Abstract
Objective: Hospital- and population-based studies demonstrate an increasing incidence of Clostridium difficile infection (CDI) in adults and children; although pediatric CDI outcomes are incompletely understood. We analysed United States National Hospital Discharge Survey (NHDS) data to study CDI in hospitalized children.
Methods: NHDS data for 2005–2009 (demographics, diagnoses and discharge status) were obtained; cases and comorbidities were identified using ICD-9 codes. Weighted univariate and multivariate analyses were performed to ascertain incidence of CDI; associations between CDI and outcomes [length of stay (LOS), colectomy, all-cause in-hospital mortality and discharge to a care facility (DTCF)].
Results: Of an estimated 13.8 million pediatric inpatients; 46 176 had CDI; median age was 3 years; overall incidence was 33.5/10 000 hospitalizations. The annual frequency of CDI did not vary from 2005 to 2009 (0.24–0.43%; P = 0.64). On univariate analyses, children with CDI had a longer median LOS (6 vs 2 days), higher rates of colectomy [odds ratio (OR) 2.0; 95% confidence interval (CI) 1.7–2.4], mortality (OR 2.5; 95% CI 2.3–2.7), and DTCF (OR 1.6; 95% CI 1.6–1.7) (all P < 0.0001). After adjusting for age, sex and comorbidities, CDI was an independent and the strongest predictor of increased LOS (adjusted mean difference, 6.4 days; 95% CI 5.4–7.4), higher rates of colectomy (OR 2.1; 95% CI 1.8–2.5), mortality (OR 2.3; 95% CI 2.2–2.5), and DTCF (OR 1.7; 95% CI 1.6–1.8) (all P < 0.0001). On excluding infants from the analysis, children with CDI had higher rates of mortality, DTCF and longer LOS than children without CDI.
Conclusions: Despite increased awareness and advancements in management, CDI remains a significant problem and is associated with increased LOS, colectomy, in-hospital mortality and DTCF in hospitalized children.
Keywords: Clostridium difficile infection, children, pediatric, outcomes, epidemiology
Introduction
Clostridium difficile is the most common healthcare-associated infection [1] and the principal cause of infectious diarrhea in hospitalized patients [2]. C. difficile infection (CDI) is associated with known risk factors, including hospitalization, advanced age, gastrointestinal surgery or procedures, and antibiotic exposure [2]. The disease spectrum of CDI ranges from mild to severe colitis and can be complicated by recurrent infection, sepsis, need for critical care, surgery or death. CDI has also emerged in populations previously considered to be at low risk and lacking the traditional risk factors for CDI [3], including in the community setting [4]. Recent studies have shown that CDI is a more common cause of infectious diarrhea in children than previously thought, both in the hospital and community settings, with growing incidence and severity [5–9]. Outbreaks of pediatric CDI have also been reported [10, 11]. An analysis of National Hospital Discharge Survey (NHDS) data from the USA showed an increasing incidence of CDI in hospitalized children from 1997 to 2006 [12]; however, there is limited information on outcomes in respect of CDI in children, including the effect of CDI on length of hospital stay, in-hospital mortality, colectomy and discharge to a care facility. In the current study, we analysed United States NHDS data from 2005–2009 to evaluate these outcomes in pediatric patients with CDI.
Materials and methods
Data source
The National Hospital Discharge Survey (NHDS) has been conducted annually in the USA since 1965 and collects hospital discharge information from non-federal short-stay hospitals [defined as average length of stay (LOS) less than 30 days] throughout the United States with a stratified random sampling process. NHDS contains diagnosis and procedure codes, demographics, admission type, LOS, all-cause in-hospital mortality, and discharge information (e.g. to home or to a short-term or long-term healthcare facility). The database is publicly available online at http://www.cdc.gov/nchs/nhds.htm. Diagnoses are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Data collection
Data extraction and statistical analysis were carried out using Statistical Analysis Software (SAS) version 9.2 and JMP version 9.01 (SAS Institute, Cary, NC, USA). Data collected and analysed for this study included age, sex, race, admission type (urgent or emergent versus elective), any diagnosis of colectomy, length of stay, type of discharge, and mortality for all patients discharged between January 1, 2005, through December 31, 2009.
Definition of variables
Patients recorded in the NHDS database from 2005–2009 with age <18 years, with an ICD-9-CM code of 008.45 listed as their primary or additional diagnosis (from 2005–2009) or diagnosis on admission (data collected 2008 onwards), were deemed to be CDI patients. Individuals born during that hospital admission (coded as newborns) were excluded. We analysed outcomes in children aged 2–17 years separately after excluding infants (aged <1 year).
Race
Data on race collected by NHDS is classified into White, Black / African American, American Indian / Alaskan Native, Asian, Native Hawaiian / Other Pacific Islander, Other, Multiple race indicated, or unknown.
Geographical region
Hospitalizations were classified according to geographical area of the United States: Northeast, Midwest, South and West. Hawaii and Alaska were included in the ‘West’ geographical area.
Admission type
Hospital admissions in pediatric patients in the NHDS database are characterized as emergency, urgent, elective or not available.
Discharge type
The type of hospital discharge is classified by the NHDS database into the following categories: routine and/or discharged home, discharged to a short-term healthcare facility, discharged to a long-term healthcare facility, unknown discharge status, left against medical advice, or death during hospitalization. To analyse the likelihood of discharge to a care-term facility (DTCF), we combined all patients discharged to short- and long-term healthcare facilities and compared them with patients who had a routine and/or home discharge. Patients who died or for whom the type of discharge was not stated were excluded from this aspect of the analysis.
Surgical procedures
ICD-9 codes that were used to determine which patients underwent partial or total colectomy included 45.71, 45.72, 45.73, 45.74, 45.75 and 45.76, 45.8, 45.81, 45.82 and 45.83. The incidence of colectomy was compared in patients with and without CDI.
Length of stay
The NHDS collects data on the LOS for all patients. These data were abstracted electronically and used to calculate differences in LOS among patients with CDI, as compared with other patients.
In-hospital mortality
Death during hospitalization was analysed as a separate clinical outcome. Mortality after release from hospital is not included in the data collected by the NHDS. All-cause mortality for CDI was compared with that for pediatric patients without CDI.
Comorbidities
Comorbid conditions were abstracted from the data using the Health-care cost and utilization guidelines at https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Clinically relevant comorbid conditions including hematological malignancies, solid organ tumors, inflammatory bowel disease and chronic lung disease were abstracted and used for statistical adjustment along with age and sex. Other comorbidities were not used due to the low frequency of those conditions in this patient population.
Statistical analyses
The summary database was converted to a JMP file (SAS Institute, Cary, NC, USA) using SAS version 9.2. Demographic and clinical outcome data were analysed using the t-test for normally distributed continuous variables and the Wilcoxon rank sum test if not normally distributed (e.g. age and LOS). For comparison of continuous data among several groups, analysis of variance was used if the data were normally distributed, and the Kruskal-Wallis test was used if data were skewed. Results of continuous variables are reported as mean or median (minimum, maximum) as appropriate. Categorical variables are reported as percentages and compared using odds ratios (ORs) and 95% confidence intervals (95% CIs). Multivariate linear and logistic regression models with weighted analyses were used to adjust for the effect of age, sex and comorbid conditions on CDI-associated outcomes. Weighted analysis was performed in order to obtain nationwide estimates and account for the stratified sampling process of the NHDS database. A P-value of less than 0.01 was considered statistically significant.
Results
Patient characteristics
From 2005–2009, the NHDS database included an estimated 13.8 million pediatric hospital admissions. These patients had a median age of 5 years (range 1–17) and 47.8% were female. Overall, 72.4% admissions were classified as emergency or urgent. The median LOS was 2 days (range 1–561) and 2.7% of all patients were dismissed to a short- or long-term care facility. The overall rate of colectomy was 0.2% and the overall all-cause in-hospital mortality was 0.5%. Detailed patient characteristics are shown in Table 1.
Table 1.
Patient characteristics and incidence of Clostridium difficile infection
| Characteristics | Entire cohort (n = 13.8 x 106) | Patients with CDI (n = 46, 176) | Patients without CDI (n = 13.7 x 106) | Incidence of CDI/ 10 000 hospitalizations | P-valuea |
|---|---|---|---|---|---|
| Median age, years | 5 (2–14) | 3 (2–11) | 5 (2–14) | ||
| Age distribution | |||||
| <1 year | 26.3 | 27.8 | 26.3 | 35.4 | <0.0001 |
| 1–17 years | 73.7 | 72.2 | 73.7 | 32.8 | |
| Race | |||||
| Caucasian | 56.7 | 52.7 | 56.7 | 31.5 | <0.0001 |
| African-American | 14.7 | 12.1 | 14.7 | 27.7 | |
| Asian | 1.4 | 2.1 | 1.4 | 50.8 | |
| Othersb | 27.2 | 33.1 | 27.2 | 40.6 | |
| Geographical region | |||||
| Northeast | 18.5 | 20.4 | 18.5 | 36.9 | <0.0001 |
| Midwest | 15.5 | 13.0 | 15.5 | 28.1 | |
| South | 38.6 | 30.9 | 38.6 | 26.8 | |
| West | 27.4 | 35.7 | 27.4 | 43.7 | |
| Admission type | |||||
| Emergency | 41.7 | 9.1 | 41.7 | 31.4 | <0.0001 |
| Urgent | 30.7 | 30.6 | 30.7 | 33.4 | |
| Elective | 19.4 | 23.2 | 19.4 | 40.0 | |
| Unknown | 8.2 | 7.1 | 8.2 | 28.9 | |
| Hospital ownership | |||||
| Proprietary | 10.4 | 7.5 | 10.4 | 24.2 | <0.0001 |
| Government | 11.3 | 7.9 | 11.3 | 23.4 | |
| Non-profit | 78.3 | 84.6 | 78.3 | 36.2 | |
| Hospital beds | |||||
| 6–99 | 15.2 | 7.9 | 15.2 | 17.5 | <0.0001 |
| 100–199 | 18.1 | 17.6 | 18.1 | 32.6 | |
| 200–299 | 30.3 | 33.4 | 30.3 | 36.9 | |
| 300–499 | 22.8 | 24.6 | 22.8 | 36.1 | |
| ≥500 | 13.6 | 16.5 | 13.6 | 40.7 | |
All data are proportion of cases unless otherwise stated.
aP-value for comparing incidence of CDI in the various groups.
b‘Others’ includes patients in whom the race was not stated or multiple races were indicated for the same patient.
CDI = C. difficile infection
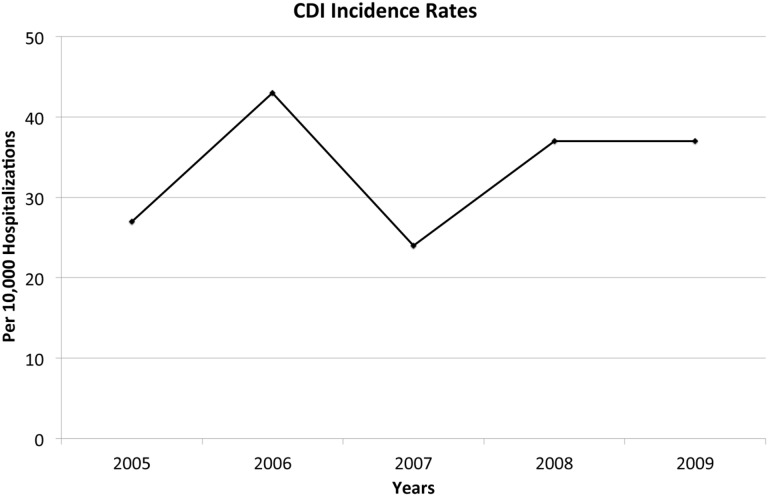
There were 46 176 pediatric CDI cases with an overall incidence of 33.5/10 000 hospitalizations. The annual incidence of CDI varied from 24 to 43 cases per 10 000 pediatric hospitalizations over the study period, with no significant trend (P = 0.64) (Figure 1). Also no significant trend was seen over the study period in the rates of colectomy, in-hospital mortality, DTCF and LOS in pediatric CDI cases. Among geographical regions, the Western region had the highest incidence of hospitalizations with CDI, followed by the Northeast. There was a significantly higher incidence of CDI in hospitals with larger numbers of beds signifying that larger hospitals have a higher CDI incidence. (Table 1).
Figure 1.
Annual rate of incidence of Clostridium difficile infection over the study period.
Of the estimated 13.8 million pediatric inpatients, 3.6 million infants accounted for 26% of the population. The rates of CDI in infants and in those aged 2–17 years were similar (35 vs 33 per 10 000 hospitalizations, respectively).
Outcomes from CDI
Length of stay
The median LOS among all hospitalized children was 2.0 days. Patients with CDI had a median LOS of 6.0 days. After adjusting for co-morbid conditions, CDI was the strongest independent predictor of increased LOS among predictors analysed (adjusted mean difference in LOS 6.4 days; 95% CI 5.4–7.4; P < 0.0001) (Table 2).
Table 2.
Comparison of outcomes in patients with and without CDI
| Outcomes | Patients with CDI | Patients without CDI | OR (95% CI) | Adjusted OR (95% CI) | P-valuea |
|---|---|---|---|---|---|
| Median length of stay (days) | 6 | 2 | Mean difference: 4 | Adjusted mean difference: 6.4 (5.4–7.4) | <0.0001 |
| In-hospital mortality, % | 1.2 | 0.5 | 2.5 (2.3–2.7) | 2.3 (2.2–2.5) | <0.0001 |
| Colectomy, % | 0.3 | 0.2 | 2.1 (1.7–2.4) | 2.1 (1.8–2.5) | <0.0001 |
| Discharge to care facility, % b | 4.3 | 2.7 | 1.6 (1.6–1.7) | 1.7 (1.6–1.8) | <0.0001 |
Data are presented as number (percentage) or median (minimum, maximum).
aP-value for the adjusted OR. Adjustment was made for age, sex, and comorbidities, including hematological malignancies, solid tumors and chronic lung disease.
bExcluding patients who left against medical advice, died or in whom discharge status was not reported.
CDI = C. difficile infection; CI = confidence interval; OR = odds ratio.
Colectomy
The overall rate of colectomy in all hospitalized patients was 0.2%. Patients with CDI showed a higher likelihood of undergoing colectomy than those without CDI. After adjusting for co-morbidities, CDI was an independent predictor of colectomy in these patients (Table 2).
All-cause in-hospital mortality
The overall in-hospital mortality was 0.5%. Patients with CDI had a higher all-cause mortality than patients without the disease. After adjusting for co-morbidities, CDI was an independent predictor of all-cause in-hospital mortality (Table 2).
Hospital discharge
The overall rate of discharge to a short- or long-term care facility was 2.7%. Patients with CDI were more likely to be discharged to a care facility than those without CDI. After adjusting for co-morbidities, CDI was an independent predictor of DTCF (Table 2).
Outcomes in children aged 2–17 years (excluding infants)
On analysing data restricting the study population to non-infants (age 2–17 years), the rates of DTCF and mortality, and the mean LOS, remained significantly higher in the CDI group than in the non-CDI group; however there was no significant difference in rates of colectomy between children with or without CDI (Table 3).
Table 3.
Comparison of outcomes in children aged 2–17 years with and without CDI
| Outcomes | Patients with CDI | Patients without CDI | OR (95% CI) | Adjusted OR (95% CI) | P-valuea |
|---|---|---|---|---|---|
| Median length of stay (days) | 6 | 2 | Mean difference: 4 | Adjusted mean difference: 5.8 (4.9–6.5) | <0.0001 |
| In-hospital mortality, % | 1.7 | 0.3 | 5.9 (5.4–6.4) | 6.2 (5.7–6.7) | <0.0001 |
| Colectomy, %b | 0.1 | 0.1 | 0.9 (0.6–1.2) | 1.0 (0.7–1.3) | – |
| Discharge to care facility, %c | 4.0 | 2.5 | 1.7 (1.6–1.8) | 1.6 (1.6–1.7) | <0.0001 |
Data are presented as number (percentage) or median (minimum, maximum).
aP-value for the adjusted OR. Adjustment was made for age, sex, and co-morbidities, including hematological malignancies, solid tumors, chronic lung disease and inflammatory bowel disease.
bNo adjustment for IBD made as no IBD and CDI cases had colectomy.
cExcluding patients who left against medical advice, died or in whom discharge status was not reported.
CDI = C. difficile infection; CI = confidence interval; OR = odds ratio.
Relationship to inflammatory bowel disease
Of the estimated 13.8 million pediatric inpatients, an estimated 31 842 had a diagnosis of inflammatory bowel disease (IBD). This equates to a rate of 23 cases per 10 000 hospitalizations. The rates of CDI were much higher in children with IBD (415/10 000 hospitalizations) than in those without (33/10 000 hospitalizations). Rates of colectomy were significantly higher in IBD patients without CDI (11.5%) than in those with CDI (0%).
Discussion
We have demonstrated, using a large national database, that CDI is associated with worse outcomes in hospitalized children including longer LOS, higher rates of colectomy, higher likelihood of DTCF and higher in-hospital mortality. Even after adjusting for comorbid conditions, CDI remained the strongest independent predictor of these adverse outcomes compared to comorbid conditions in the multivariate analysis.
Numerous studies have shown rising rates of adult and pediatric CDI over the past decade [7, 13–15]. There have been reports that incidence of CDI has peaked and is now reaching a plateau, and there was no significant increase in the incidence of CDI in other large studies in children [5, 16]. Change in the incidence of CDI over time may represent a true increase due to the emergence of a hypervirulent strain, an increase in ‘at risk’ population owing to increasing use of antibiotics, more sensitive testing methods and better coding over time. An increase in incidence was not demonstrated in our study, perhaps reflecting the relatively short 5-year study period and the slowing of the CDI epidemic in the latter half of the last decade. Increased incidence of CDI in larger hospitals was noted; this could be explained by more frequent testing for C. difficile in larger hospitals, or the presentation of sicker children with CDI to these institutions, or lapses in infection control practices.
Whether pediatric CDI is merely an extension of adult CDI, or has its own specific epidemiology—and might actually be a source of- and magnify adult CDI—is debatable. Among the different pediatric age groups, a maximum increase in incidence has been noted in the diaper-wearing 1–4 year-old population [14]. Finding similar C. difficile strains in pediatric and adult patients, and increasing community-acquired CDI (CA-CDI) in adults, lend support to the hypothesis that pediatric patients can act as sources for adult CDI [17]. A case control study identified close contact with children under the age of 2 years as a potential risk factor for CA-CDI [18]. A plausible role for infants and young children acting as reservoirs and vectors for C. difficile is supported by data that several toxigenic and non-toxigenic strains are carried by infants, although none was found to carry the hypervirulent 027 or 078 strains [19, 20]. Regular diaper changing, by mothers, in babies carrying C. difficile has been hypothesized to partially explain the female predilection of CA-CDI [21].
The large difference between colectomy rates in IBD patients without CDI (11.5%) and with CDI (0%) is interesting and may indicate the predominantly non-surgical therapy for CDI in children, primarily in IBD patients and how sick these children with IBD and CDI may be, precluding them from getting a colectomy. The fact that children aged 2–17 years with CDI did not have higher rates of colectomy than children without CDI probably points at the rarity of colectomy as a therapeutic agent for CDI in children (10/10 000 hospitalizations in both groups).
Reports on the severity of CDI and outcomes in children have demonstrated heterogenous results. Our group has previously shown a high rate of adverse outcomes in children with CDI in a population-based study [13]. Other reports have shown low rates of CDI-associated complications [16]. An increase in complications over time has not been noted in previous longitudinal studies [7, 22]. One source of heterogeneity may be the use of non-standardized definitions for severe infection [23]; defined criteria for severity of CDI exist for adults but are lacking for children [24].
Analyses have in the past excluded infants [8]. Pediatric diarrhea has historically been considered primarily a viral disease, but a recent study reported C. difficile as the most common single organism isolated from the stool of children with healthcare-associated diarrhea [25]. Although originally thought to be non-pathogenic in infants [26], CDI has been shown to afflict infants, as shown by the isolation of C. difficile from their stools, absence of any other identified cause of diarrhea, and response to metronidazole therapy. The American Academy of Pediatrics recommends testing for CDI if the clinical suspicion is high but recommends first considering alternate diagnoses. We included all inpatients—other than newborns—in our study, identically to other studies [7], but also analysed data after excluding infants. Due to the risk of colonization, the significance of positive C. difficile testing in infants remains unclear, and such findings should be interpreted with caution [27]. Thirty to forty percent of adult inpatients with a positive stool assay for C. difficile are colonized but do not have colitis (asymptomatic carriers).
Length of hospital stay has a dual relationship with CDI as increased length of hospital stay is a well-known risk factor for CDI, and patients with CDI tend to stay longer in the hospital.
There are several limitations to our study. The data were analysed as part of a large national survey, and longitudinal follow-up for patients is not available. Being an administrative database, the NHDS lacks information on risk factors such as exposure to antibiotics or PPIs, CDI treatment, C. difficile strain, severity of CDI, healthcare-associated vs CA-CDI, and important laboratory parameters such as serum creatinine or leukocyte count. Several studies have failed to demonstrate an association between use of PPIs and CDI in children [15, 28, 29] and, although a majority of children with CDI had received antibiotics in a large population-based study from Olmsted County, MN, USA, a fifth did not receive antibiotics or PPIs, while low rates of antibiotic usage have been reported in other studies [5, 13]. There is limited information on CDI colonization vs true infection, as the data are based on ICD-9 codes. Nevertheless, the ICD-9-CM code for CDI diagnosis has been validated in previous studies, and has been shown to correlate well with the results of C. difficile toxin assay [30, 31].
Conclusions
Despite increased awareness of CDI in children and advancements in management and infection prevention and control practices, CDI remains a problem in hospitalized children, and is an independent predictor of increased LOS and poor outcomes such as colectomy, in-hospital mortality and DTCF. Several actions are needed, including more aggressive policies on infection control, antimicrobial stewardship and education to enhance the early recognition and prompt treatment of CDI, hopefully to prevent adverse outcomes and reduce the spread of infection.
Funding
None
Conflict of interest statement: none declared.
Author contributions
AG, DSP, LMB and SK designed the research, analysed the data, wrote the paper and critically revised the manuscript for important intellectual content.
Data sharing statement
no additional data available.
IRB statement
IRB approval was not required as the research data is held in the public domain.
Informed consent statement
Data were obtained from the NHDS, which is in the public domain.
References
- 1.Magill SS, Edwards JR, Bamberg W. et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile: more difficult than ever. N Engl J Med 2008;359:1932–40. [DOI] [PubMed] [Google Scholar]
- 3.Khanna S, Pardi DS, Aronson SL. et al. Outcomes in community-acquired Clostridium difficile infection. Aliment Pharmacol Ther 2012;35:613–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Khanna S. Community-acquired infection: an increasing public health threat. Infect Drug Resist 2014;7:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson L, Song X, Campos J. et al. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 2007;28:1233–35. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Shaklee JF, Smathers S. et al. Risk factors and outcomes associated with severe clostridium difficile infection in children. Pediatr Infect Disease J 2012;31:134–38. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Smathers SA, Prasad P. et al. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001–2006. Pediatrics 2008;122:1266–70. [DOI] [PubMed] [Google Scholar]
- 8.Nylund CM, Goudie A, Garza JM. et al. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med 2011;165:451–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis 2008;14:929–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferroni A, Merckx J, Ancelle T. et al. Nosocomial outbreak of Clostridium difficile diarrhea in a pediatric service. Eur J Clin Microbiol Infect Dis 1997;16:928–33. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Brandmarker SA, Guandalini S. Pediatric Clostridium difficile: a phantom menace or clinical reality? J Pediatr Gastroenterol Nutr 2000;31:220–31. [DOI] [PubMed] [Google Scholar]
- 12.Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerg Infect Dis 2010;16:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna S, Baddour LM, Huskins WC. et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013;56:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000–2005. Pediatr Infect Dis J 2008;27:1111–13. [DOI] [PubMed] [Google Scholar]
- 15.Rexach CE, Tang-Feldman YJ, Cantrell MC. et al. Epidemiologic surveillance of Clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn Microbiol Infect Dis 2006;56:109–14. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz KL, Darwish I, Richardson SE. et al. Severe clinical outcome is uncommon in Clostridium difficile infection in children: a retrospective cohort study. BMC Pediatr 2014;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marwick CA, Yu N, Lockhart MC. et al. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case-control. J Antimicrob Chemother 2013;68:2927–33. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox MH, Mooney L, Bendall R. et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 2008;62:388–96. [DOI] [PubMed] [Google Scholar]
- 19.Stoesser N, Crook DW, Fung R. et al. Molecular epidemiology of Clostridium difficile strains in children compared with that of strains circulating in adults with Clostridium difficile-associated infection. J Clin Microbiol 2011;49:3994–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rousseau C, Lemee L, Le Monnier A. et al. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol 2011;60(Pt 8):1112–18. [DOI] [PubMed] [Google Scholar]
- 21.Leffler DA, Lamont JT. Editorial: not so nosocomial anymore: the growing threat of community-acquired Clostridium difficile. Am J Gastroenterol 2012;107:96–98. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande A, Pant C, Anderson MP. et al. Clostridium difficile infection in the hospitalized pediatric population: increasing trend in disease incidence. Pediatr Infect Dis J 2013;32:1138–40. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Shaklee JF, Smathers S. et al. Risk factors and outcomes associated with severe clostridium difficile infection in children. Pediatr Infect Dis J 2012;31:134–38. [DOI] [PubMed] [Google Scholar]
- 24.Cohen SH, Gerding DN, Johnson S. et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 25.Langley JM, LeBlanc JC, Hanakowski M. et al. The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect Control Hosp Epidemiol 2002;23:660–64. [DOI] [PubMed] [Google Scholar]
- 26.Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr 2010;51:2–7. [DOI] [PubMed] [Google Scholar]
- 27.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA pediatrics 2013;167:567–73. [DOI] [PubMed] [Google Scholar]
- 28.Sandora TJ, Fung M, Flaherty K. et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J 2011;30:580–84. [DOI] [PubMed] [Google Scholar]
- 29.Turco R, Martinelli M, Miele E. et al. Proton pump inhibitors as a risk factor for paediatric Clostridium difficile infection. Aliment Pharmacol Ther 2010;31:754–59. [DOI] [PubMed] [Google Scholar]
- 30.Dubberke ER, Reske KA, McDonald LC. et al. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis 2006;12:1576–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaklee J, Zerr DM, Elward A. et al. Improving surveillance for pediatric Clostridium difficile infection: derivation and validation of an accurate case-finding tool. Pediatr Infect Dis J 2011;30:e38–e40. [DOI] [PubMed] [Google Scholar]