-87 It is becoming obvious that plants, and their endogenous anesthetics, will turn out to be highly relevant for our understanding of the evolutionary origins of anesthesia. Very relevant in this respect are local anesthetics most of which are derived from the plant alkaloid cocaine.88,89 Interestingly, there are also other plant alkaloids with properties of local anesthetics; such as atropine,90 menthol,91,92 and several other alkaloids.93,94
The relevance of endogenous controls over anesthesia for behavior and survival
A coherent and robust concept is emerging from the data discussed in this paper, which suggests that endogenous anesthetics are essential for the survival of plants, allowing them to cope with stress, to enter and break dormancy, as well as to generate tasteful fruits ‘designed’ to be eaten alive by animals and humans (for the sake of effective reproduction of flowering plants). Similarly, bacteria and fungi generate ethylene under stressful situations and there are several indications of endogenous anesthetics in animals and humans. In metabolic human diseases, several metabolites with anesthetic features accumulate in such amounts that they can impose temporary or permanent loss of consciousness (reviewed in ref. 9). Relevant in this respect is the well-known phenomena of transient loss of consciousness (sometimes referred as syncope, fainting, or blackout) which can be induced by diverse stress situations, serious wounding, as well as by powerful emotional stresses.95-99 Transient loss of consciousness apparently has relevance to survival. It was proposed that this phenomenon has evolved in ancient times as an effective defense mechanism;97,99 providing, in addition, protection against sensory overload. Moreover, it might also be the case that some of the numerous examples of so-called apparent death (thanatopsis) behavior in predator-threatened animals are not the result of deceptive/ mimicry behavior by the animal, (as typically interpreted) but rather due to the syncope-like transient loss of consciousness. Even at the cellular level, both stress and anesthetics can have similar paralyzing outcomes.100-102
Since ancient times, humans have been using natural anesthetics produced by plants and fungi to impose anesthesia, induce altered sensory states as well as psychedelic experiences.60,61,63-106 In fact, human evolution is well known to be shaped by the consumption of alcohol.107-109 This feature is shared not only with apes110 but also with other animals such as wild treeshrews,111 and even insects, which can also develop alcoholism under stress challenges.112-114
Furthermore, there are several similarities between the deep phases of sleep (REM sleep) and the state of anesthesia.115-117 Although sleep and anesthesia are different phenomena, the underlying neuronal processes are common for both forms of the loss of consciouseness.117-119 Although the precise roles of sleep and anesthesia are not fully understood yet, it is clear that sleep is essential for cognitive and survival reasons.120-122 For example, REM sleep was proposed to generate a proto-conscious states relevant for the formation of the full-blown waking consciousness.123 Similarly sleep and anesthesia are under endogenous control,124 suggesting that the state of anesthesia has important, albeit still elusive, functions for organisms. Importantly, the sleep/ waking cycle controls sensitivity to anesthetics in Drosophila.125 Relevant in this respect are also numerous examples where pain perceptions have been shown to be affected by expectations, cognition and meditation.126-128 Moreover, pain responses in humans are mediated not only via conscious but also via unconscious processes.129-131 Finally, there are close similarities between anesthesia and coma.132
Genes are not involved in switching off/on of consciousness via anesthetics
Anesthetics provided in the appropriate concentrations switch-off human-specific consciousness within a few seconds, precluding any role whatsoever for DNA and gene expression in those actions (Fig. 1). If anesthetics are maintained at their active levels, loss of consciousness is permanent as long as the anesthetics are present at critical levels. After their removal, the recovery of consciousness is often very rapid. Although some anesthetics have side effects, and can even be toxic (e.g. chloroform), the most effective ones – for example xenon, ethylene, and vinyl ether allow fast and smooth recovery. Again, the speed of regaining the consciousness precludes any active role for gene expression also in this process (Fig. 1). Of course, genes are relevant for the sensitivity of organisms to anesthetics and several mutants have been characterized which are less sensitive, or even not sensitive at all, to some of the anesthetics. But this is just due to the modifications or absence of critical proteins and membranes (Fig. 1) which induce the anesthetic action. The absence of gene involvement should not necessarily be surprising. Human erythrocytes, which have no nucleus and therefore no genes, still undergo the complex cellular process of circadian rhythm.133 Genes may act to adjust circadian rhythm for variation in the regularity of the day/ night cycle, as, for example, incurred by traveling to other time zones, but they are not causal in circadian rhythm itself. This is clearly demonstrated by incubating 3 enzymes extracted from cyanobacteria with ATP: a relatively temperature independent 24 hour cycle of phosphorylation of one of the enzymes is observed.134
Figure 1.

Fast loss and gain of consciousness after exposure and removal of anesthetics is based on primary processes linked to the plasma membrane (ion fluxes, electric activities, endocytic vesicle recycling), whereas changes in gene expression are playing only secondary roles.
Why are neurons exquisitely sensitive to anesthetics: clues from plants and chloroplasts?
There are several mysteries associated with anesthetics and anesthesia. One of them is the fact that neurons are more sensitive to anesthetics compared to other cells. Claude Bernard was the first to realize that depending upon concentration of an anesthetic, there are several stages of anesthesia. The first is loss of awareness and pain perception, but all vital biochemical processes are unaffected, the second is inhibition of respiration and other biochemical processes, the third is the loss of ability of all cells to react to stimuli and the cessation cilia movements and heart beating.11,12 In humans, minimal alveolar concentrations (MAC) concept was introduced to characterize the potency of inhalational anesthetics.135 At 0.1- 0.3 MAC, most humans show sensory distortions, memory loss and sleepiness; at 0.3- 0.5 MAC, responses to verbal commands cease and loss of consciousness occurs; at about 1.0 MAC, insensitivity to noxious stimuli occurs.68,135 This marked concentration dependency of anesthetic potency suggests that some cells, or cellular processes, are more sensitive than others due to perhaps a higher number of the critical binding sites for the anesthetics. In neuronal assemblies, there are large numbers of excitable cells organized, via synaptic cell-cell adhesions, into higher order cellular assemblies. These communicate via action potentials and accomplish synchronous and coherent oscillations dependent on sensory stimuli. It is perhaps important that the plasma membrane at synapses is actively engaged in the endocytic vesicle recycling, amplifying the critical area of membranes significantly and underlying the high excitability of neuronal membranes.136-138 The high electrical activity of neurons is related to their active maintenance of the physical properties of the lipid bilayer and the internal physico-chemical properties in the fluctuating cellular environments. Anesthetics and action potentials are highly relevant in this respect.8,136,139 Surprisingly, although plant cells are sometimes considered not to be excitable,136,137 the opposite is true.12,139-147 In plant cells, action potentials, vesicle recycling, and sensitivity to anesthetics seem to be related to the active protection of the plasma membrane against ionic and structural disturbances.12,139,147-153 These issues are prominent especially in neurons and specialized root apex cells both of which are very active in both electrical activities and endocytic vesicle recycling.143,147 For both neurons and specialized plant cells, the endocytic (synaptic) recycling apparatus enhances the sensitivity to anesthetics due to large area of plasma membrane and the associated recycling vesicles, which is supported by the dynamic cytoskeleton.147,154-156 It is emerging that these unique properties of brain neurons and plant root apex cells makes them exquisitely sensitive to anesthetics (Fig. 2).
Figure 2.
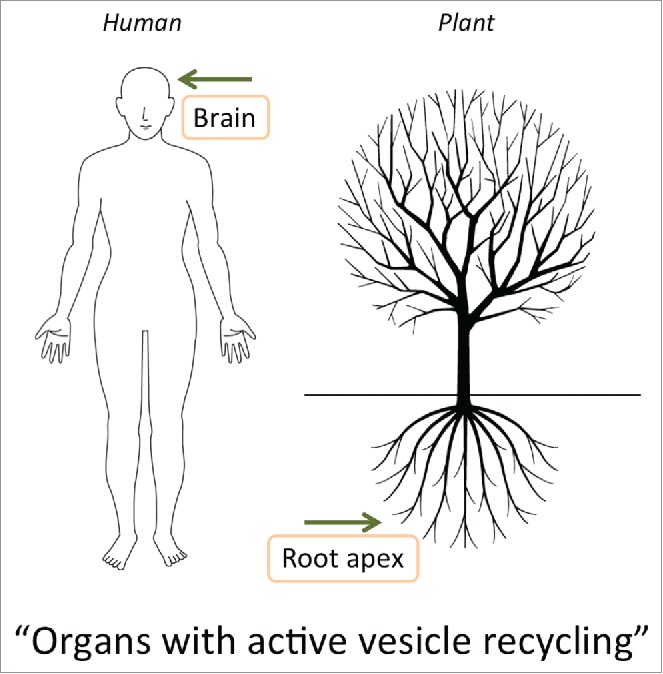
Both in animals and plants, organs with the highest activities of endocytic vesicle recycling and electric activities are implied in loss of consciousness (motility, sensitivity, and behavior).
Claude Bernard was the first to note that chloroplasts and their photosynthetic pathways are more sensitive to anesthetics than mitochondria and their respiratory pathways.12,25 One of the striking differences between chloroplasts and mitochondria is stacking of internal chloroplast membranes into synaptic-like assemblies known as thylakoid grana which are essential for the photosynthetic pathways.157 Intriguingly, in this respect, synaptic-like MORN-domain proteins have been reported for the thylakoid proteome.158 On the other hand, internal membranes of mitochondria are much simpler and never form such prominent membrane stacks. Moreover, synaptic-like proteins including synaptotagmin-like E-SYT, yeast tricalbins and plant SYTs localize to membrane adhesions between intracellular organelles including plasma membranes, nuclei, mitochondria, chloroplasts, peroxisomes, ER membranes, and lipid bodies.159-161 We are proposing that synaptic-like157 stacking of thylakoid membranes into prominent chloroplast grana162,163 is behind the higher sensitivity of the chloroplast photosynthesis to anesthetics25 in comparison to the mitochondrial respiration (Fig. 3).
Figure 3.
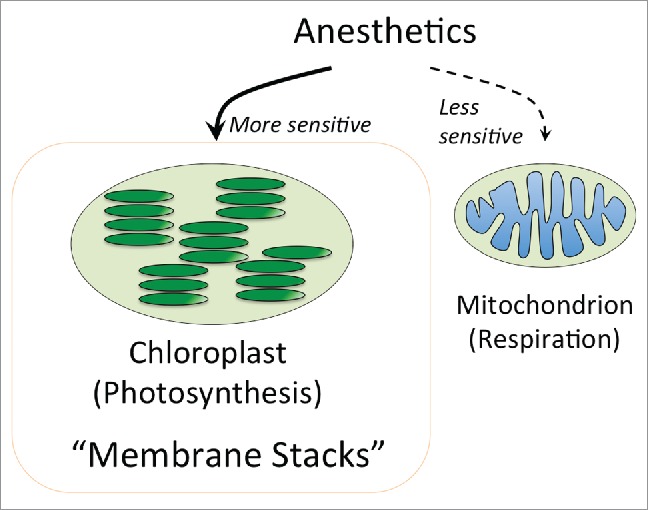
Claude Bernard discovered higher sensitivity of photosynthesis to anesthetics in comparison to respiration.12,25 Chloroplast accomplish photosynthesis on stacked membranes know as thylakoid grana162,163 which can be considered for inter-organellar synapses.157 We propose that these stacked membranes are not only essential for photosynthesis but also makes this process more sensitive to anesthetics.
Physical nature of anesthesia and consciousness
The unique aspect of anesthesia and consciousness is that these deep mysteries challenge both biology and physics. In fact, there are many aspects of the actions of anesthetics on all life which implicate a profound physical basis for both anesthesia and consciousness. Action potentials have not only electrical, but also mechanical aspects, as they change significantly plasma membrane thickness and even the length of electrically active neurons.164,167 Moreover, protein activity is also highly dependent on physical factors. For example, the tertiary structures of proteins are easily deformed and this affects their activity.
Interestingly, action potentials also generate heat in excited membranes168-170 which then unfolds proteins and fluidizes lipid bilayers.171 Also anesthetics unfold proteins and induce fluidization of lipid bilayers.171-173 Importantly, the potency of anesthetics in inducing the loss of motility and consciousness decreases with the temperature increasing.174-176
Recent advances with xenon anesthesia strongly suggest that the currently favored proteins/ receptors model of anesthetics actions will require a serious update toward including the lipid bilayer as the primary target of xenon. This unique simple noble gas is chemically inert and acts on membranes only through its physical properties.177,178 The physical nature of both anesthesia and consciousness is implicated also with the reversal of anesthetic induced loss of consciousness by high pressure. This pressure reversal was discovered by Johnson and Flagler who showed that anesthesized tadpoles regain activities at pressures of about 50 bar.178 Since then this observation has been confirmed with many other organisms and also with diverse anesthetics. With respect to xenon, high pressure was shown to prevent free xenon diffusion within lipid bilayers and xenon was pushed out to accumulate in the middle of the lipid bilayer.177 Furthermore, xenon was found to modulate the bilayer lateral pressure profile in a reversible fashion.178 Xenon-induced changes to the critical proteins may be only the secondary consequences of these physical effects of xenon on lipid bilayers.178,180 Alternatively, there might also be a genuine direct effect of xenon on proteins via their hydrophobic pockets. Intriguingly, xenon rapidly reverses electron spins and these electron spin effects were found to be different in Drosophila mutants which did not respond to the anesthetics.10 The next perplexing finding is that both local and general anesthetics show similar physical effects on membranes by lowering their melting temperatures and this anesthetic effect is reversed by high pressure.167,181,182 There are further issues suggesting that physical phenomena are related to both anesthesia and consciousness based on quantum aspects of physical reality.7 In conclusion, solving of theses mysteries will be based on both, biology and physics, and relevant to both. This fundamental advance in our knowledge will help to understand the basic question of biology posed by the famous physicist Erwin Schrödinger: ‘what is life?’.183
The implications of a lack of a role for genes in consciousness
As noted above, the rapidity of the switching on and off of consciousness by anesthetics precludes a role for the necessarily slower action of gene expression. It can be argued that genes are in fact only necessary to 2 quite small, but nevertheless important, aspects of the life process, namely reproduction and the storage of necessary data (base sequence) to transcribe the peptides essential for the cell to function.184 On this model, organisms as we know them today, were preceded by metabolizing proto-cells based on proteins; which regulated themselves in order to realize a proto-phenotype with the ability to engage in purposeful behavior. Such cells could not replicate themselves, but divided due to stresses on the cell membrane. The crucial step to true life as we know it was to recruit nucleobases to encode peptide sequences in DNA, thus allowing true replication and evolution to more complex organisms to commence. It has long been known that even the most primitive of organisms are capable of purposeful behavior185-187 and that this can only be due to cellular proteins processing environmental information detected at the cell membrane.188 Enucleated cells commutate with nucleated cells, obtaining small molecules to correct the deficient cells,189 but are also able to survive up to several months190,191 and organize their circadian clocks without any DNA and gene expression.133 These considerations lend weight to the proposal that anesthetics act by disrupting the activity of proteins as enzymes,5 specifically the (mostly not understood) processes in protein information processing, perhaps with those associated with membranes.
Outlook
Although it is not clearly stated by most of the authors discussing consciousness, and some even claim that consciousness is an epiphenomenon, it is very obvious that consciousness is essential for survival and life in general.192,193 Anyone of us losing consciousness would not be able to survive a few days without the devoted assistance and help from our fellows. Similarly, life is not possible without recognizing danger via pain194 and other kinds of negative experiences safeguarding survival,195-197 all based on consciousness. It is obvious that consciousness is essential for any organism to have online access to their sensory information about their environments.193,198 Importantly, consciousness gives all organisms ability to act as agents of their own interest199,200 which is essential for their survival. This is essential for organisms to navigate successfully in complex environments that challenge their survival.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci 2004; 5:709-72; http://dx.doi.org/ 10.1038/nrn1496 [DOI] [PubMed] [Google Scholar]
- [2].Perouansky M. The quest for a unified model of anesthetic action. A century in Claude Bernard's shadow. Anesthesiology 2012; 117:465-74; http://dx.doi.org/ 10.1097/ALN.0b013e318264492e [DOI] [PubMed] [Google Scholar]
- [3].Rinaldi A. Reawakening anaesthesia research. EMBO Rep 2014; 15:1113-8; http://dx.doi.org/ 10.15252/embr.201439593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bancroft WD, Richter GH. Claude Bernard's theory of narcosis. Proc Natl Acad Sci USA 1930; 16:573-577; http://dx.doi.org/ 10.1073/pnas.16.9.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature 1984; 310:599-601; http://dx.doi.org/ 10.1038/310599a0 [DOI] [PubMed] [Google Scholar]
- [6].Hameroff SR. The entwined mysteries of anesthesia and consciousness: is there a common underlying mechanism? Anesthesiology 2006; 105:400-12; http://dx.doi.org/ 10.1097/00000542-200608000-00024 [DOI] [PubMed] [Google Scholar]
- [7].Hameroff SR, Craddock TJ, Tuszynski JA. Quantum effects in the understanding of consciousness. J Integr Neurosci 2014; 13:229-52; http://dx.doi.org/ 10.1142/S0219635214400093 [DOI] [PubMed] [Google Scholar]
- [8].Sonner JM. A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesth Analg 2008; 107:849-854; http://dx.doi.org/ 10.1213/ane.0b013e31817ee684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sonner JM, Cantor RS. Molecular mechanisms of drug action: an emerging view. Ann Rev Biophys 2013; 42:143-67; http://dx.doi.org/ 10.1146/annurev-biophys-083012-130341 [DOI] [PubMed] [Google Scholar]
- [10].Turin L, Skoulakis EMC, Horsfield AP. Electron spin changes during general anesthesia in Drosophila. Proc Natl Acad Sci USA 2014; 111:E3524-33; http://dx.doi.org/ 10.1073/pnas.1404387111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernard C. Lectures on the Phenomena of Life Common to Animals and Plants. Charles C Thomas Pub Ltd (June 1974); 1878. [Google Scholar]
- [12].Grémiaux A, Yokawa K, Mancuso S, Baluška F. Plant anesthesia supports similarities between animals and plants: Claude Bernard's forgotten studies. Plant Signal Behav 2014; 9:e27886; http://dx.doi.org/ 10.4161/psb.27886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lloyd FE. Some effects of narcotics on Spirogyra. Anesth Analg 1924; 3:9-19; http://dx.doi.org/ 10.1213/00000539-192402000-00003 [DOI] [Google Scholar]
- [14].White DC, Dundas CR. Effect of anaesthetics on emission of light by luminous bacteria. Nature 1970; 226:456-8; http://dx.doi.org/ 10.1038/226456a0 [DOI] [PubMed] [Google Scholar]
- [15].De Luccia TP. Mimosa pudica, Dionaea muscipula and anesthetics. Plant Signal Behav 2012; 7:1163-7; http://dx.doi.org/ 10.4161/psb.21000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hall GM, Kirtland SJ, Baum H. The inhibition of mitochondrial respiration by inhalational anaesthetic agents. Br J Anaesth 1973; 45:1005-9; http://dx.doi.org/ 10.1093/bja/45.10.1005 [DOI] [PubMed] [Google Scholar]
- [17].Cohen PJ. Effect of anesthetics on mitochondrial function. Anesthesiology 1973; 39:153-64; http://dx.doi.org/ 10.1097/00000542-197308000-00007 [DOI] [PubMed] [Google Scholar]
- [18].Brunner EA, Cheng SC, Berman ML. Effects of anesthesia on intermediary metabolism. Annu Rev Med 1975; 26:391-401; http://dx.doi.org/ 10.1146/annurev.me.26.020175.002135 [DOI] [PubMed] [Google Scholar]
- [19].Nahrwold ML, Cohen PJ. Anesthetics and mitochondrial respiration. Clin Anesth 1975; 11:25-44 [PubMed] [Google Scholar]
- [20].Nakao H, Ogli K, Yokono S, Ono J, Miyatake A. The effect of volatile anesthetics on light-induced phosphorylation in spinach chloroplasts. Toxicol Lett 1998; 100-101:135-8; http://dx.doi.org/ 10.1016/S0378-4274(98)00177-5 [DOI] [PubMed] [Google Scholar]
- [21].La Monaca E, Fodale V. Effects of anesthetics on mitochondrial signaling and function. Curr Drug Saf 2012; 7:126-39; http://dx.doi.org/ 10.2174/157488612802715681 [DOI] [PubMed] [Google Scholar]
- [22].Ingram LO. Adaptation of membrane lipids to alcohols. J Bacteriol 1976; 125:670-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt) 2008; 9:205-13; http://dx.doi.org/ 10.1089/sur.2007.036 [DOI] [PubMed] [Google Scholar]
- [24].Weng Y, Yang L, Corringer PJ, Sonner JM. Anesthetic sensitivity of theGloeobacter violaceus proton-gated ion channel. Anesth Analg 2010; 110:59-63; http://dx.doi.org/ 10.1213/ANE.0b013e3181c4bc69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bernard C. Leçons sur les anesthésiques et sur l'asphyxie. Librairie J-B Baillière et Fils. 1875 [Google Scholar]
- [26].Ewart AJ. On the Physics and Physiology of Protoplasmic Streaming in Plants. Oxford; 1903. [Google Scholar]
- [27].Osterhout WJV. Some aspects of protoplasmic motion. J Gen Physiol 1951; 35:519-27; http://dx.doi.org/ 10.1085/jgp.35.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garcia-Sierra F, Frixione E. Lidocaine, a local anesthetic, reversibly inhibits cytoplasmic streaming in Vallisneria mesophyll cells. Protoplasma 1993; 175:153-60; http://dx.doi.org/ 10.1007/BF01385014 [DOI] [Google Scholar]
- [29].Kiefer RT, Ploppa A, Krueger WA, Plank M, Nohé B, Haeberle HA, Unertl K, Dieterich HJ. Local anesthetics impair human granulocyte phagocytosis activity, oxidative burst, and CD11b expression in response toStaphylococcus aureus. Anesthesiology 2003; 98:842-8; http://dx.doi.org/ 10.1097/00000542-200304000-00009 [DOI] [PubMed] [Google Scholar]
- [30].Hameroff S. Why anesthetic mechanism research has failed, and what to do about it. http://anesth.medicine.arizona.edu/system/files/pdfs, Downloaded June 2015 [Google Scholar]
- [31].Sonner JM. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg 2002; 95:609-14 [DOI] [PubMed] [Google Scholar]
- [32].Brosnan RJ, Yang L, Milutinovic PS, Zhao J, Laster MJ, Eger EI 2nd, Sonner JM. Ammonia has anesthetic properties. Anesth Analg 2007; 104:430-3; http://dx.doi.org/ 10.1213/01.ane.0000264072.97705.0f [DOI] [PubMed] [Google Scholar]
- [33].Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI 2nd, Sonner JM. Anesthetic properties of the ketone bodies β-hydroxybutyric acid and acetone. Anesth Analg 2007; 105:673-9; http://dx.doi.org/ 10.1213/01.ane.0000278127.68312.dc [DOI] [PubMed] [Google Scholar]
- [34].Weng Y, Hsu TT, Zhao J, Nishimura S, Fuller GG, Sonner JM. Isovaleric, methylmalonic, and propionic acid decrease anesthetic EC50 in tadpoles, modulate glycine receptor function, and, interact with the lipid 1,2-dipalmitoyl-Sn-glycero-3-phosphocholine. Anesth Analg 2009; 108:1538-45; PMID:19372333; http://dx.doi.org/ 10.1213/ane.0b013e31819cd964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eger EI 2nd, Raines DE, Shafer SL, Hemmings HC Jr, Sonner JM. Is a new paradigm neededto explain how inhaled anesthetics produce immobility? Anesth Analg 2008; 107:832-48; http://dx.doi.org/ 10.1213/ane.0b013e318182aedb [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection, by volatile anesthetics. Anesthesiology 2004;100:707-21; PMID:15108989; http://dx.doi.org/ 10.1097/00000542-200403000-00035 [DOI] [PubMed] [Google Scholar]
- [37].De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg 2005; 100:1584-93; PMID:15920178; http://dx.doi.org/ 10.1213/01.ANE.0000153483.61170.0C [DOI] [PubMed] [Google Scholar]
- [38].Agarwal B, Stowe DF, Dash RK, Bosnjak ZJ, Camara AK. Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol 2014; 5:341; PMID:25278902; http://dx.doi.org/ 10.3389/fphys.2014.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Keller C, Grimm C, Wenzel A, Hafezi F, Remé C. Protective effect of halothane anesthesiaon retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest Ophthalmol Vis Sci 2001; 42:476-80 [PubMed] [Google Scholar]
- [40].Stevenson GW, Hall S, Rudnick SJ, Alvord G, Rossio J, Urban W, Leventhal JB, Miller P, Seleny F, Stevenson HC. Halothane anesthesia decreases human monocyte hydrogen peroxide generation. Protection of monocytes by activation with gamma interferon. Immunopharmacol Immunotoxicol 1987; 9:489-510; PMID:3125239; http://dx.doi.org/ 10.3109/08923978709035228 [DOI] [PubMed] [Google Scholar]
- [41].Barodka VM, Acheampong E, Powell G, Lobach L, Logan DA, Parveen Z, Armstead V, Mukhtar M. Antimicrobial effects of liquid anesthetic isoflurane on Candida albicans. J Transl Med 2006; 4:46; PMID:17094810; http://dx.doi.org/ 10.1186/1479-5876-4-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Manley SL. Phytogenesis of halomethanes: a production of selection or a metabolic accident? Biogeochemistry 2002; 60:163-80; http://dx.doi.org/ 10.1023/A:1019859922489 [DOI] [Google Scholar]
- [43].Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN. Biogenic volatile organic compounds in the Earth system. New Phytol 2009; 183:27-51; PMID:19422541; http://dx.doi.org/ 10.1111/j.1469-8137.2009.02859.x [DOI] [PubMed] [Google Scholar]
- [44].Redeker KR, Wang N, Low JC, McMillan A, Tyler SC, Cicerone RJ. Emissions of methyl halides and methane from rice paddies. Science 2000; 290:966-9; PMID:11062125; http://dx.doi.org/ 10.1126/science.290.5493.966 [DOI] [PubMed] [Google Scholar]
- [45].Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 2010; 15:176-84; PMID:20144557; http://dx.doi.org/ 10.1016/j.tplants.2010.01.006 [DOI] [PubMed] [Google Scholar]
- [46].Loreto F, Schnitzler JP. 2010. Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154-66; PMID:20133178; http://dx.doi.org/ 10.1016/j.tplants.2009.12.006 [DOI] [PubMed] [Google Scholar]
- [47].Peñuelas J, Staudt M. BVOCs and global change. Trends Plant Sci 2010; 15:133-144; http://dx.doi.org/ 10.1016/j.tplants.2009.12.005 [DOI] [PubMed] [Google Scholar]
- [48].Kegge W, Pierik R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci 2010; 15:126-32; PMID:20036599; http://dx.doi.org/ 10.1016/j.tplants.2009.11.007 [DOI] [PubMed] [Google Scholar]
- [49].Fang Z, Ionescu P, Chortkoff BS, Kandel L, Sonner J, Laster MJ, Eger EI 2nd. Anestheticpotencies of n-alkanols: results of additivity and solubility studies suggest a mechanism of action similar to that for conventional inhaled anesthetics. Anesth Analg 1997; 84:1042-8; PMID:9141929; http://dx.doi.org/ 10.1213/00000539-199705000-00017 [DOI] [PubMed] [Google Scholar]
- [50].Hau KM, Connell DW, Richardson BJ. A study of the biological partitioning behavior of n-alkanes and n-alkanols in causing anesthetic effects. Regul Toxicol Pharmacol 2002; 35:273-9; http://dx.doi.org/ 10.1006/rtph.2001.1531 [DOI] [PubMed] [Google Scholar]
- [51].Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 2013; 368:20130122; PMID:23713120; http://dx.doi.org/ 10.1098/rstb.2013.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Könneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T, Schada von Borzyskowski L, Erb TJ, Stahl DA, Berg IA. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc Natl Acad Sci USA 2014; 111:8239-44; PMID:24843170; http://dx.doi.org/ 10.1073/pnas.1402028111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hu HW, Chen D, He JZ. Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 2015; 39:729-49 [DOI] [PubMed] [Google Scholar]
- [54].Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Curr Opin Plant Biol 2002, 5:230-6; PMID:11960741; http://dx.doi.org/ 10.1016/S1369-5266(02)00250-9 [DOI] [PubMed] [Google Scholar]
- [55].Chechetkin IR, Mukhitova FK, Blufard AS, Yarin AY, Antsygina LL, Grechkin AN. Unprecedented pathogen-inducible complex oxylipins from flax linolipins A and B. FEBS J 2009; 276:4463-72; PMID:19645727; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07153.x [DOI] [PubMed] [Google Scholar]
- [56].Wang F, Cui X, Sun Y, Dong CH. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep 2013; 32:1099-109; PMID:23525746; http://dx.doi.org/ 10.1007/s00299-013-1421-6 [DOI] [PubMed] [Google Scholar]
- [57].Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 2015; 20:219-29; PMID:25731753; http://dx.doi.org/ 10.1016/j.tplants.2015.02.001 [DOI] [PubMed] [Google Scholar]
- [58].Calixto JB, Beirith A, Ferreira J, Santos AR, Filho VC, Yunes RA. Naturally occurring antinociceptive substances from plants. Phytother Res 2000; 14:401-18; PMID:10960893; http://dx.doi.org/ 10.1002/1099-1573(200009)14:6%3c401::AID-PTR762%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- [59].Askitopoulou H, Ramoutsa IA, Konsolaki E. Analgesia and anesthesia: etymology and literary history of related greek words. Anesth Analg 2000; 91:486-91; PMID:10910873 [DOI] [PubMed] [Google Scholar]
- [60].Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr 2011; 2:32-50; PMID:22211188; http://dx.doi.org/ 10.3945/an.110.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kennedy DO. Plants and the Human Brain. Oxford University Press, 2014 [Google Scholar]
- [62].Meotti FC, Lemos de Andrade E, Calixto JB. TRP modulation by natural compounds. Handb Exp Pharmacol 2014; 223:1177-238; PMID:24961985; http://dx.doi.org/ 10.1007/978-3-319-05161-1_19 [DOI] [PubMed] [Google Scholar]
- [63].Dolferus R, Jacobs M, Peacock WJ, Dennis ES. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol 1994; 105:1075-87; PMID:7972489; http://dx.doi.org/ 10.1104/pp.105.4.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Strommer J. The plant ADH gene family. Plant J 2011; 66:128-42; PMID:21443628; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04458.x [DOI] [PubMed] [Google Scholar]
- [65].Taylorson RB, Hendricks SB. Overcoming dormancy, in seeds with ethanol and other anesthetics. Planta 1979; 145:507-10; PMID:24317868 [DOI] [PubMed] [Google Scholar]
- [66].Dillard MM. Ethylene – the new general anesthetic. J Natl Med Assoc 1930; 22:10-11; PMID:20892343 [PMC free article] [PubMed] [Google Scholar]
- [67].Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. New Engl J Med 2003; 348:2110-24; PMID:12761368; http://dx.doi.org/ 10.1056/NEJMra021261 [DOI] [PubMed] [Google Scholar]
- [68].Kopp Lugli A, Yost CS, Kindler CH. Anaesthetic mechanisms: update on the challenge of unravelling the mystery of anaesthesia. Eur J Anaesthesiol 2009; 26:807-20; PMID:19494779; http://dx.doi.org/ 10.1097/EJA.0b013e32832d6b0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Freebairn HT, Buddendenhagen IW. Ethylene production by Pseudomonas solanacearum. Nature 1964; 202:313-4; PMID:14167811; http://dx.doi.org/ 10.1038/202313a0 [DOI] [PubMed] [Google Scholar]
- [70].Smith AM. Ethylene as a cause of soil fungistasis. Nature 1973; 246:311-3; PMID:4586317; http://dx.doi.org/ 10.1038/246311a0 [DOI] [PubMed] [Google Scholar]
- [71].Lynch JM, Harper SHT. Formation of ethylene by a soil fungus. J Gen Microbiol 1974; 80:187-95; http://dx.doi.org/ 10.1099/00221287-80-1-187 [DOI] [PubMed] [Google Scholar]
- [72].Smith AM, James Cook R. Implications of ethylene production by bacteria for biological balance of soil. Nature 1974; 252:703-5; PMID:4437621; http://dx.doi.org/ 10.1038/252703b04437621 [DOI] [Google Scholar]
- [73].Primrose SB, Dilworth MJ. Ethylene production by bacteria. J Gen Microbiol 1976; 93:177-81; PMID:772166; http://dx.doi.org/ 10.1099/00221287-93-1-177 [DOI] [PubMed] [Google Scholar]
- [74].Considine PJ, Flynn N, Patching JW. Ethylene production by soil microorganisms. Appl Environ Microbiol 1977; 33:977-9; PMID:869541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Graham JH, Linderman RG. Ethylene production by ectomycorrhizal fungi, Fusarium oxysporum f. sp. pini, and by aseptically synthesized ectomycorrhizae and Fusarium-infected Douglas-fir roots. Can J Microbiol 1980; 26:1340-7; PMID:7214223; http://dx.doi.org/ 10.1139/m80-222 [DOI] [PubMed] [Google Scholar]
- [76].Huang TC, Chow TJ. Ethylene production by blue-green algae. Bot Bull Acad Sinica 1984; 25:81-6 [Google Scholar]
- [77].Kauppi N, Kauppi A, Garty J. Ethylene produced by the lichen Cladina stellaris exposed to sulphur and heavy metal-containing solutions under acidic conditions. New Phytol 1998; 139:537-47; http://dx.doi.org/ 10.1046/j.1469-8137.1998.00214.x [DOI] [Google Scholar]
- [78].Chagué V, Elad Y, Barakat R, Tudzynski P, Sharon A. Ethylene biosynthesis inBotrytis cinerea. FEMS Microbiol Ecol 2002; 40:143-9; http://dx.doi.org/ 10.1111/j.1574-6941.2002.tb00946.x [DOI] [PubMed] [Google Scholar]
- [79].Plettner I, Steinke M, Malin G. Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environm 2005; 28:1136-45; http://dx.doi.org/ 10.1111/j.1365-3040.2005.01351.x [DOI] [Google Scholar]
- [80].Quadir A, Hewett EW, Long PG, Dilley DR. A non-ACC pathway for ethylene biosynthesis in Botrytis cinerea. Postharv Biol Technol 2011; 62:314-8; http://dx.doi.org/ 10.1016/j.postharvbio.2011.06.003 [DOI] [Google Scholar]
- [81].Whalen FX, Bacon DR, Smith HM. Inhaled anesthetics: an historical overview. Best Pract Res Clin Anaesthesiol 2005; 19:323-30; PMID:16013684; http://dx.doi.org/ 10.1016/j.bpa.2005.02.001 [DOI] [PubMed] [Google Scholar]
- [82].Mazurek MJ. Dr. Chauncey Leake and the development of divinyl oxide from bench to bedside. CSA Bull 2007; 86-9 [Google Scholar]
- [83].Finer B. Divinyl ether. Brit J Anaesth 1965; 37:661-6; PMID:5320087; http://dx.doi.org/ 10.1093/bja/37.9.661 [DOI] [PubMed] [Google Scholar]
- [84].Itoh A, Howe GA. Molecular cloning of a divinyl ether synthase. J Biol Chem 2001; 276:3620-27; PMID:11060314; http://dx.doi.org/ 10.1074/jbc.M008964200 [DOI] [PubMed] [Google Scholar]
- [85].Stumpe M, Carsjens JG, Göbel C, Feussner I. Divinyl ether synthesis in garlic bulbs. J Exp Bot 2008; 59:907-15; PMID:18326559; http://dx.doi.org/ 10.1093/jxb/ern010 [DOI] [PubMed] [Google Scholar]
- [86].Fammartino A, Verdaguer B, Fournier J, Tamietti G, Carbonne F, Esquerré-Tugayé MT, Cardinale F. Coordinated transcriptional regulation of the divinyl ether biosynthetic genes in tobacco by signal molecules related to defense. Plant Physiol Biochem 2010; 48:225-31; PMID:20137961; http://dx.doi.org/ 10.1016/j.plaphy.2010.01.012 [DOI] [PubMed] [Google Scholar]
- [87].Fammartino A, Cardinale F, Göbel C, Mène-, Saffrané L, Fournier J, Feussner I, Esquerré-Tugayé MT. Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol 2007; 143:378-88; PMID:17085514; http://dx.doi.org/ 10.1104/pp.106.087304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dasta J. Local anesthetics: evolving to a new standard of care. Pharm Pract New Spec Rep 2013; January:1-8. [Google Scholar]
- [89].Golembiewski J, Dasta J. Evolving role of local anesthetics in managing postsurgical analgesia. Clin Therap 2015; 37:1354-1371; http://dx.doi.org/ 10.1016/j.clinthera.2015.03.017 [DOI] [PubMed] [Google Scholar]
- [90].Behçet Al. The source-synthesis: history and use of atropine. J Acad Emerg Med 2014; 13:2-3; http://dx.doi.org/ 10.5152/jaem.2014.1120141 [DOI] [Google Scholar]
- [91].Façanha MF, Gomes LC. Efficacy of menthol as an anesthetic for tambaqui (Colossoma macropomum, Characiformes: Characidae). Acta Amazon 2005; 35:71-5; http://dx.doi.org/ 10.1590/S0044-59672005000100011 [DOI] [Google Scholar]
- [92].Watt EE, Betts BA, Kotey FO, Humbert DJ, Griffith TN, Kelly EW, Veneskey KC, Gill N, Rowan KC, Jenkins A, Hall AC. Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. Eur J Pharmacol 2008; 590:120-6; PMID:18593637; http://dx.doi.org/ 10.1016/j.ejphar.2008.06.003 [DOI] [PubMed] [Google Scholar]
- [93].Ghelardini C, Galeotti N, Mazzanti G. Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Med 2001; 67:564-6; PMID:11509984; http://dx.doi.org/ 10.1055/s-2001-16475 [DOI] [PubMed] [Google Scholar]
- [94].de Lima Silva L, Thomas da Silva D, Garlet QI, Cunha MA, Mallmann CA, Baldisserotto B, Longhi SJ, Soares Pereira AM, Heinzmann BA. Anesthetic activity of Brazilian native plants in silver catfish (Rhamdia quelen). Neotrop Ichthyol 2013; 11:443-51; http://dx.doi.org/ 10.1590/S1679-62252013000200014 [DOI] [Google Scholar]
- [95].Burton RR. G-induced loss of consciousness: definition, history, current status. Aviat Space Environ Med 1988; 59:2-5; PMID:3281645 [PubMed] [Google Scholar]
- [96].Johanson DC, Pheeny HT. A new look at the loss of consciousness experience within the US. Naval forces. Aviat Space Environ Med 1988; 59:6-8; PMID:3355469 [PubMed] [Google Scholar]
- [97].Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res 2005; 15:126-129; PMID:15834770; http://dx.doi.org/ 10.1007/s10286-005-0244-0 [DOI] [PubMed] [Google Scholar]
- [98].Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atrial Fibril 2014; 7:97-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Rose da Silva MFL. Syncope: epidemiology, etiology, and prognosis. Front Physiol 2014; 5:4; PMID:24478717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Palmer LK, Rannels SL, Kimball SR, Jefferson LS, Keil RL. Inhibition of mammalian translation initiation by volatile anesthetics. Am J Physiol Endocrinol Metab 2006; 290:E1267-75; PMID:16434554; http://dx.doi.org/ 10.1152/ajpendo.00463.2005 [DOI] [PubMed] [Google Scholar]
- [101].Uesono Y. Environmental stresses and clinical drugs paralyze a cell. Commun Integr Biol 2009; 2:275-8; PMID:19641750; http://dx.doi.org/ 10.4161/cib.2.3.8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Uesono Y, Toh EA, Kikuchi Y, Terashima I. Structural analysis of compounds with actions similar to local anesthetics and antipsychotic phenothiazines in yeast. Yeast 2011; 28:391-404; PMID:21374719; http://dx.doi.org/ 10.1002/yea.1846 [DOI] [PubMed] [Google Scholar]
- [103].Safford WE. An Aztec narcotic. J Hered 1915; 6:291-311 [Google Scholar]
- [104].Osmond H. Ololiuqui: The ancient Aztec narcotic. J Men Dis 1955; 101:526-37 [DOI] [PubMed] [Google Scholar]
- [105].Spiller HA, Hale JR, De Boer JZ. The Delphic Oracle: A multidisciplinary defense of the gaseous vent theory. Clin Toxicol 2002; 40:189-96 [DOI] [PubMed] [Google Scholar]
- [106].Icaza EE, Mashour GA. Altered states. Psychedelics and anesthetics. Anesthesiology 2013; 119:1255-60; PMID:24061599; http://dx.doi.org/ 10.1097/01.anes.0000435635.42332.ee [DOI] [PubMed] [Google Scholar]
- [107].Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr Comp Biol 2004; 44:315-23; PMID:21676715; http://dx.doi.org/ 10.1093/icb/44.4.315 [DOI] [PubMed] [Google Scholar]
- [108].McGovern PE, Mirzoian A, Hall GR. Ancient Egyptian herbal wines. Proc Natl Acad Sci USA 2013; 106:7361-6; http://dx.doi.org/ 10.1073/pnas.0811578106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Carrigan MA, Uryasev O, Frye CB, Eckman BL, Myers CR, Hurley TD, Benner SA. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc Natl Acad Sci USA 2015; 112:458-63; PMID:25453080; http://dx.doi.org/ 10.1073/pnas.1404167111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hockings KJ, Bryson-Morrison N, Carvalho S, Fujisawa M, Humle T, McGrew WC, Nakamura M, Ohashi G, Yamanashi Y, Yamakoshi G, et al.. Tools to tipple: ethanol ingestion by wild chimpanzees using leaf-sponges. R Soc Open Sci 2015; 2:150150; PMID:26543588; http://dx.doi.org/ 10.1098/rsos.150150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wiens F, Zitzmann A, Lachance MA, Yegles M, Pragst F, Wurst FM, von Holst D, Guan SL, Spanagel R. Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci USA 2008; 105:10426-31; PMID:18663222; http://dx.doi.org/ 10.1073/pnas.0801628105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Devineni AV, Heberlein U. Addiction-like behavior in Drosophila. Commun Integr Biol 2010; 3:357-9; PMID:20798826; http://dx.doi.org/ 10.4161/cib.3.4.11885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science 2012; 335:1351-5; PMID:22422983; http://dx.doi.org/ 10.1126/science.1215932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Peru Y, Colón de Portugal RL, Ojelade SA, Penninti PS, Dove RJ, Nye MJ, Acevedo SF, Lopez A, Rodan AR, Rothenfluh A. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict Biol 2014; 19:392-401; PMID:24164972; http://dx.doi.org/ 10.1111/adb.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 2005; 103:1268-95; PMID:16306742; http://dx.doi.org/ 10.1097/00000542-200512000-00024 [DOI] [PubMed] [Google Scholar]
- [116].Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science 2008; 322:876-80; PMID:18988836; http://dx.doi.org/; http://dx.doi.org/ 10.1126/science.1149213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hutt A. Sleep and anesthesia. Front Neurosci 2009; 3:408-9 [Google Scholar]
- [118].Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 2008; 9:370-86; PMID:18425091; http://dx.doi.org/ 10.1038/nrn2372 [DOI] [PubMed] [Google Scholar]
- [119].Franks NP, Zecharia AY. Sleep and general anesthesia. Can J Anaesth 2011; 58:139-48; PMID:21170623; http://dx.doi.org/ 10.1007/s12630-010-9420-3 [DOI] [PubMed] [Google Scholar]
- [120].McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci 2003; 23:9687-95; PMID:14573548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Walker MP. The role of sleep in cognition and emotion. Ann NY Acad Sci 2009; 1156:168-97; PMID:19338508; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- [122].Rasch B, Born J. About sleep's role in memory. Physiol Rev 2013; 93:681-766; PMID:23589831; http://dx.doi.org/ 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci 2009; 10:803-13; PMID:19794431 [DOI] [PubMed] [Google Scholar]
- [124].Choi S, Yu E, Lee S, Llinás RR. Altered thalamocortical rhythmicity and connectivity in mice lacking CaV3.1 T-type Ca2+ channels in unconsciousness. Proc Natl Acad Sci USA 2015; pii: 201420983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kottler B, Bao H, Zalucki O, Imlach W, Troup M, van Alphen B, Paulk A, Zhang B, van Swinderen B. A sleep/wake circuit controls isoflurane sensitivity in Drosophila. Curr Biol 2013; 23:594-8; PMID:23499534; http://dx.doi.org/; http://dx.doi.org/ 10.1016/j.cub.2013.02.021 [DOI] [PubMed] [Google Scholar]
- [126].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000; 288:1769-72; PMID:10846154; http://dx.doi.org/ 10.1126/science.288.5472.1769 [DOI] [PubMed] [Google Scholar]
- [127].Wager TD, Atlas LY. How Is Pain influenced by cognition? Neuroimaging weighs in. Persp Psych Sci 2013; 8:91-7; http://dx.doi.org/ 10.1177/1745691612469631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zeidan F, Martucci KT, Kraft RA, McHaffie JG, Coghill RC. Neural correlates of mindfulness meditation-related anxiety relief. Soc Cogn Affect Neurosci 2014; 9:751-9; PMID:23615765; http://dx.doi.org/ 10.1093/scan/nst041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci USA 2012; 109:15959-64; PMID:23019380; http://dx.doi.org/ 10.1073/pnas.1202056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jensen K, Kirsch I, Odmalm S, Kaptchuk TJ, Ingvar M. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci USA 2015; 112:7863-7; http://dx.doi.org/ 10.1073/pnas.1504567112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Price DD. Unconscious and conscious mediation of analgesia and hyperalgesia. Proc Natl Acad Sci USA 2015; 112:7624-5; PMID:26056258; http://dx.doi.org/ 10.1073/pnas.1508765112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010; 363:2638-50; PMID:21190458; http://dx.doi.org/ 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature 2011; 469:498-503; PMID:21270888; http://dx.doi.org/ 10.1038/nature09702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 2005; 308:414-15; PMID:15831759; http://dx.doi.org/ 10.1126/science.1108451 [DOI] [PubMed] [Google Scholar]
- [135].Eger EI, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 1965; 26:756-63; PMID:5844267; http://dx.doi.org/ 10.1097/00000542-196511000-00010 [DOI] [PubMed] [Google Scholar]
- [136].Cook ND. The neuron-level phenomena underlying cognition and consciousness: synaptic activity and the action potential. Neuroscience 2008; 153:556-70; PMID:18406536; http://dx.doi.org/ 10.1016/j.neuroscience.2008.02.042 [DOI] [PubMed] [Google Scholar]
- [137].Cook ND, Carvalho GB, Damasio A. From membrane excitability to metazoan psychology. Trends Neurosci 2014; 37:698-705; PMID:25176475; http://dx.doi.org/ 10.1016/j.tins.2014.07.011 [DOI] [PubMed] [Google Scholar]
- [138].Llinás RR. Intrinsic electrical properties of mammalian neurons and CNS function: a historical perspective. Front Cell Neurosci 2014; 8:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Baluška F, Wan YL. Physical control over endocytosis In: Endocytosis in Plants. Šamaj J. (ed), Springer Verlag; 2012. [Google Scholar]
- [140].Wayne R. Excitability in plant cells. Am Sci 1993; 81:140-51. [Google Scholar]
- [141].Wayne R. The excitability of plant cells: With a special emphasis on Characean internodal cells. Bot Rev 1994; 60:265-7; PMID:11539934; http://dx.doi.org/ 10.1007/BF02960261 [DOI] [PubMed] [Google Scholar]
- [142].Beilby MJ. Action potential in Charophytes. Int Rev Cytol 2007; 257:43-83; PMID:17280895; http://dx.doi.org/ 10.1016/S0074-7696(07)57002-6 [DOI] [PubMed] [Google Scholar]
- [143].Masi E, Ciszak M, Stefano G, Renna L, Azzarello E, Pandolfi C, Mugnai S, Baluška F, Arecchi FT, Mancuso S. Spatio-temporal dynamics of the electrical network activity in the root apex. Proc Natl Acad Sci USA 2009; 106:4048-53; PMID:19234119; http://dx.doi.org/ 10.1073/pnas.0804640106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hedrich R. Ion channels in plants. Physiol Rev 2012; 92:1777-811; PMID:23073631; http://dx.doi.org/ 10.1152/physrev.00038.2011 [DOI] [PubMed] [Google Scholar]
- [145].Król E, Dziubinska H, Trębacz K. What do plants need action potentials for? In:Action Potential: Biophysical and Cellular Context, Initiation, Phases and Propagation. DuBois ML. (ed), 2010; 1-26 [Google Scholar]
- [146].Volkov A. Plant Electrophysiology: Signaling and Responses. Springer Verlag; 2012. [Google Scholar]
- [147].Baluška F, Mancuso S. Root apex transition zone as oscillatory zone. Front Plant Sci 2013; 4:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Goldsworthy A. The evolution of plant action potentials. J Theor Biol 1983; 103:645-8; http://dx.doi.org/ 10.1016/0022-5193(83)90287-4 [DOI] [Google Scholar]
- [149].Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular, mechanism similar to neurotransmitter release. Science 1994; 263:390-3; PMID:7904084; http://dx.doi.org/ 10.1126/science.7904084 [DOI] [PubMed] [Google Scholar]
- [150].Andrews NW, Chakrabarti S. There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol 2005; 15:626-31; PMID:16168654; http://dx.doi.org/ 10.1016/j.tcb.2005.09.001 [DOI] [PubMed] [Google Scholar]
- [151].Fu D, Vissavajjhala P, Hemmings HC Jr. Volatile anaesthetic effects on phospholipid binding to synaptotagmin 1, a presynaptic Ca2+ sensor. Br J Anaesth 2005; 95:216-21; PMID:15923266; http://dx.doi.org/ 10.1093/bja/aei163 [DOI] [PubMed] [Google Scholar]
- [152].Schapire AL, Valpuesta V, Botella MA. Plasma membrane repair in plants. Trends Plant Sci 2009; 14:645-52; PMID:19819752; http://dx.doi.org/ 10.1016/j.tplants.2009.09.004 [DOI] [PubMed] [Google Scholar]
- [153].Schapire AL, Voigt B, Jasik J, Rosado A, Lopez-Cobollo R, Menzel D, Salinas J, Mancuso S, Valpuesta V, Baluška F, Botella MA. Arabidopsis synaptotagmin 1 is required for themaintenance of plasma membrane integrity and cell viability. Plant Cell 2009; 20:3374-88; http://dx.doi.org/ 10.1105/tpc.108.063859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic 2005; 6:725-40; PMID:16101677; http://dx.doi.org/ 10.1111/j.1600-0854.2005.00318.x [DOI] [PubMed] [Google Scholar]
- [155].Pristerá A, Okuse K. Building excitable membranes: lipid rafts and multiple controls on trafficking of electrogenic molecules. Neuroscientist 2012; 18:70-81; http://dx.doi.org/ 10.1177/1073858410393977 [DOI] [PubMed] [Google Scholar]
- [156].Zhao X, Li R, Lu C, Baluška F, Wan Y. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiol Biochem 2015; 87:53-60; PMID:25549979; http://dx.doi.org/ 10.1016/j.plaphy.2014.12.015 [DOI] [PubMed] [Google Scholar]
- [157].Baluška F, Mancuso S. Synaptic view of eukaryotic cell. Int J Gen Syst 2014; 43:740-56; http://dx.doi.org/ 10.1080/03081079.2014.920999 [DOI] [Google Scholar]
- [158].Peltier JB, Ytterberg AJ, Sun Q, van Wijk KJ. New functions of the thylakoid membrane proteome of Arabidopsis thaliana revealed by a simple, fast, and versatile fractionation strategy. J Biol Chem 2004; 279:49367-83; PMID:15322131; http://dx.doi.org/ 10.1074/jbc.M406763200 [DOI] [PubMed] [Google Scholar]
- [159].Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. J Cell Sci 2012; 125:49-58; PMID:22250200; http://dx.doi.org/ 10.1242/jcs.085118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pérez-Lara A, Jahn R. Extended synaptotagmins (E-Syts): architecture and dynamics of membrane contact sites revealed. Proc Natl Acad Sci USA 2015; 112:4837-8; http://dx.doi.org/; http://dx.doi.org/ 10.1073/pnas.1504487112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Pérez-Sancho J, Vanneste S, Lee E, McFarlane HE, Esteban Del Valle A, Valpuesta V, Friml J, Botella MA, Rosado A. The Arabidopsis Synaptotagmin1 is enriched in endoplasmic reticulum-plasma membrane contact sites and confers cellular resistance to mechanical stresses. Plant Physiol 2015; 168:132-43; http://dx.doi.org/ 10.1104/pp.15.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mustardy L, Garab G. Granum revisited. A three-dimensional model – where things fall into place. Trends Plant Sci 2003; 8: 117-22; PMID:12663221; http://dx.doi.org/ 10.1016/S1360-1385(03)00015-3 [DOI] [PubMed] [Google Scholar]
- [163].Kim EH, Chow WS, Horton P, Anderson JM. Entropy-assisted stacking of thylakoid membranes. Biochim Biophys Acta 2005; 1708:187-95; PMID:15953475; http://dx.doi.org/ 10.1016/j.bbabio.2005.03.011 [DOI] [PubMed] [Google Scholar]
- [164].Iwasa K, Tasaki I. Mechanical changes in squid giantaxons associated with production of action potentials. Biochem Biophys Research Comm 1980; 95:1328-31; http://dx.doi.org/ 10.1016/0006-291X(80)91619-8 [DOI] [PubMed] [Google Scholar]
- [165].Iwasa K, Tasaki I, Gibbons RC. Swelling of nerve fibres associated with action potentials. Science 1980; 210:338-9; PMID:7423196; http://dx.doi.org/ 10.1126/science.7423196 [DOI] [PubMed] [Google Scholar]
- [166].Terakawa S. Changes in intracellular pressure in squid giant axons associated with production of action potentials. Biochem Biophys Res Commun 1983; 114:1006-10; PMID:6615499; http://dx.doi.org/ 10.1016/0006-291X(83)90661-7 [DOI] [PubMed] [Google Scholar]
- [167].Heimburg T. Nerves and anaesthesia: A physics perspective on medicine. Anest Ratown 2014; 8:252-63 [Google Scholar]
- [168].Abbott BC, Hill AV, Howarth JV. The positive and negative heat production associated with a nerve impulse. Proc Roy Soc London B 1958; 148:149-87; http://dx.doi.org/ 10.1098/rspb.1958.0012 [DOI] [PubMed] [Google Scholar]
- [169].Ritchie JM, Keynes RD. The production and absorption of heat associated with electrical activity in nerve and electric organ. Q Rev Biophys 1985; 18:451-76; PMID:3916342; http://dx.doi.org/ 10.1017/S0033583500005382 [DOI] [PubMed] [Google Scholar]
- [170].Tasaki I, Byrne PM. Heat production associated with synaptic transmission in the bullfrog spinal cord. Brain Res 1987; 407:386-9; PMID:3032367; http://dx.doi.org/ 10.1016/0006-8993(87)91119-X [DOI] [PubMed] [Google Scholar]
- [171].Ueda I, Yoshida T. Hydration of lipid membranes and the action mechanisms of anesthetics and alcohols. Chem Phys Lip 1999; 101:65-79; http://dx.doi.org/ 10.1016/S0009-3084(99)00056-0 [DOI] [PubMed] [Google Scholar]
- [172].Papahadjopoulos D, Jacobson K, Poste G, Shepherd G. Effects of local anesthetics on membrane properties. I. Changes in the fluidity of phospholipid bilayers. Biochim Biophys Acta 1975; 394: 504-19; PMID:1148230; http://dx.doi.org/ 10.1016/0005-2736(75)90137-6 [DOI] [PubMed] [Google Scholar]
- [173].Zapata-Morin PA, Sierra-Valdez FJ, Ruiz-Suárez JC. The interaction of local anesthetics with lipid membranes. J Mol Graph Model 2014; 53:200-5; PMID:25181454; http://dx.doi.org/ 10.1016/j.jmgm.2014.08.001 [DOI] [PubMed] [Google Scholar]
- [174].Cherkin A, Catchpool JF. Temperature dependence of anesthesia in goldfish. Science 1964; 144:1460-2; PMID:14171540; http://dx.doi.org/ 10.1126/science.144.3625.1460 [DOI] [PubMed] [Google Scholar]
- [175].Martin BJ. Evaluation of hypothermia for anesthesia in reptiles and amphibians. ILAR J 1995; 37:186-90; PMID:11528038; http://dx.doi.org/ 10.1093/ilar.37.4.186 [DOI] [PubMed] [Google Scholar]
- [176].Franks NP, Lieb WR. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology 1996; 84:716-20; PMID:8659800; http://dx.doi.org/ 10.1097/00000542-199603000-00027 [DOI] [PubMed] [Google Scholar]
- [177].Yamamoto E, Akimoto T, Shimizu H, Hirano Y, Yasui M, Yasuoka K. Diffusive natureof xenon anesthetic changes properties of a lipid bilayer: molecular dynamics simulations. J Phys Chem B 2012; 116:8989-95; PMID:22715916; http://dx.doi.org/ 10.1021/jp303330c [DOI] [PubMed] [Google Scholar]
- [178].Booker RD, Sum AK. Biophysical changes induced by xenon on phospholipid bilayers. Biochim Biophys Acta 2013; 1828:1347-56; PMID:23376329; http://dx.doi.org/ 10.1016/j.bbamem.2013.01.016 [DOI] [PubMed] [Google Scholar]
- [179].Johnson FH, Flagler EA. Hydrostatic pressure reversal of narcosis in tadpoles. Science 1950; 112:91-2; PMID:15442245; http://dx.doi.org/ 10.1126/science.112.2899.91-a [DOI] [PubMed] [Google Scholar]
- [180].Lundbaek JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface 2010; 7:373-95; PMID:19940001; http://dx.doi.org/ 10.1098/rsif.2009.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Heimburg T, Jackson AD. The thermodynamics of general anesthesia. Biophys J 2007; 92:3159-65; PMID:17293400; http://dx.doi.org/ 10.1529/biophysj.106.099754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Graesbøll K, Sasse-Middelhoff H, Heimburg T. The thermodynamics of general and local anesthesia. Biophys J 2014; 106:2143-56; PMID:24853743; http://dx.doi.org/ 10.1016/j.bpj.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Schrödinger E. What Is Life? The Physical Aspect of the Living Cell. Based on lectures delivered under the auspices of the Dublin Institute for Advanced Studies at Trinity College, Dublin, in February 1943. Cambridge University Press; 1944. [Google Scholar]
- [184].Annila A, Baverstock K. Genes without prominence: a reappraisal of the foundations of biology. J R Soc Interface 2014; 11:20131017; PMID:24554573; http://dx.doi.org/ 10.1098/rsif.2013.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Ben Jacob E, Becker I, Shapira Y, Levine H. Bacterial linguistic communication and social intelligence. Trends Microbiol 2004; 12:366-72; PMID:15276612; http://dx.doi.org/ 10.1016/j.tim.2004.06.006 [DOI] [PubMed] [Google Scholar]
- [186].Ben Jacob E, Shapira Y, Tauber AI. Seeking the foundations of cognition in bacteria: from Schrödinger's negative entropy to latent information. Physica A 2006; 359:495-524. [Google Scholar]
- [187].Ben-Jacob E, Lu M, Schultz D, Onuchic JN. The physics of bacterial decision making. Front Cell Infect Microbiol 2014; 4:154; PMID:25401094; http://dx.doi.org/ 10.3389/fcimb.2014.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Bray D. Wetware: a Computer in Every Living Cell. 2009; New Haven; London: Yale University Press. [Google Scholar]
- [189].Cox RP, Krauss MR, Balis ME, Dancis J. Studies on cell communication with enucleated human fibroblasts. J Cell Biol 1976; 71:693-703; PMID:993266; http://dx.doi.org/ 10.1083/jcb.71.3.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Hämmerling J. Nucleo-cytoplasmic interactions in Acetabularia and other cells. Ann Rev Plant Physiol 1963; 14:65-92; http://dx.doi.org/ 10.1146/annurev.pp.14.060163.000433 [DOI] [Google Scholar]
- [191].Chapman CJ, Nugent NA, Schreiber RW. Nucleic acid synthesis in the chloroplasts of Acetabularia mediterranea. Plant Physiol 1966; 41:589-92; PMID:5932403; http://dx.doi.org/ 10.1104/pp.41.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Nagel T. Mind and Cosmos: Why the Materialist Neo-Darwinian Conception of Nature is Almost Certainly False. Oxford University Press; 2012. [Google Scholar]
- [193].Searle J. Theory of mind and Darwin's legacy. Proc Natl Acad Sci USA 2013; 110: 10343-8; PMID:23754416; http://dx.doi.org/ 10.1073/pnas.1301214110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Bastian B, Jetten J, Hornsey MJ, Leknes S. The positive consequences of pain: a biopsychosocial approach. Pers Soc Psychol Rev 2014; 18:256-279; PMID:24727972; http://dx.doi.org/ 10.1177/1088868314527831 [DOI] [PubMed] [Google Scholar]
- [195].Heil M. Damaged-self recognition as a general strategy for injury detection. Plant Signal Behav 2012; 7:576-80; PMID:22516811; http://dx.doi.org/ 10.4161/psb.19921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Heil M, Land WG. Danger signals - damaged-self recognition across the tree of life. Front Plant Sci 2014; 5:578; PMID:25400647; http://dx.doi.org/ 10.3389/fpls.2014.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Heil M, Ibarra-Laclette E, Adame-Álvarez RM, Martínez O, Ramirez-Chávez E, Molina-Torres J, Herrera-Estrella L. How plants sense wounds: damaged-self recognition is based on plant-derived elicitors and induces octadecanoid signaling. PLoS One 2012; 7:e30537; PMID:22347382; http://dx.doi.org/ 10.1371/journal.pone.0030537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Trewavas AJ, Baluška F. The ubiquity of consciousness. EMBO Rep 2011; 12:1221-5; PMID:22094270; http://dx.doi.org/ 10.1038/embor.2011.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Vandekerckhove M, Bulnes LC, Panksepp J. The emergence of primary anoetic consciousness in episodic memory. Front Behav Neurosci 2014; 7:210; PMID:24427125; http://dx.doi.org/ 10.3389/fnbeh.2013.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Fabbro F, Aglioti SM, Bergamasco M, Clarici A, Panksepp J. Evolutionary aspects of self- and world consciousness in vertebrates. Front Hum Neurosci 2015; 9:157; PMID:25859205; http://dx.doi.org/ 10.3389/fnhum.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]


