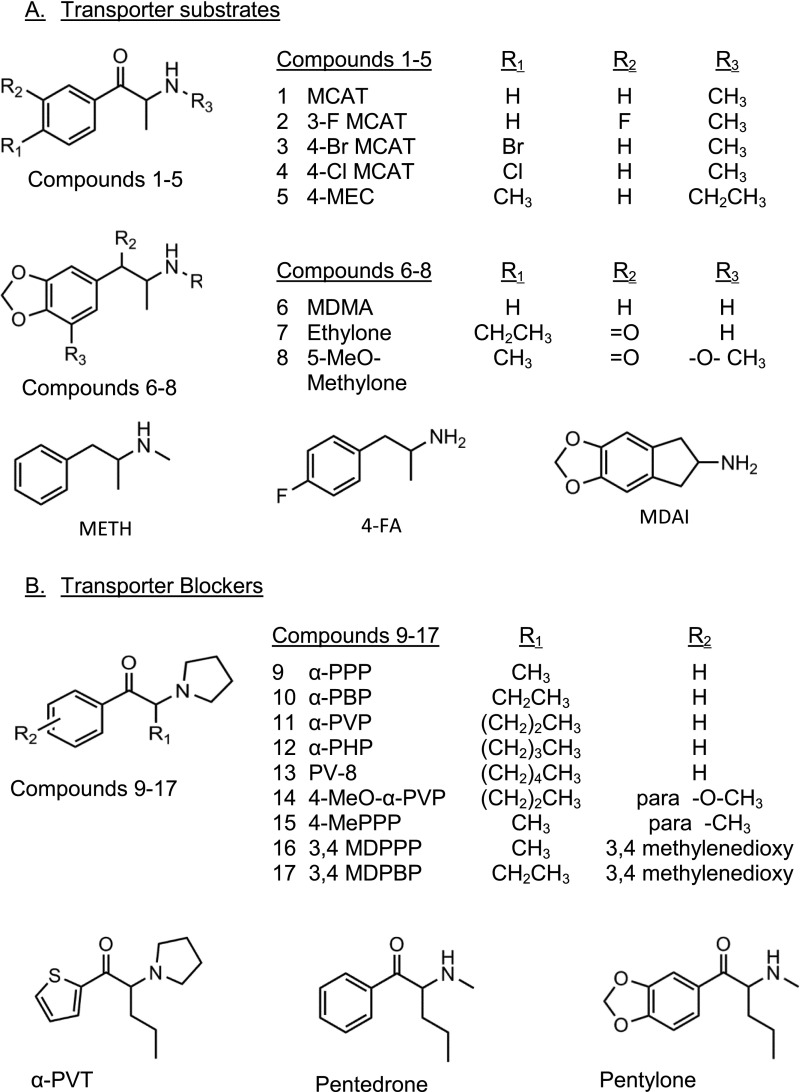
Abstract
Synthetic cathinones are components of “bath salts” and have physical and psychologic side effects, including hypertension, paranoia, and hallucinations. Here, we report interactions of 20 “bath salt” components with human dopamine, serotonin, and norepinephrine transporters [human dopamine transporter (hDAT), human serotonin transporter (hSERT), and human norepinephrine transporter (hNET), respectively] heterologously expressed in human embryonic kidney 293 cells. Transporter inhibitors had nanomolar to micromolar affinities (Ki values) at radioligand binding sites, with relative affinities of hDAT>hNET>hSERT for α-pyrrolidinopropiophenone (α-PPP), α-pyrrolidinobutiophenone, α-pyrrolidinohexiophenone, 1-phenyl-2-(1-pyrrolidinyl)-1-heptanone, 3,4-methylenedioxy-α-pyrrolidinopropiophenone, 3,4-methylenedioxy-α-pyrrolidinobutiophenone, 4-methyl-α-pyrrolidinopropiophenone, α-pyrrolidinovalerophenone, 4-methoxy-α-pyrrolidinovalerophenone, α-pyrrolidinopentiothiophenone (alpha-PVT), and α-methylaminovalerophenone, and hDAT>hSERT>hNET for methylenedioxypentedrone. Increasing the α-carbon chain length increased the affinity and potency of the α-pyrrolidinophenones. Uptake inhibitors had relative potencies of hDAT>hNET>hSERT except α-PPP and α-PVT, which had highest potencies at hNET. They did not induce [3H]neurotransmitter release. Substrates can enter presynaptic neurons via transporters, and the substrates methamphetamine and 3,4-methylenedioxymethylamphetamine are neurotoxic. We determined that 3-fluoro-, 4-bromo-, 4-chloro-methcathinone, and 4-fluoroamphetamine were substrates at all three transporters; 5,6-methylenedioxy-2-aminoindane (MDAI) and 4-methylethcathinone (4-MEC) were substrates primarily at hSERT and hNET; and 3,4-methylenedioxy-N-ethylcathinone (ethylone) and 5-methoxy-methylone were substrates only at hSERT and induced [3H]neurotransmitter release. Significant correlations between potencies for inhibition of uptake and for inducing release were observed for these and additional substrates. The excellent correlation of efficacy at stimulating release versus Ki/IC50 ratios suggested thresholds of binding/uptake ratios above which compounds were likely to be substrates. Based on their potencies at hDAT, most of these compounds have potential for abuse and addiction. 4-Bromomethcathinone, 4-MEC, 5-methoxy-methylone, ethylone, and MDAI, which have higher potencies at hSERT than hDAT, may have empathogen psychoactivity.
Introduction
New psychoactive substances, including substituted cathinones, are public health and regulatory challenges (Spiller et al., 2011; Griffiths et al., 2013; Baumann and Volkow, 2016). Some compounds, marketed as “bath salts” or “plant food,” have similar activity as the stimulants (+)methamphetamine (METH) and cocaine or the empathogen 3,4-methylenedioxymethylamphetamine (MDMA) and circumvent legal restrictions of the latter compounds (Iversen et al., 2013). Adverse physical and neuropsychiatric sequelae include tachycardia, brain swelling, hallucinations, and paranoia and can result in death (Prosser and Nelson, 2012; Miotto et al., 2013; Sykutera et al., 2015). Herein, we report the pharmacology of second-generation substituted cathinones, and two compounds that target monoamine transporters, 5,6-methylenedioxy-2-aminoindane (MDAI) and 4-fluoroamphetamine (4-FA). Substituted cathinones, structurally related to METH and MDMA, have a β-position ketone, as do methcathinone (MCAT) (Fig. 1) and (−)cathinone, the active ingredient in the psychoactive plant product khat (Glennon et al., 1987; Kelly, 2011). At least 10 substituted cathinones are schedule 1 substances, having no therapeutic uses and high potential for abuse (Drug Enforcement Administration, Department of Justice, 2016).
Fig. 1.
Structures of substituted cathinones, MDMA, METH, 4-FA, and MDAI. (A). Structures of compounds that were substrates at one or more transporters. (B) Structures of compounds that were inhibitors at hDAT, hSERT and hNET.
The biochemical pharmacology and behavioral effects of first-generation substituted cathinones have been reported. These include butylone, 4-fluoromethcathinone (4-F MCAT; flephedrone), mephedrone, MCAT, 3,4-methylenedioxypyrovalerone (MDPV), naphthylpyrovalerone (naphyrone), and pyrovalerone (Hadlock et al., 2011; Baumann et al., 2012; Lopez-Arnau et al., 2012; Cameron et al., 2013; Eshleman et al., 2013; Gatch et al., 2013; Iversen et al., 2013; Simmler et al., 2014a), and the results are summarized in German et al. (2014). Their mechanisms of action are similar, with the compounds primarily binding, with varying affinities and selectivities, to dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET). Some compounds are transporter blockers, similar to cocaine, whereas others are substrates, similar to METH. They generally have lower affinities for neurotransmitter receptors than for transporters.
Second-generation substituted cathinones are synthesized and available on the Internet. 3-Fluoromethcathinone (3-F MCAT) and 4-methylethcathinone (4-MEC) were identified in “plant food” being marketed and used as recreational drugs (Archer, 2009; Caudevilla-Galligo et al., 2013; Odoardi et al., 2016). Methylenedioxypentedrone (pentylone) and 4-methyl-α-pyrrolidinopropiophenone (4-MePPP) were found in samples of “NRG-1” and “NRG-3” available from websites (Brandt et al., 2011), and pentylone, α-pyrrolidinovalerophenone (α-PVP), 4-MEC, MDAI, 3,4-methylenedioxy-α-pyrrolidinobutiophenone (3,4-MDPBP), and α-methylaminovalerophenone (pentedrone) were confirmed in human blood specimens (Marinetti and Antonides, 2013; Elliott and Evans, 2014).
Behavioral assays indicate abuse potential of these compounds. 4-MEC, pentedrone, pentylone, 4-MePPP, 3-F MCAT, α-pyrrolidinobutiophenone (α-PBP), and α-PVP stimulate locomotion and substitute, at varying doses, for discriminative stimulus effects of cocaine and METH (Gatch et al., 2015a,b; Naylor et al., 2015). 3-F MCAT increases mouse locomotor activity, head weaving, and salivation, and decreases rotorod performance (Marusich et al., 2012). MDAI, an indane analog of MDMA first synthesized in 1990, substitutes for MDMA and cocaine in drug discrimination assays and produces conditioned place preference (Nichols et al., 1990; Oberlender and Nichols, 1991; Gatch et al., 2016). In rats, intraperitoneal injections of 4-chloromethcathinone (4-Cl MCAT) and 4-bromomethcathinone (4-Br MCAT) produce increases in nucleus accumbens extracellular dopamine (DA) and serotonin [5-hydroxy-tryptamine (5-HT)] levels (Suyama et al., 2016) but cause minimal effects on intracranial self-stimulation (ICSS), whereas both 4-MEC and α-PVP affect ICSS similar to METH (Watterson et al., 2014). Rats self-administer α-PVP (Aarde et al., 2015).
In pharmacological assays, 4-MEC, 3-F MCAT, 4-FA, pentylone, pentedrone, 3,4-methylenedioxy-N-ethylcathinone (ethylone), MDAI, and 4-Br MCAT inhibit uptake via NET, DAT, and SERT, and at 100 μM, 3-F MCAT, 4-FA, and 4-Br MCAT induce neurotransmitter release via these transporters, whereas MDAI, ethylone, pentylone, and 4-MEC induce release mainly via SERT (Iversen et al., 2013; Simmler et al., 2013, 2014a,b; Rickli et al., 2015). 3,4-Methylenedioxy-α-pyrrolidinopropiophenone (3,4-MDPPP), 3,4-MDPBP, and α-PVP (O-2387) had similar potencies for inhibition of uptake at DAT and NET and much lower potency at SERT (Meltzer et al., 2006; Rickli et al., 2015). 4-MEC and 4-MePPP have differential effects on DAT and SERT function: both are uptake inhibitors at DAT but are inactive at releasing [3H]1-methyl-4-phenylpyridinium+ via DAT from rat synaptosomes, whereas at SERT, 4-MEC inhibits uptake and stimulates release but 4-MePPP is inactive (Saha et al., 2015).
The objective of these experiments was to determine and, in some cases, confirm interactions with monoamine transporters by substituted cathinones that indicate abuse liability. We investigated the structure-activity relationship of 10 α-pyrrolidinophenones at human dopamine transporter (hDAT), human serotonin transporter (hSERT), and norepinephrine transporter (hNET) and the effect of ortho- and parasubstitutions on the phenyl ring of MCAT. We queried if the relationship between binding affinity and potency for inhibition of uptake predicts whether a compound is a transporter inhibitor or substrate. In addition, the relationship between potencies at inhibition of uptake and stimulation of release was assessed.
Materials and Methods
Drugs and Materials
[125I]methyl (1R,2S,3S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate ([125I]RTI-55), [3H]DA, [3H]5-HT, and [3H]norepinephrine (NE) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Cocaine, METH, MDMA, and MCAT were generously supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). 3-F MCAT, MDAI, 4-MEC, ethylone HCl, 4-MePPP, α-PBP, α-pyrrolidinohexiophenone (α-PHP), α-pyrrolidinopropiophenone (α-PPP), α-PVP, α-pyrrolidinopentiothiophenone, 3,4-MDPBP, pentedrone, pentylone, and 4-FA were supplied by the National Institute on Drug Abuse (Bethesda, MD). 4-Br MCAT HCl, 4-Cl MCAT HCl, 5-methoxy-methylone (5-MeO-methylone) HCl, 3,4-MDPPP HCl, 4-methoxy-PVP (4-MeO-α-PVP) HCl, 1-phenyl-2-(1-pyrrolidinyl)-1-heptanone (PV-8) HCl, and α-PHP HCl were purchased from Cayman Chemical (Ann Arbor, MI). FetalClone and bovine calf serum were purchased from HyClone and pcDNA1 was purchased from Invitrogen (Thermo Fisher, Waltham, MA). HEK cells transfected with pcDNA3-hNET cells were generously supplied by Dr. Randy Blakely (Florida Atlantic Univ). Most other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Inhibition of [125I]RTI-55 Binding to, and [3H]Neurotransmitter Uptake by, hDAT, hSERT, or hNET in Clonal Cells
The methods for characterizing radioligand binding and the functional uptake assays have been described (Eshleman et al., 1999, 2013). Human embryonic kidney (HEK) 293 cells stably expressing the recombinant hDAT (HEK-hDAT), hSERT (HEK-hSERT), or hNET (HEK-hNET) were used, with the expression vectors pcDNA1 with pBabepuro (Morgenstern and Land, 1990) for antibiotic resistance (hDAT and hSERT) and pcDNA3 (hNET). Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 5% FetalClone, 5% bovine calf serum, penicillin/streptomycin, and 2 µg/ml puromycin (hDAT and hSERT) or 10% FetalClone, penicillin/streptomycin, and 300 µg/ml G418 (hNET). For [125I]RTI-55 competition binding assays, cells were grown to confluence on 150-mm-diameter tissue culture dishes. The medium was removed, and cells were rinsed with calcium- and magnesium-free phosphate-buffered saline. Lysis buffer (2 mM HEPES with 1 mM EDTA) was added. After 10 minutes, cells were scraped from plates and centrifuged at 30,000 × g for 20 minutes. The supernatant was removed, and the pellet was resuspended in 50, 15, or 5 ml (hDAT, hSERT, or hNET, respectively) of 0.32 M sucrose/cell culture plate using a Polytron (VWR, Radnor, PA) at setting 7 for 10 seconds. The resuspension volume was selected to reflect binding of 10% or less of the total radioactivity.
Binding Assay Conditions.
Each assay tube contained 50 µl of membrane preparation (about 5–15 µg of protein); 25 µl of unknown, compound used to define nonspecific binding, or buffer [Krebs-HEPES (pH 7.4), 122 mM NaCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 10 μM pargyline, 100 µM tropolone, 0.2% glucose, and 0.02% ascorbic acid, buffered with 25 mM HEPES]; and 25 µl of [125I]RTI-55 (40–80 pM final concentration) in a final volume of 250 µl. Membranes were preincubated with unknowns for 10 minutes prior to the addition of [125I]RTI-55. Following a 90-minute incubation at 18–20°C, binding was terminated by filtration over Filtermat A filters (PerkinElmer) using a 96-well cell harvester (Tomtec, Hamden, CT). Filters were washed for 6 seconds with ice-cold saline. Radioactivity remaining on the filter was determined using a PerkinElmer µbeta-plate reader. Specific binding was defined as the difference in binding observed in the presence and absence of 5 µM mazindol (HEK-hDAT and HEK-hNET) or 5 µM imipramine (HEK-hSERT).
For [3H]neurotransmitter uptake assays, cells were grown to confluence as described earlier. The medium was removed, and cells were washed with calcium- and magnesium-free phosphate-buffered saline. Cells from each plate were gently scraped and then triturated with a pipette in 3 ml of Krebs-HEPES. Two cell plates provided enough cells for four concentration-response curves.
Uptake Inhibition Assay Conditions.
Krebs-HEPES and test compound, compound used to define nonspecific uptake, or buffer and cells (50 µl) were added to vials and placed in a 25°C water bath. Cells were preincubated with unknowns for 10 minutes prior to the addition of [3H]dopamine (hDAT), [3H]serotonin (hSERT), or [3H]norepinephrine (hNET) (20 nM final concentration) in a final volume of 500 µl. Specific uptake was defined as the difference in uptake observed in the presence and absence of 5 µM mazindol (HEK-hDAT and HEK-hNET) or 5 µM imipramine (HEK-hSERT). Filtration using a Tomtec 96-well cell harvester through Filtermat A filters presoaked in 0.05% polyethylenimine was used to terminate uptake after 10 minutes.
Biogenic Amine Transporters: [3H]Neurotrans-mitter Release
The methods for characterizing drug-induced release of preloaded [3H]neurotransmitter from HEK-hDAT, HEK-hSERT, and HEK-hNET cells have been described previously (Gatch et al., 2011; Eshleman et al., 2013). A 20-channel Suprafusion 2500 device (Brandel, Gaithersburg, MD) was used, with a perfusion speed to yield a flow rate of 0.8 ml/min. For HEK-hDAT and HEK-hSERT assays, a 150-mm-diameter plate of cells was scraped into 8 ml of Krebs-HEPES buffer, centrifuged at 500 rpm for 7 minutes, and resuspended in 8 ml of Krebs-HEPES. For HEK-hNET assays, three plates of cells were prepared as previously described. To load the cells with [3H]neurotransmitter, uptake was conducted for 15 minutes at 30°C with 120 nM [3H]DA (9.9 Ci/mmol, hDAT), 30 minutes at 25°C with 20 nM [3H]5-HT (29 Ci/mmol, hSERT), or 15 minutes at 30°C with 120 nM [3H]NE (6.5 Ci/mmol, hNET). Cells were centrifuged, resuspended in 7 ml of Krebs-HEPES, and cell suspension (280 μl) was added to the superfusion device, using polyethylene discs (Brandel) in the reaction tubes. Release was conducted at 30°C (hDAT) or 25°C (hSERT and hNET). Buffer was perfused for 12 minutes (hDAT and hSERT) or 15 minutes (hNET), with the last 6 minutes (3 × 2-minute fractions) collected. The cells were then perfused with drug solutions, and 22 minutes (11 × 2-minute fractions) of effluent were collected. To release the remaining radioactivity in the cells, SDS (1%) was perfused, and four fractions (2.5 minutes each) were collected. Radioactivity in the samples was determined using conventional liquid scintillation spectrometry.
Data Analysis
For competition binding results, data were normalized to the specific binding in the absence of drug. Three or more independent competition experiments were conducted with duplicate determinations. GraphPad Prism (GraphPad Software, La Jolla, CA) was used to analyze the ensuing data, with IC50 values converted to Ki values using the Cheng-Prusoff (Cheng and Prusoff, 1973) equation (Ki = IC50/(1 + ([drug*]/Kd drug*))), where drug* was [125I]RTI-55, and the Kd was determined using the described assay conditions. The Kd values used in the equations are given by Eshleman et al. (2013). Differences in affinities were assessed by one-way analysis of variance (ANOVA) using the logarithms of the Ki values for test compounds and standards. Dunnett’s multiple comparison test was used to compare test compounds to a drug standard, typically METH.
For release assays, fractional release was defined as the amount of radioactivity in a fraction divided by the total radioactivity remaining in the sample, which was determined by summing the counts per minute in that fraction and all later fractions. For each time course, the area under the curve (AUC) was calculated using GraphPad Prism, with the baseline defined as the average of the two lowest fractions of that time course. For each experiment, the AUC of basal release in the absence of drug was subtracted from each AUC before normalizing to the percentage of maximal METH-stimulated release [or maximal p-chloroamphetamine (PCA)–stimulated release for some hSERT experiments]. For functional assays, GraphPad Prism was used to calculate either IC50 (uptake) or EC50 (release) values using sigmoidal dose-response nonlinear regression, with data expressed as the percentage of total specific uptake or percentage of maximal METH-stimulated release. Dunnett’s multiple comparison test was used to compare test compounds to a drug standard, typically METH. Spearman nonparametric correlation was used to analyze the relationships among affinity for the binding site on the transporters and the potencies for inhibition of uptake and stimulation of release.
Results
α-Pyrrolidinophenones at hDAT, hSERT, and hNET: Inhibition of [125I]RTI-55 Binding and [3H]Neurotransmitter Uptake.
The affinities of the 18 substituted cathinones, MDAI, 4-FA, METH, MDMA, MCAT, and the reference compounds cocaine and mazindol for the hDAT, hSERT, and hNET expressed in HEK cells were assessed by inhibition of [125I]RTI-55 binding (Table 1). The rank order of affinity for the compounds differed among the transporters. For example, the five compounds with the highest affinity at hDAT and hNET were PV-8, α-PHP, α-PVP, MDPBP, and α-PBP, albeit in different rank order, whereas at hSERT, the highest-affinity compounds were pentylone, 4-MeO-α-PVP, 4-Br MCAT, 4-MEC, and pentedrone.
TABLE 1.
Affinity and potency of substituted cathinones and standard compounds at hDAT, hSERT, and hNET
Data are normalized to specific binding or specific uptake in the absence of drugs. Drugs were tested in binding and uptake assays at concentrations ranging from 1 nM to 10 μM, 100 μM, or 1 mM. Hill slopes for binding ranged from −0.51 to −1.69.
| Drug |
Inhibition of [125I]RTI-55 Binding Ki ± S.E.M. (n) |
Inhibition of [3H]Neurotransmitter Uptake IC50 ± S.E.M. (n) |
||||
|---|---|---|---|---|---|---|
| hDAT |
hSERT |
hNET |
hDAT [3H]DA |
hSERT [3H]5-HT |
hNET [3H]NE |
|
| μM | μM | μM | μM | μM | μM | |
| α- Pyrrolidinophenones | ||||||
| α-PPP | 1.29 ± 0.50 (3) | 161.4 ± 6.7 (3) | 2.04 ± 0.50 (4) | 0.540 ± 0.076 (3) | 188 ± 12 (4) | 0.305 ± 0.057 (3) |
| α-PBP | 0.145 ± 0.034 (7) | 163 ± 41 (5) | 0.51 ± 0.13 (5) | 0.078 ± 0.011 (3) | 67 ± 14 (5) | 0.143 ± 0.030 (5) |
| α-PVP | 0.0222 ± 0.0053 (7) | 68 ± 18 (3) | 0.122 ± 0.029 (3) | 0.0197 ± 0.0048 (5) | 57 ± 14 (3) | 0.046 ± 0.018 (5) |
| α-PHP | 0.0160 ± 0.0044 (8) | 33 ± 12 (5) | 0.339 ± 0.037 (9) | 0.0216 ± 0.0035 (6) | 40 ± 11 (3) | 0.0363 ± 0.0077 (7) |
| PV-8 | 0.0148 ± 0.0041 (5) | 39 ± 11 (5) | 0.63 ± 0.14 (5) | 0.0145 ± 0.0016 (4) | 26.8 ± 7.2 (3) | 0.0552 ± 0.0054 (4) |
| 4-MePPP | 1.12 ± 0.14 (3) | 50.01 ± 0.68 (3) | 5.87 ± 0.45 (3) | 0.566 ± 0.098 (3) | 10.34 ± 0.68 (3) | 1.07 ± 0.39 (3) |
| 4-MeO-α-PVP | 0.317 ± 0.090 (5) | 6.07 ± 0.98 (5) | 1.12 ± 0.26 (5) | 0.1126 ± 0.0094 (3) | 4.18 ± 0.28 (3) | 0.222 ± 0.054 (3) |
| 3,4-MDPPP | 1.10 ± 0.26 (5) | 18.7 ± 4.9 (3) | 4.9 ± 1.1 (5) | 0.409 ± 0.068 (3) | 12.8 ± 1.7 (3) | 0.881 ± 0.074 (3) |
| 3,4-MDPBP | 0.069 ± 0.020 (3) | 10.14 ± 0.33 (3) | 0.76 ± 0.15 (5) | 0.057 ± 0.14 (4) | 3.1 ± 1.0 (3) | 0.146 ± 0.040 (3) |
| α-PVT | 0.630 ± 0.054 (4) | 165.4 ± 5.0 (3) | 2.26 ± 0.31 (3) | 0.3428 ± 0.0049 (3) | 242 ± 41 (4) | 0.175 ± 0.038 (4) |
| Other substituted cathinones | ||||||
| MCAT | 4.78 ± 0.83 (18) | 313 ± 46 (14) | 3.59 ± 0.52 (21) | 0.152 ± 0.015 (18) | 20.6 ± 3.6 (20) | 0.0297 ± 0.0029 (19) |
| 3-F-MCAT | 3.25 ± 0.77 (5) | 211 ± 48 (3) | 2.92 ± 0.54 (3) | 0.214 ± 0.056 (4) | 12.9 ± 3.4 (5) | 0.0212 ± 0.0010 (3) |
| 4-Cl-MCAT | 9.41 ± 0.65 (4) | 28.7 ± 5.2 (4) | 19.6 ± 2.6 (4) | 0.208 ± 0.036 (3) | 0.67 ± 0.13 (3) | 0.0755 ± 0.0072 (3) |
| 4-Br-MCAT | 7.87 ± 0.28 (3) | 9.24 ± 0.93 (3) | 17.1 ± 1.8 (3) | 0.471 ± 0.093 (4) | 0.45 ± 0.11 (8) | 0.070 ± 0.016 (4) |
| 4-MEC | 3.00 ± 0.52 (3) | 12.6 ± 2.8 (4) | 15.8 ± 3.9 (4) | 0.96 ± 0.15 (3) | 0.218 ± 050 (4) | 0.93 ± 0.23 (3) |
| 5-MeO-methylone | 144 ± 54 (4) | 27.9 ± 4.2 (3) | 76 ± 14 (3) | >92 (4) | 1.90 ± 0.11 (4) | 19.0 ± 3.8 (4) |
| Ethylone | 5.0 ± 1.6 (3) | 23.7 ± 2.1 (4) | 18.8 ± 3.0 (3) | 1.72 ± 0.45 (3) | 0.464 ± 0.075 (4) | 1.42 ± 0.20 (3) |
| Pentedrone | 0.339 ± 0.098 (7) | 16.4 ± 4.6 (3) | 1.95 ± 0.56 (5) | 0.1762 ± 0.0089 (3) | 9.0 ± 2.3 (3) | 0.239 ± 0.067 (3) |
| Pentylone | 0.3942 ± 0.0040 (3) | 3.38 ± 0.52 (4) | 8.19 ± 0.57 (3) | 0.167 ± 0.037 (3) | 0.81 ± 0.14 (4) | 0.65 ± 0.16 (3) |
| Noncathinones | ||||||
| MDAI | 41.3 ± 3.5 (3) | 119.1 ± 5.8 (3) | 48 ± 11 (4) | 3.48 ± 0.46 (3) | 0.59 ± 0.13 (4) | 0.192 ± 0.036 (3) |
| 4-FA | 33.6 ± 3.1 (4) | 75.8 ± 6.6 (4) | 18.5 ± 4.2 (3) | 0.091 ± 0.025 (3) | 3.12 ± 0.66 (4) | 0.0426 ± 0.0044 (3) |
| METH | 4.58 ± 0.76 (23) | 213 ± 29 (20) | 1.82 ± 0.23 (19) | 0.0667 ± 0.0082 (26) | 7.4 ± 1.1 (27) | 0.0165 ± 0.0017 (23) |
| MDMA | 32.4 ± 4.9 (15) | 23.9 ± 5.5 (14) | 26.6 ± 6.4 (16) | 0.410 ± 0.045 (13) | 0.362 ± 0.053 (14) | 0.108 ± 0.015 (13) |
| Cocaine | 0.494 ± 0.037 (29) | 0.617 ± 0.045 (27) | 1.91 ± 0.23 (25) | 0.256 ± 0.022 (26) | 0.320 ± 0.034 (33) | 0.272 ± 0.024 (23) |
| Mazindol | 0.0296 ± 0.0039 (20) | 0.085 ± 0.012 (22) | 0.0071 ± 0.0014 (19) | 0.0146 ± 0.0016 (21) | 0.0435 ± 0.0040 (23) | 0.00143 ± 0.00017 (25) |
n, number of independent experiments conducted in duplicate.
The compounds with highest affinities for hDAT and hNET were α-pyrrolidinophenones, which are cathinones with a pyrrolidine group, with varying carbon chain length on the α-carbon. In general, this group of compounds was relatively selective for hDAT. At hDAT, the affinity increased with the length of the carbon chain (Ki in µM): methyl, α-PPP (1.29); ethyl, α-PBP (0.145); propyl, α-PVP (0.0222); butyl, α-PHP (0.016); and pentyl, PV-8 (0.0148) (Table 1). A similar structure-activity relationship was seen when measuring drug potency for inhibition of [3H]DA uptake. The addition of a 4-methyl group to α-PPP, forming 4-MePPP, had no effect on affinity or potency. The addition of a 4-methoxy group to α-PVP, forming 4-MeO-α-PVP, decreased affinity and potency 6- to 10-fold. The addition of a 3,4-methylenedioxy moiety to the phenyl ring of α-PPP to form 3,4-MDPPP did not change affinity or potency, whereas this addition to α-PBP to form 3,4-MDPBP and to α-PVP to form MDPV (Eshleman, et al., 2013) increased affinity but had little effect on potency. The substitution of a thiophene group for the phenyl of α-PVP to form α-PVT decreased affinity 30-fold and potency 15-fold.
The α-pyrrolidinophenones had lower affinity for hNET than for hDAT, and there was a trend for affinity to increase with increasing chain length, with the 3-carbon α-PVP having the highest affinity. Substitutions at the 4 position or addition of a 3,4-methylenedioxy group either had no effect or decreased potency in the [3H]NE uptake assay.
In general, the α-pyrrolidinophenones had very low affinity for and potency at hSERT (Table 1). As with hDAT, the affinity increased with the length of carbon chain: α-PPP Ki = 161.4 µM to α-PHP Ki = 33 µM (Table 1). Only 4-MeO-α-PVP had a Ki value below 10 µM, with 10-fold higher affinity than α-PVP. Addition of a 3,4-methylenedioxy group increased affinity 10-fold (3,4-MDPPP) and 16-fold (3,4-MDPBP) above α-PPP and α-PBP, respectively. This group of compounds also had very low potency at inhibition of [3H]5-HT uptake. The uptake potency ratio of hDAT to hSERT indicates a very high selectivity for hDAT, ranging from ∼2900 for α -PVP to ∼18 for 4-MePPP (Table 2). Addition of 4-methyl, 4-MeO-, and 3,4-methylenedioxy groups generally increased potency for inhibition of [3H]5-HT uptake.
TABLE 2.
hDAT uptake selectivity and ratio of binding affinity to uptake potency of substituted cathinones and standard compounds at hDAT, hSERT, and hNET
| Drug |
hDAT Uptake Selectivitya | Ratio of Ki for Inhibition of [125I]RTI-55 Binding/IC50 for Inhibition of [3H]Neurotransmitter Uptake | ||
|---|---|---|---|---|
| hDAT |
hSERT |
hNET |
||
| α-Pyrrolidinophenones | ||||
| α-PPP | 348 | 2.4 | 0.9 | 6.7 |
| α-PBP | 859 | 1.9 | 2.4 | 3.6 |
| α-PVP | 2893 | 1.1 | 1.2 | 2.7 |
| α-PHP | 1852 | 0.7 | 0.8 | 9.3 |
| PV-8 | 1848 | 1.0 | 1.5 | 11.4 |
| 4-MePPP | 18.3 | 2.0 | 4.8 | 5.5 |
| 4-MeO-α-PVP | 37.1 | 2.8 | 1.5 | 5.0 |
| 3,4-MDPPP | 31.3 | 2.7 | 1.5 | 5.6 |
| 3,4 MDPBP | 54.4 | 1.2 | 3.3 | 5.2 |
| α-PVT | 706 | 1.8 | 0.7 | 12.9 |
| MDPV | 110b | 1.5b | 0.91b | 5.7b |
| Naphyrone | 4.7b | 0.85b | 0.25b | 1.7b |
| Other substituted cathinones | ||||
| MCAT | 136 | 31.4 | 15.2 | 120.9 |
| 3-F MCAT | 60.3 | 15.2 | 16.4 | 137.7 |
| 4-Cl MCAT | 3.22 | 45.2 | 42.8 | 259.6 |
| 4-Br MCAT | 0.96 | 16.7 | 20.5 | 244.3 |
| 4-MEC | 0.23 | 3.1 | 57.8 | 17.0 |
| 5-MeO-methylone | <0.02 | <1.6 | 14.7 | 4.0 |
| Ethylone | 0.27 | 2.9 | 51.1 | 13.2 |
| Pentedrone | 51.1 | 1.9 | 1.8 | 8.2 |
| Pentylone | 4.9 | 2.4 | 4.2 | 12.6 |
| 4-F MCAT | >36b | 38b | ND | 276b |
| Butylone | 9.3b | 8.2b | 5.3b | 7.5b |
| Mephedrone | 5.2b | 49b | 41b | 220b |
| Methylone | 5.6b | 15b | 50b | 71b |
| Noncathinones | ||||
| MDAI | 0.17 | 11.9 | 201.9 | 250 |
| 4-FA | 34.3 | 369.2 | 24.3 | 434.3 |
| METH | 111 | 68.7 | 28.8 | 110.3 |
| MDMA | 0.88 | 79.0 | 66.0 | 246.3 |
| Cocaine | 1.25 | 1.9 | 1.9 | 7.0 |
| Mazindol | 3.0 | 2.0 | 2.0 | 5.0 |
4-F MCAT, 4-fluoromethcathinone; Mephedrone, 4-methyl-N-methylcathinone; ND, ratio could not be determined.
hDAT uptake selectivity is calculated as 1/hDAT IC50:1/hSERT IC50.
Data from Eshleman et al. (2013).
Pentedrone is structurally similar to α-PVP, with the pyrrolidine moiety replaced by a secondary nitrogen with a methyl group. Pentedrone has lower affinity and potency at hDAT and hNET, but higher affinity and potency for hSERT, compared with α-PVP. The addition of a 3,4-methylenedioxy group to pentedrone, forming pentylone, had no effect on the affinity at hDAT, but increased affinity and potency at hSERT 5- to 10-fold and decreased affinity at hNET 4-fold. Although both compounds had the same order of potency at blocking the transporters (hDAT>hNET>hSERT) as α-PVP, the uptake potency ratios of hDAT to hSERT for pentedrone and pentylone are 51.1 and 4.9, reflecting their increased potency at hSERT (Table 2). For α-PVP, the uptake potency ratio of hDAT to hSERT is 2893.
For α-PBP, α-PVP, 4-MePPP, 4-MeO-α-PVP, 3,4-MDPPP, and 3,4-MDPBP, the ratio of the Ki value to the IC50 value at each of the transporters is less than 6, and for α-PPP, α-PHP, PV-8, α-PVT, pentedrone, and pentylone, the ratio is less than 6 at hDAT and hSERT and less than 13 at hNET (Table 2), indicating that binding affinity is relatively predictive of uptake potency. This is also observed for cocaine and mazindol, which are pure uptake blockers at the transporters. Thus, this pharmacological pattern suggests that these compounds may primarily interact with the transporters as uptake blockers.
Other Substituted Cathinones, MDAI, and 4-FA at hDAT, hSERT, and hNET: Inhibition of [125I]RTI-55 Binding and [3H]Neurotransmitter uptake.
MCAT had much higher affinity at hDAT and hNET compared with hSERT and a rank order of potency for inhibition of uptake of hNET>hDAT>hSERT. Addition of 3-fluoro, 4-chloro, or 4-bromo substituents to MCAT had a minimal effect on affinity or potency at hDAT (Table 1). However, the 4-chloro and 4-bromo moieties increased both affinity and potency at the hSERT, and decreased affinity and potency at the hNET, compared with MCAT. Compared with MCAT, ethylone and 4-MEC had similar affinity but lower potency (6- to 11-fold) at hDAT, increased affinity (13- to 25-fold) and potency (44- to 94-fold) at hSERT, and decreased affinity (4- to 5-fold) and potency (31- to 48-fold) at hNET.
In contrast to the α-pyrrolidinophenones, 3-F MCAT, MDAI, 4-MEC, 4-Br MCAT, 4-Cl MCAT, 5-MeO-methylone, ethylone, and 4-FA have low affinities for the [125I]RTI-55 binding site (micromolar range) of hDAT, hSERT, and hNET (Table 1). METH, MDMA, and MCAT also have low affinities for the [125I]RTI-55 binding site on the transporters, and these compounds have higher potency at inhibiting uptake at the transporters compared with their Ki values (Tables 1 and 2). For 3-F MCAT, the ratios of binding Ki to uptake IC50 at the three transporters were all greater than 15, and for MDAI, the ratio was 11.9 for hDAT and greater than 200 for hSERT and hNET. For 4-MEC, 5-MeO-methylone, and ethylone, the ratios for hDAT (3.1, <1.6, and 2.9) and hNET (17, 4, and 13.2) were similar to that of the blockers (discussed earlier), but for hSERT, the ratios were much higher. METH and MDMA are substrates at the transporters, and we and others have previously observed that substrates have higher potency at inhibition of uptake compared with affinity for radioligand binding sites (Eshleman et al., 1999, 2013; Rothman et al., 2000). Thus, the results suggest that the compounds in this subset are substrates at some or all of the transporters.
hDAT, hSERT, and hNET: Substituted Cathinone Efficacy and Potency at Stimulating Release of Preloaded [3H]Neurotransmitter.
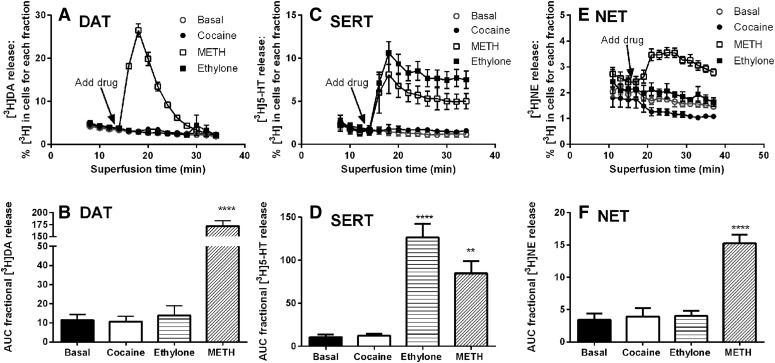
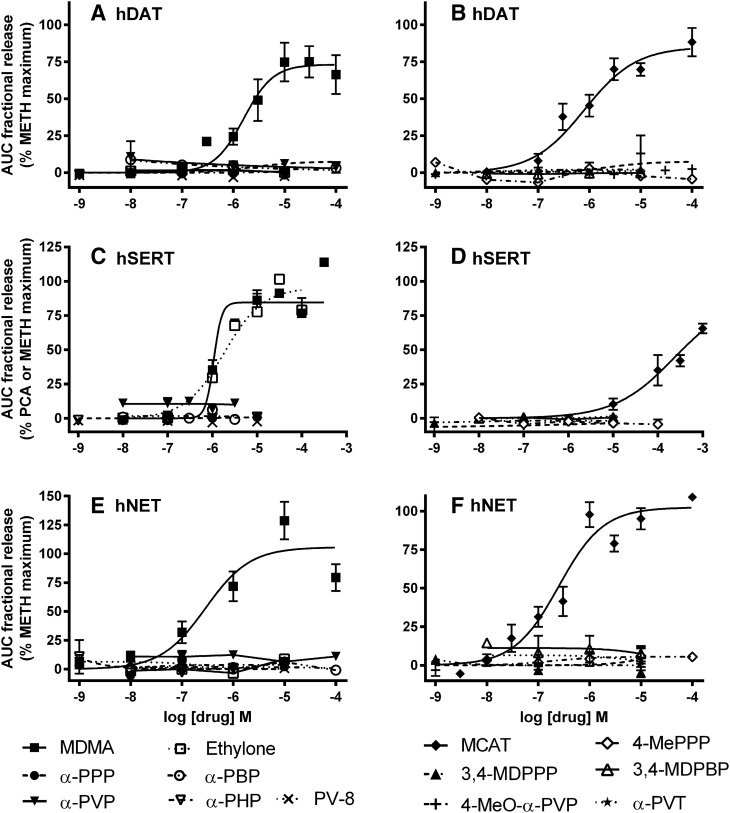
To determine if the substituted cathinones, MDAI, and 4-FA stimulate neurotransmitter release via the transporters, intact cells were preloaded with [3H]neurotransmitter, and release assays were conducted with a superfusion device, as described in Materials and Methods. This method continually removes released [3H]neurotransmitter from the extracellular buffer and allows release to proceed with minimal or no concurrent reuptake of the released [3H]neurotransmitter. With hDAT, hSERT, and hNET cells, release in the presence of cocaine, a transporter blocker, was similar to basal release in the absence of drug, indicating that minimal or no released [3H]neurotransmitter was available for reuptake (Fig. 2, P > 0.05, one-way ANOVA followed by Dunnett’s multiple comparison test). In contrast, METH induced robust release via the three transporters, and ethylone induced release only via SERT.
Fig. 2.
Effect of cocaine, ethylone, and METH on [3H]neurotransmitter release. hDAT (A), hSERT (C), and hNET (E) show the time courses of the average fractional release (mean ± S.E.M.) in the presence of buffer, cocaine (10 µM), ethylone (30 µM), or the maximally effective concentrations of METH (10 µM, hDAT; 100–300 µM, hSERT; 1 µM, hNET). hDAT (B), hSERT (D), and hNET (F) show the average AUC for fractional release for each condition tested. N = 3–6. **P < 0.01 and ****P < 0.0001 compared with basal release, one-way ANOVA followed by Dunnett’s multiple comparison test.
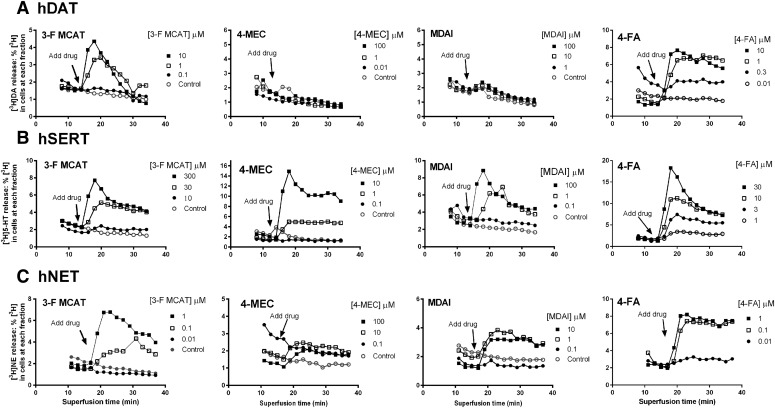
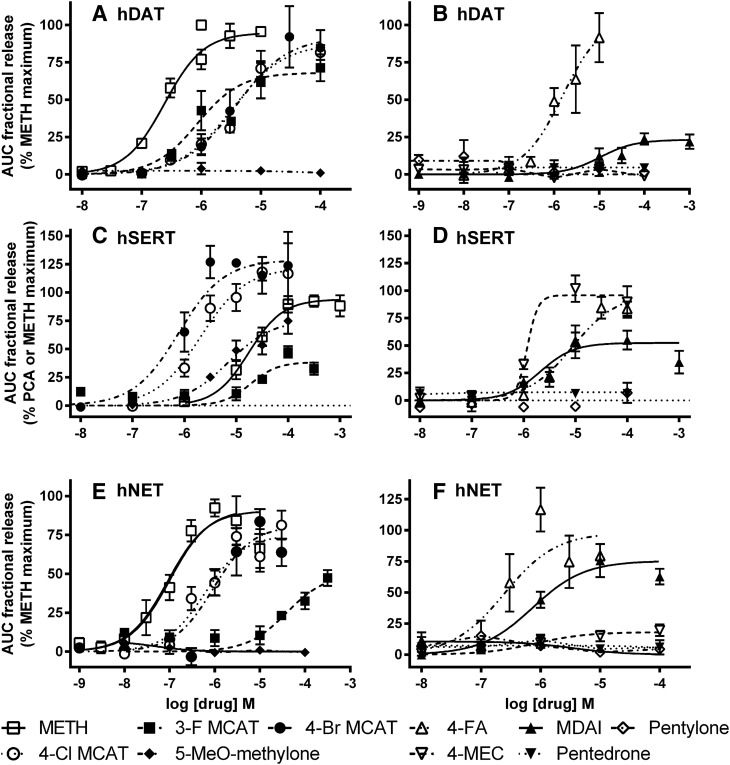
At hDAT, 3-F MCAT (300 nM to 100 μM) and 4-FA (10 nM to 10 μM) elicited [3H]DA release that peaked about 6 minutes after addition of drug, with all concentrations above 100 nM having some effect (Fig. 3A). 4-Cl MCAT, 4-Br MCAT, 4-FA, and MDAI (Fig. 4, A and B) were partially or fully efficacious. In contrast, the α-pyrrolidinophenones, pentedrone, pentylone, 4-MEC, 5-MeO-methylone, and ethylone at concentrations ranging from 1 nM to 100 μM (Figs. 4, A and B, and 5, A and B; Table 3) had little to no effect. Comparing the maximal drug-induced releases (Fig. 4, A and B and 5, A and B; Table 3, P < 0.0001, one-way ANOVA), MDMA, MCAT, 4-Br MCAT, 4-Cl MCAT, and 4-FA elicited maximal [3H]DA release, similar to METH (ps > 0.05, Dunnett’s multiple comparison test), whereas MDAI and 3-F MCAT were less efficacious (ps < 0.05). Comparing the potencies of drugs to induce [3H]DA release (Table 3, P < 0.0001, one-way ANOVA), 3-F MCAT had similar potency to METH, while MDAI (55-fold), MDMA (17-fold), MCAT (5.3-fold), 4-FA (3.2 fold), 4-Br MCAT (7.6-fold), and 4-Cl MCAT (6.6-fold) were less potent than METH (ps < 0.05–0.001).
Fig. 3.
Time courses of [3H]neurotransmitter release induced by the substituted cathinones 3-F MCAT and 4-MEC, the aminoindane MDAI, and the substituted amphetamine 4-FA. hDAT (A), hSERT (B), and hNET (C) cells. Data are representative experiments with a subset of concentrations shown for clarity. Data are normalized to percentage release of [3H]neurotransmitter remaining in cells at each time point. The last three buffer fractions prior to addition of drug and the 11 fractions in the presence of drug are shown.
Fig. 4.
Concentration-response curves of [3H]neurotransmitter release by METH, 3-F MCAT, 4-Cl MCAT, 4-Br MCAT, 4-FA, MDAI, 4-MEC, pentedrone, pentylone, and 5-MeO-methylone. hDAT (A and B), hSERT (C and D), and hNET (E and F) cells. The AUC for each drug concentration was normalized to the maximal effect of METH for that experiment (hDAT, hNET, and hSERT) or the maximal effect of PCA (some hSERT). The maximal stimulation of [3H]5-HT release by PCA and METH was similar in experiments when both were tested. Data are the mean and S.E.M. of three or more experiments, except when a drug had no releasing efficacy, in which case the data are the mean and range of two experiments.
Fig. 5.
Concentration-response curves of [3H]neurotransmitter release by MDMA, MCAT, ethylone, α-PPP, α-PBP, α-PVP, α-PHP, PV-8, 4-MePPP, 3,4-MDPPP, 3,4-MDPBP, 4-MeO-α-PVP, and α-PVT. hDAT (A and B), hSERT (C and D), and hNET (E and F) cells. The AUC for each drug concentration was normalized to the maximal effect of METH for that experiment (hDAT, hNET, and hSERT) or the maximal effect of PCA (some hSERT). The maximal stimulation of [3H]5-HT by PCA and METH was similar in experiments when both were tested. Data are the mean and S.E.M. of three or more experiments, except when a drug had no releasing efficacy, in which case the data are the mean and range of two experiments.
TABLE 3.
Potency and efficacy of substituted methcathinones and standard compounds to release preloaded [3H]neurotransmitter from HEK-hDAT, HEK-hSERT, and HEK-hNET cells
When the data could not be fit by nonlinear regression (EC50 > 10 or 100 μM), the maximum release is the average of release at the three highest concentrations tested.
| Drug | Drug-Induced Release of [3H]Neurotransmitter EC50 ± S.E.M. (μM) (n) % Maximum Release ± S.E.M.a | ||
|---|---|---|---|
| hDAT [3H]DA |
hSERT [3H]5-HT |
hNET [3H]NE |
|
| α-Pyrrolidinophenones | |||
| α-PPP | >10 µM (2) | >10 µM (2) | >10 µM (2) |
| −0.51 ± 2.06% | −0.58 ± 0.35% | 3.7 ± 4.3% | |
| α-PBP | >100 μM (2) | >100 μM (2) | >100 μM (2) |
| −1.6 ± 2.8% | 1.8 ± 1.6%b | −4.1 ± 4.3% | |
| α-PVP | >100 μM (2) | >100 μM (2) | >100 μM (2) |
| 4.5 ± 1.6% | 9.9 ± 1.5%b | −1.7 ± 2.9% | |
| α-PHP | >10 µM (2) | >10 µM (2) | >10 µM (2) |
| 1.47 ± 0.59% | 3.2 ± 3.6% | −0.5 ± 1.2% | |
| PV-8 | >10 µM (2) | >10 µM (2) | >10 µM (2) |
| −2.2 ± 0.73% | −0.53 ± 1.24% | 17 ± 11% | |
| 4-MePPP | >100 μM (2) | >100 μM (2) | >100 μM (3) |
| −1.3 ± 1.7% | −2.1 ± 1.7%b | 5.2 ± 2.1% | |
| 4-MeO-α-PVP | >9.5 (5) | >10 µM (2) | >10 µM (2) |
| 13 ± 12% | −0.5 ± 1.2% | 0.24 ± 0.54% | |
| 3,4-MDPPP | >10 µM (2) | >10 µM (2) | >10 µM (2) |
| 1.34 ± 0.32% | −1.12 ± 0.61% | −0.9 ± 2.7% | |
| 3,4-MDPBP | >10 µM (2) | >10 µM (2) | >10 µM (2) |
| −0.13 ± 0.24% | 0.04 ± 0.54% | 10.2 ± 2.4% | |
| α-PVT | >10 μM (2) | >10 μM (2) | >10 μM (2) |
| 0.88 ± 0.02% | 0.43 ± 0.14% | 4.8 ± 2.7% | |
| Other substituted cathinones | |||
| MCAT | 2.31 ± 0.76 (11) | 107 ± 36 (7) | 0.64 ± 0.24 (9) |
| 85.4 ± 6.0% | 65 ± 10% | 93.5 ± 8.2% | |
| 3-F MCAT | 1.29 ± 0.42 (6) | 39.0 ± 4.3 (3) | 0.13 ± 0.05 (5) |
| 63.3 ± 8.4% | 50.8 ± 1.4%b | 96 ± 11% | |
| 4-Cl MCAT | 2.89 ± 0.99 (3) | 1.98 ± 0.42 (4) | 1.24 ± 0.44 (6) |
| 83.5 ± 6.5% | 124 ± 10% | 84.9 ± 7.0% | |
| 4-Br MCAT | 3.3 ± 1.1 (3) | 0.96 ± 0.13 (4) | 1.50 ± 0.63 (5) |
| 90 ± 13% | 131 ± 13% | 91.8 ± 6.9% | |
| 4-MEC | >100 μM (3) | 1.52 ± 0.42 (3) | 0.71 ± 0.20 (5) |
| 0.10 ± 2.0% | 99 ± 10% | 17.5 ± 4.2% | |
| 5-MeO-methylone | >100 μM (2) | 3.89 ± 0.41 (3) | >100 μM (2) |
| 2.1 ± 0.9% | 67.4 ± 2.5% | 1.6 ± 1.3% | |
| Ethylone | >10 µM (2) | 1.48 ± 0.25 (4) | >10 µM (2) |
| 1.22 ± 0.62% | 86 ± 15%b | 1.3 ± 1.7% | |
| Pentedrone | >100 μM (2) | >100 μM (2) | >100 μM (2) |
| 4.2 ± 1.6% | 7.4 ± 2.4%b | −1.1 ± 4.4% | |
| Pentylone | >100 μM (2) | >100 μM (2) | >100 μM (2) |
| 2.4 ± 1.2% | −5.2 ± 2.1%b | 4.6 ± 1.5% | |
| Noncathinones | |||
| MDAI | 24 ± 11 (4) | 2.9 ± 1.2 (5) | 0.57 ± 0.20 (4) |
| 25.0 ± 4.9% | 61 ± 12%b | 68 ± 18% | |
| 4-FA | 1.40 ± 0.41 (5) | 11.1 ± 1.5 (4) | 0.144 ± 0.051 (5) |
| 96 ± 15% | 99.5 ± 6.2% | 145 ± 25% | |
| METH | 0.435 ± 0.074 (13) | 23.3 ± 4.2 (10) | 0.125 ± 0.024 (8) |
| 101.3 ± 2.9 | 93.0 ± 7.5% | 102.6 ± 3.0% | |
| MDMA | 7.5 ± 2.3 (10) | 1.10 ± 0.29 (9) | 0.360 ± 0.092 (7) |
| 94 ± 10% | 85.1 ± 7.8% | 147 ± 15% | |
| PCA | 1.25 ± 0.34 (6) | ||
| 105.5 ± 1.6%b | |||
n, number of independent experiments.
Maximum release is defined as the maximum release (maximal AUC) induced by METH (1 or 10 μM, hDAT; 0.3 or 1 μM, hNET; 0.3–1 mM, hSERT) for each experiment.
Maximum release is defined as the maximum release (maximal AUC) induced by p-chloroamphetamine (PCA, 10 μM, hSERT) for each experiment.
At hSERT, 3-F MCAT, 4-Br MCAT, 4-Cl MCAT, 4-FA, MDAI, ethylone, 5-MeO-methylone, and 4-MEC elicited release of [3H]5-HT that peaked about 6 minutes after addition of drug (Figs. 3B, 4, C and D; and 5C; Table 3). In contrast, the α-pyrrolidinophenones pentedrone and pentylone, had minimal releasing efficacy at concentrations ranging from 1 nM to 100 μM (Figs. 4D and 5C and 5D). Compared with the maximal release elicited by METH or PCA (Figs. 4, C and D, and 5, C and D; Table 3, P < 0.0001, one-way ANOVA), MDMA, MCAT, 4-Cl MCAT, MDAI, ethylone, 5-MeO-methylone, and 4-MEC elicited similar levels of [3H]5-HT release (p > 0.05, Dunnett’s test, Table 3), whereas 3-F MCAT was less efficacious, and 4-Br MCAT was significantly more efficacious (p < 0.05). Compared with the potency of METH (Fig. 4C; Table 3, P < 0.0001, one-way ANOVA), MDMA (21-fold), 4-Br MCAT (24-fold), 4-Cl MCAT (11-fold), ethylone (15-fold), MDAI (8-fold), 4-MEC (15-fold), and 5-MeO-methylone (6-fold) had higher potencies (ps < 0.01), whereas 3-F MCAT and 4-FA had similar potency to METH (p > 0.05) and MCAT had lower potency (4.6-fold).
At hNET, 3-F MCAT, 4-MEC, MDAI, and 4-FA elicited release of [3H]NE that peaked 8–16 minutes after addition of drug (Fig. 3C). MCAT, MDAI, 3-F MCAT, 4-FA, 4-Br MCAT, and 4-Cl MCAT had similar efficacy compared with METH (Figs. 4, E and F, and 5, E and F; Table 3), whereas 4-MEC had lower efficacy (p < 0.01), and MDMA had higher efficacy (p < 0.05). Compared with the potency of METH (Fig. 4E; Table 3, P < 0.001, one-way ANOVA), 4-Br MCAT (12-fold) and 4-Cl MCAT (10-fold) had lower potency, and the rest of the substrates had similar potency to METH (p > 0.05). In contrast, the α-pyrrolidinophenones pentedrone, pentylone, and ethylone had minimal releasing efficacy (Figs. 4F, and 5, E and F).
For compounds that stimulated release via the transporters, 3-F MCAT, 4-FA, and MCAT had relative potency of hNET>hDAT>hSERT similar to that of METH; MDAI had relative potency of hNET>hSERT>hDAT similar to that of MDMA; and 4-MEC had relative potency of hNET>hSERT>>hDAT. Ethylone and 5-MeO-methylone stimulated release only via hSERT. 4-Br MCAT and 4-Cl MCAT had similar potencies for release at the three transporters. Because only a subset of the drugs had robust effects on release, it is difficult to establish structure-activity relationships across transporters.
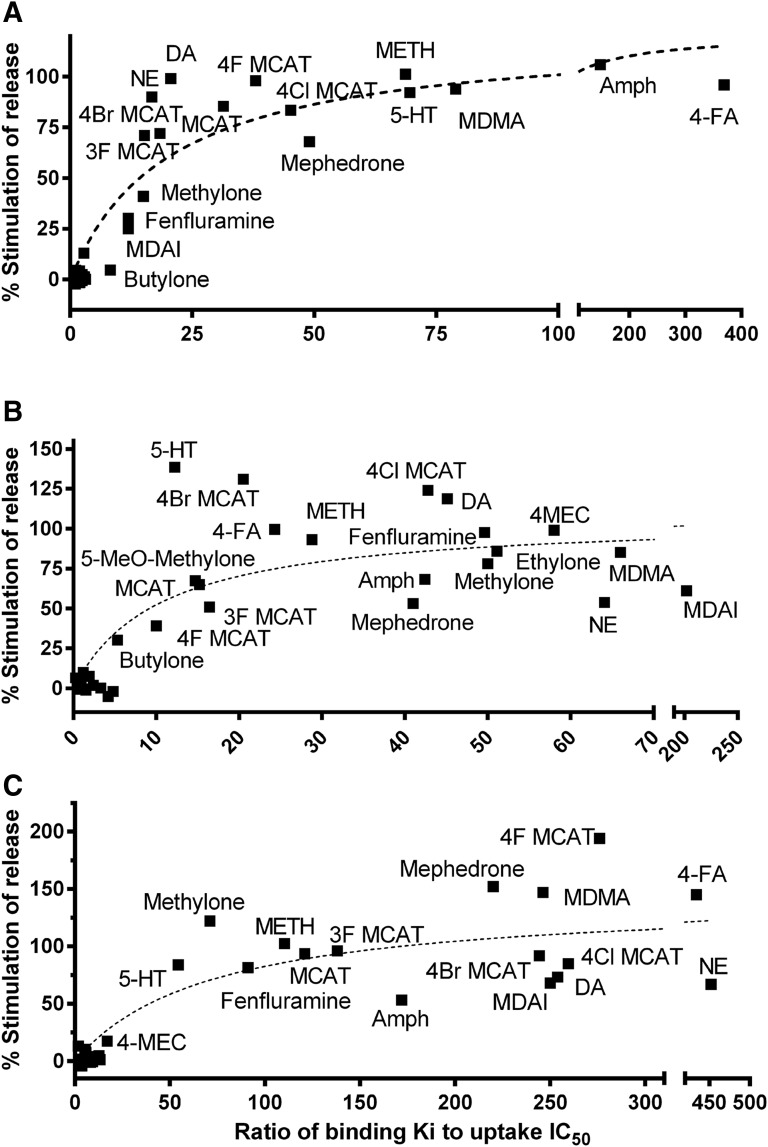
As noted earlier, substrates, defined here as those compounds that elicit nonvesicular release via a transporter, have higher potency in uptake assays compared with their affinity for the [125I]RTI-55 binding site. We queried whether there is a binding/uptake ratio above which we could predict, with some assurance, whether a compound is a substrate. Figure 6 shows the relationship between the affinity/potency ratios (listed in Table 2) and the percentage of maximal release for the 20 compounds reported here, six substituted methcathinones reported earlier (Eshleman et al., 2013), METH, MCAT, MDMA, S(+)amphetamine, (+)fenfluramine, DA, 5-HT, and NE at the hDAT, hSERT, and hNET. As shown, the relationship was generally hyperbolic, as there was an asymptote for the maximal amount of [3H]neurotransmitter that could be released. Spearman’s r correlation coefficient was significant for all three transporters. At binding/uptake ratios above approximately 12 for hDAT, all compounds were substrates. For hSERT and hNET, at binding ratios above approximately 10 and 50 (respectively), all compounds were substrates, releasing at least 30% of [3H]hSERT or [3H]hNET release, respectively, induced by METH (or PCA for a subset of compounds at hSERT). The highest affinity/potency ratios observed were about 400 for 4-FA at hDAT and hNET and about 200 for MDAI at hSERT. There are 17, 14, and 16 compounds represented in the cluster near the y-axis for hDAT, hSERT, and hNET, respectively, that had very low affinity ratios and induced little to no release.
Fig. 6.
Correlation between the ratio of affinity for the transporter (Ki) and the potency for inhibition of uptake (IC50) to the maximal releasing efficacy of the test compounds. hDAT (A), hSERT (B), and hNET (C). The data were fit to a hyperbolic equation. Values for nonparametric Spearman’s correlation (r) and significance are as follows: DAT r = 0.84, P < 0.0001 (A); SERT r = 0.73, P < 0.0001 (B); and NET r = 0.78, P < 0.0001 (C). All of the correlations are highly significant. Amph, (+)-amphetamine.
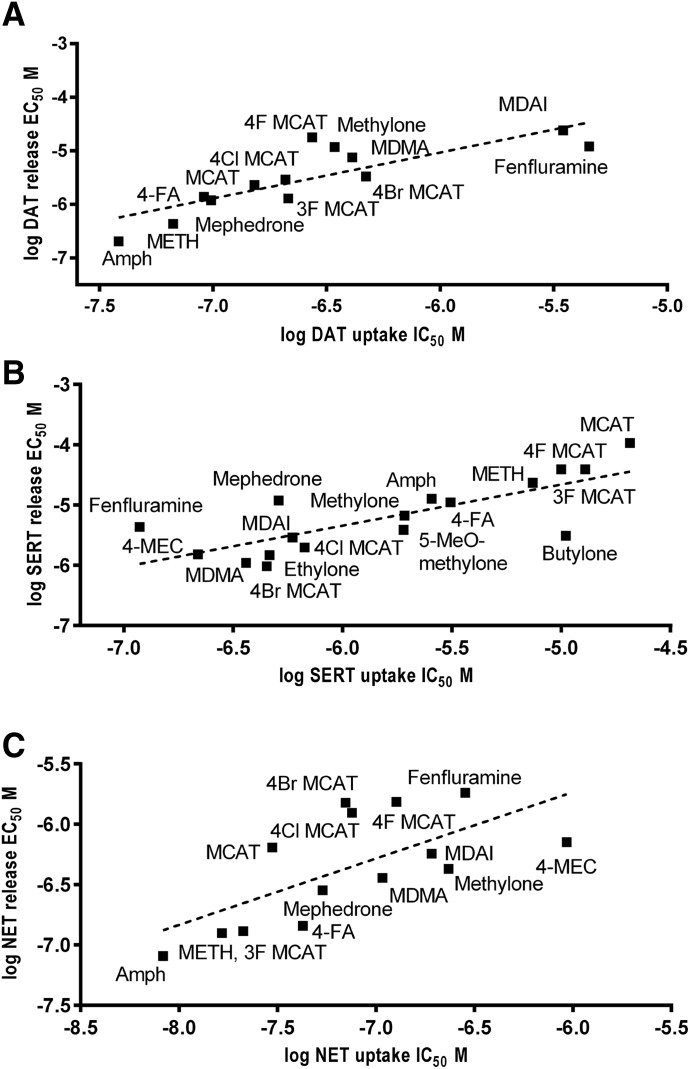
In addition, we wanted to determine if, for substrates, there was a relationship between the potencies to inhibit [3H]neurotransmitter uptake and to induce [3H]neurotransmitter release via a given transporter. The relationship between these two values is shown in Fig. 7, and there were excellent correlations between these two measures at all three transporters. In general, the EC50 values for release were higher (lower potency) than the IC50 values for uptake. Butylone at hSERT and 4-MEC at hNET were the only compounds that were more potent at inducing release than inhibiting uptake. On average, the EC50 values for release via hDAT were 16.9 ± 4.3 times higher than the uptake IC50 values, with a range of 2.7 for (+)fenfluramine to 65 for 4-F MCAT. At hSERT, the EC50 values for release were 7.2 ± 2.2 times higher than the uptake IC50 values, with a range of 0.3 for butylone to 36 for (+)fenfluramine. At hNET, the EC50 values for release were 8.2 ± 1.6 times higher than the uptake IC50 values, with a range of 0.76 for 4-MEC to 21.6 for MCAT
Fig. 7.
Correlation between potencies at inhibition of uptake and stimulation of release. hDAT (A), hSERT (B), and hNET (C). The linear regression line is shown. Values for nonparametric Spearman’s correlation (r) and significance are as follows: DAT r = 0.87, P < 0.001 (A); SERT r = 0.75, P < 0.001 (B); and NET r = 0.71, P < 0.006 (C). All of the correlations are highly significant. Amph, (+)-amphetamine.
Discussion
We characterized the binding affinities and functional activities of 18 substituted cathinones and two structurally related compounds at the hDAT, hSERT, and hNET.
For α-pyrrolidinophenones, increasing the chain length from one to five carbons (α-PPP to PV-8) increased affinity for hDAT ∼100-fold. These results, with increasing affinity and potency at hDAT correlating with increasing carbon chain length off the α carbon, are in agreement with those of Marusich et al. (2014) and Kolanos et al. (2015), who found that the potencies for inhibition at DAT correlate with both the volume of the α-substituent and the lipophilic character. We further extended this finding to include hNET, with an ∼17-fold increase in affinity from one to three carbons (α-PVP) and a correlation between affinity at hSERT and α-substituent chain length, although all of these compounds had very low affinity for hSERT. Rickli et al. (2015) indicated the same trend for 3,4-methylenedioxy–substituted pyrovalerones. The n-pyrrolidino substituent on 4-MePPP is sterically repulsed by bulky side chains in SERT that are found in the orthosteric binding pocket as delineated by the Leu-T structural model, which may account for the low affinity of this entire series for SERT (Saha et al., 2015). The pure transporter inhibitor activity of this group may be due to the n-pyrrolidino substituent, as Sandtner et al. (2016) observed that interactions with critical aspartate residues of DAT and SERT are necessary for substrate activity, and secondary or tertiary substitution on nitrogen inhibits interaction with these residues. The high selectivity for hDAT over hSERT suggests that these compounds may have high abuse liability, consistent with the ability of α-PBP and α-PVP to fully substitute for discriminative stimulus effects of both cocaine and METH and to produce conditioned place preference (Gatch et al., 2015a). In contrast, 4-MePPP fully substitutes only for METH, does not produce conditioned place preference, and in the current study had less selectivity for hDAT, all of which suggest a lower abuse liability as compared with other α-pyrrolidinophenones characterized here. Substrates and inhibitors of the transporters can fully cross-substitute for discriminative stimulus effects due to the commonality of increased extracellular neurotransmitter following drug administration (Camp et al., 1994).
Pentedrone and pentylone were two additional transporter inhibitors that had moderate affinity for hDAT. They produce long-lasting locomotor stimulant effects and fully substitute for discriminative stimulus effects of both cocaine and METH, and pentedrone increases conditioned place preference in mice and is self-administered by rats (Gatch et al., 2015b; Hwang et al., 2015). Both compounds may have abuse potential.
Differing from the α-pyrrolidinophenones, most of the other substituted cathinones were substrates at (some) transporters. Drug substrates for the transporters have several functions, competing with extracellular neurotransmitter for reuptake into the presynaptic neuron and inducing release of intracellular neurotransmitter. There are several theories for the molecular mechanism of substrate-induced release, including forward and reverse transport by the same protein, referred to as the “facilitated exchange diffusion model,” and forward and reverse transport mediated by different proteins within an oligomeric complex (reviewed by Sitte and Freissmuth, 2015). Another theory is the channel model, whereby substrates are depolarizing and excitatory, causing neurotransmitter release by vesicle fusion (reviewed by De Felice et al., 2014). Certain aspects of this “channel mode” of transporter function are present in our cell expression model. For example, substrates induce inward sodium currents in HEK cells expressing the hNET or hDAT, thereby elevating intracellular sodium to drive reverse transport (Galli et al., 1995; Sitte et al., 1998). However, as HEK cells do not have synaptic vesicles (Hu et al., 2013), release via vesicle fusion is not operational in our model system.
These potentially depolarizing transporter-mediated substrate-induced sodium currents (Sonders et al., 1997; Sitte et al., 1998) cause reverse transport or release of neurotransmitter. The exogenous substrate-induced sodium currents can put cells at risk, as (+)fenfluramine is associated with neuronal depletion of 5-HT (Baumann et al., 2014), and amphetamine can indirectly activate voltage-gated Ca2+ channels (Cameron et al., 2015). The substrates identified here, especially those that had efficacies similar to METH, may cause depolarization in neurons, as evidenced by the dose-dependent currents elicited by 4-MEC in Xenopus oocytes expressing hSERT (Saha et al., 2015).
Diagnostic assays that assess whether a compound is a substrate include electrophysiological measurement of transporter-mediated currents and testing reverse transport under control and intracellular high-sodium conditions (Sitte and Freissmuth, 2015). For example, evidence for substrate versus inhibitor action is the inward depolarizing current elicited by the substrates mephedrone and METH, whereas blockers MDPV and cocaine elicit an outward hyperpolarizing current via DAT expressed in Xenopus oocytes (Cameron et al., 2013). The data in Fig. 6 indicate that the ratio of binding affinity to uptake inhibition potency predicts releasing efficacy in this model of transporters expressed in HEK cells with [125I]RTI-55 as the radioligand. For example, the low ratios at hDAT and hNET and much higher ratios at hSERT for 4-MEC, 5-MeO-methylone, and ethylone (Table 2) predict that these compounds would be blockers at hDAT and hNET but substrates at hSERT. As anticipated, these compounds were partially to fully efficacious releasers at hSERT (67–99%, Table 3), whereas none elicited release via hDAT, and only 4-MEC minimally released (17%) via hNET. These results agree with Saha et al. (2015), who reported that 4-MEC displays unique activity as a SERT substrate while being a DAT blocker. The binding-to-uptake ratios may be a quick metric to discriminate inhibitors from substrates, as they can be determined more quickly and easily than other methods, such as the in vitro efflux methods reported here or the voltage-clamp current measures in Xenopus oocytes.
Drugs that are hDAT-selective produce more stimulant and abuse-related effects than substances with mixed action at hDAT and hSERT (Wee et al., 2005; Baumann et al., 2011). The halide substitutions at either the para or ortho position of MCAT decrease the hDAT:hSERT uptake selectivity, as MCAT had 136-fold higher potency at hDAT, whereas 3-F MCAT, 4-Cl MCAT, and 4-Br MCAT had 60.3-, 3.22-, and 0.96-fold higher hDAT selectivity, respectively (Table 2). Similar effects are seen with the hDAT:hSERT release selectivity, with MCAT, 3-F MCAT, 4-Cl MCAT, and 4-Br MCAT having 46-, 30-, 0.68-, and 0.29-fold higher hDAT selectivity, respectively (Tables 1 and 3). These results are consistent with para halide MCAT substitutions decreasing the DAT:SERT release potencies from rat synaptosomes (Bonano et al., 2015). MCAT robustly increases extracellular DA, whereas 4-F MCAT and 4-Cl MCAT robustly and 4-Br MCAT modestly increase both extracellular DA and 5-HT, as measured by microdialysis in rats (Suyama et al., 2016). The ratios of (in vivo) potencies to release DA and 5-HT correlate positively with maximal ICSS, a procedure that evaluates abuse potential, and negatively with the steric bulk of the compounds (Suyama et al., 2016). Thus, the abuse potential may decrease, whereas more MDMA-like empathogenic activity may increase, within this series.
With the in vitro model used here, all substrates, except butylone at hSERT and 4-MEC at hNET, had 7-17 times lower potency, on average, at inducing release than at inhibition of uptake (Fig. 7). This finding may not apply to all model systems. Sandtner et al. (2016) reported higher potencies for release than for uptake for MDMA at the three transporters using rat brain synaptosomes, whereas MDMA was 3-, 3-, and 18-fold less potent for release compared with uptake at hNET, hSERT, and hDAT, respectively (Tables 2 and 3). The release models are quite different, with HEK cells expressing a single transporter with no blocking agents required, and the superfusion assay involved continuous removal of released substrate from the reaction, compared with rat brain synaptosomes that require blockade of VMAT2 as well as blockade of other transporters, and the “static” release assay does not remove released neurotransmitter from the reaction. Another difference between the assays is incubation time, as the release assay with synaptosomes can take up to 30 minutes, whereas in the superfusion assay, cells are exposed to drug for 22 minutes, and the peak of release occurs at 6–8 minutes after drug addition. Both models have similar results discriminating between inhibitors and substrates. The absence of cocaine-induced release at SERT (Fig. 2) differs from the findings of Scholze et al. (2000), who observed paroxetine-induced “pseudorelease” due to reuptake blockade using attached HEK-hSERT cells with a high concentration of [3H]5-HT and longer washout time.
In summary, except for ethylone, MDAI, and 5-MeO-methylone, all substituted cathinones and 4-FA had high to moderate potencies for inhibition of uptake at hDAT (less than 1 µM). Compounds with higher potency at hDAT than at hSERT, including all α-pyrrolidinophenones, 3-F MCAT, pentedrone, and 4-FA, have a high likelihood of abuse and addiction and stimulant properties similar to METH and/or cocaine. Increasing α-pyrrolidinophenone substituent chain length increases potency at the hDAT and may increase abuse potential. Compounds with similar potencies at hDAT and hSERT, or higher potency at hSERT than hDAT (4-Cl-MCAT, 4-Br MCAT, 4-MEC, 5-MeO-methylone, ethylone, pentylone, and MDAI), may have more empathogenic activity similar to MDMA, but also have some potential for abuse.
Acknowledgments
The authors thank the Research Triangle Institute and the National Institute on Drug Abuse drug supply program for providing many of the drugs used in the studies. The authors thank Yuan Chou for technical expertise.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- 4-Br MCAT
4-bromomethcathinone
- butylone
β-keto-methylbenzodioxolylbutanamine
- 4-Cl MCAT
4-chloromethcathinone
- DA
dopamine
- DAT
dopamine transporter
- ethylone
3,4-methylenedioxy-N-ethylcathinone (bk-MDEA)
- 4-FA
4-fluoroamphetamine
- 3-F MCAT
3-fluoromethcathinone
- 4-F MCAT
4-fluoromethcathinone
- hDAT
human dopamine transporter
- HEK
human embryonic kidney
- hNET
human norepinephrine transporter
- hSERT
human serotonin transporter
- 5-HT
5-hydroxy-tryptamine, serotonin
- ICSS
intracranial self-stimulation
- MCAT
methcathinone
- MDAI
5,6-methylenedioxy-2-aminoindane
- MDMA
3,4-methylenedioxymethylamphetamine
- 3,4-MDPBP
3,4-methylenedioxy-α-pyrrolidinobutiophenone
- 3,4-MDPPP
3,4-methylenedioxy-α-pyrrolidinopropiophenone
- MDPV
3,4-methylenedioxypyrovalerone
- 4-MEC
4-methylethcathinone
- 5-MeO-methylone
5-methoxy-methylone (2-A1MP)
- 4-MeO-α-PVP
4-methoxy-α-pyrrolidinovalerophenone
- 4-MePPP
4-methyl-α-pyrrolidinopropiophenone
- METH
(+)methamphetamine
- NE
norepinephrine
- NET
norepinephrine transporter
- α-PBP
α-pyrrolidinobutiophenone
- PCA
p-chloroamphetamine
- pentedrone
α-methylaminovalerophenone
- pentylone
methylenedioxypentedrone
- α-PHP
α-pyrrolidinohexiophenone (PV-7)
- α-PPP
α-pyrrolidinopropiophenone
- PV-8
1-phenyl-2-(1-pyrrolidinyl)-1-heptanone
- α-PVP
α-pyrrolidinovalerophenone
- α-PVT
α-pyrrolidinopentiothiophenone
- RTI-55
methyl (1R,2S,3S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- SERT
serotonin transporter
Authorship Contributions
Participated in research design: Eshleman, Janowsky.
Conducted experiments: Eshleman, Wolfrum, Reed, Swanson, Kim, Johnson.
Performed data analysis: Eshleman, Wolfrum, Reed, Swanson, Kim, Johnson.
Wrote or contributed to the writing of the manuscript: Eshleman, Janowsky.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Interagency Agreement ADA12013 (A.J., A.J.E.) and Grant P50 DA018165 (A.J)], the Department of Justice Drug Enforcement Administration [Interagency Agreement D-15-OD-0002], and Veterans Affairs Merit Review and Career Scientist programs (A.J.).
The authors have no actual or potential conflicts of interest.
Portions of this work were presented at Eshleman, AJ, Wolfrum KM, Reed JF, Chou YK and Janowsky A. (2013) Synthetic methcathinones: Correlation of potencies for inhibition of uptake and stimulation of neurotransmitter release via biogenic amine transporters. Society for Neuroscience abstract. 213.05, Pharmacology of psychoactive designer “bath salts”, AJ Eshleman et al., abstract #150.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. (2015) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 232:3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer RP. (2009) Fluoromethcathinone, a new substance of abuse. Forensic Sci Int 185:10–20. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bulling S, Benaderet TS, Saha K, Ayestas MA, Partilla JS, Ali SF, Stockner T, Rothman RB, Sandtner W, et al. (2014) Evidence for a role of transporter-mediated currents in the depletion of brain serotonin induced by serotonin transporter substrates. Neuropsychopharmacology 39:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. (2011) In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther 337:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Volkow ND. (2016) Abuse of New Psychoactive Substances: Threats and Solutions. Neuropsychopharmacology 41:663–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, Partilla JS, Baumann MH, Negus SS. (2015) Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol 172:2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. (2011) Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Test Anal 3:569–575. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA. (2013) Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 227:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Solis E, Jr, Ruchala I, De Felice LJ, Eltit JM. (2015) Amphetamine activates calcium channels through dopamine transporter-mediated depolarization. Cell Calcium 58:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. (1994) The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res 668:180–193. [DOI] [PubMed] [Google Scholar]
- Caudevilla-Gálligo F, Ventura M, Indave Ruiz BI, Fornís I. (2013) Presence and composition of cathinone derivatives in drug samples taken from a drug test service in Spain (2010-2012). Hum Psychopharmacol 28:341–344. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. (2014) Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci 97:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice (2016) Schedules of controlled substances: Extension of temporary placement of 10 synthetic cathinones in schedule I of the Controlled Substances Act. Final order. Fed Regist 81:11429–11431. [PubMed] [Google Scholar]
- Elliott S, Evans J. (2014) A 3-year review of new psychoactive substances in casework. Forensic Sci Int 243:55–60. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. (1999) Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther 289:877–885. [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, DeFelice LJ, Duke BJ, Moore KR, Blakely RD. (1995) Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol 198:2197–2212. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. (2015a) Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther 354:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. (2016) Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav Pharmacol 27:497–505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. (2011) Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther 338:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. (2015b) Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 232:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. (2014) Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 97:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. (1987) Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav 26:547–551. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Evans-Brown M, Sedefov R. (2013) Getting up to speed with the public health and regulatory challenges posed by new psychoactive substances in the information age. Addiction 108:1700–1703. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, et al. (2011) 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther 339:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Henke A, Karpowicz RJ, Jr, Sonders MS, Farrimond F, Edwards R, Sulzer D, Sames D. (2013) New fluorescent substrate enables quantitative and high-throughput examination of vesicular monoamine transporter 2 (VMAT2). ACS Chem Biol 8:1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JY, Kim JS, Oh JH, Hong SI, Ma SX, Jung YH, Ko YH, Lee SY, Kim HC, Jang CG. (2015) The new stimulant designer compound pentedrone exhibits rewarding properties and affects dopaminergic activity. Addict Biol DOI: 10.1111/adb.12299[published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. (2013) Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. (2011) Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal 3:439–453. [DOI] [PubMed] [Google Scholar]
- Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA. (2015) Structural Modification of the Designer Stimulant α-Pyrrolidinovalerophenone (α-PVP) Influences Potency at Dopamine Transporters. ACS Chem Neurosci 6:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. (2012) Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol 167:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. (2013) Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J Anal Toxicol 37:135–146. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. (2012) Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. (2006) 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem 49:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto K, Striebel J, Cho AK, Wang C. (2013) Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug Alcohol Depend 132:1–12. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. (1990) Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 18:3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL. (2015) Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Brewster WK, Johnson MP, Oberlender R, Riggs RM. (1990) Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA). J Med Chem 33:703–710. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. (1991) Structural variation and (+)-amphetamine-like discriminative stimulus properties. Pharmacol Biochem Behav 38:581–586. [DOI] [PubMed] [Google Scholar]
- Odoardi S, Romolo FS, Strano-Rossi S. (2016) A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013-2015. Forensic Sci Int 265:116–120. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. (2012) The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 8:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME. (2015) Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol 25:365–376. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Partilla JS, Baumann MH, Dersch CM, Carroll FI, Rice KC. (2000) Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse. Synapse 35:222–227. [DOI] [PubMed] [Google Scholar]
- Saha K, Partilla JS, Lehner KR, Seddik A, Stockner T, Holy M, Sandtner W, Ecker GF, Sitte HH, Baumann MH. (2015) ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology 40:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandtner W, Stockner T, Hasenhuetl PS, Partilla JS, Seddik A, Zhang YW, Cao J, Holy M, Steinkellner T, Rudnick G, et al. (2016) Binding Mode Selection Determines the Action of Ecstasy Homologs at Monoamine Transporters. Mol Pharmacol 89:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH. (2000) Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J Pharmacol Exp Ther 293:870–878. [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. (2014a) Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79:152–160. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME. (2014b) Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem Pharmacol 88:237–244. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. (2015) Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci 36:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. (1998) Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem 71:1289–1297. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. (1997) Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci 17:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. [DOI] [PubMed] [Google Scholar]
- Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML. (2016) Abuse-Related Neurochemical Effects of Para-Substituted Methcathinone Analogs in Rats: Microdialysis Studies of Nucleus Accumbens Dopamine and Serotonin. J Pharmacol Exp Ther 356:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykutera M, Cychowska M, Bloch-Boguslawska E. (2015) A Fatal Case of Pentedrone and α-Pyrrolidinovalerophenone Poisoning. J Anal Toxicol 39:324–329. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Burrows BT, Hernandez RD, Moore KN, Grabenauer M, Marusich JA, Olive MF. (2014) Effects of α-pyrrolidinopentiophenone and 4-methyl-N-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int J Neuropsychopharmacol 18:pyu014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854. [DOI] [PubMed] [Google Scholar]