Abstract
Exposure to organophosphorus toxins induces seizures that progress to status epilepticus (SE), which can cause brain damage or death. Seizures are generated by hyperstimulation of muscarinic receptors, subsequent to inhibition of acetylcholinesterase; this is followed by glutamatergic hyperactivity, which sustains and reinforces seizure activity. It has been unclear which muscarinic receptor subtypes are involved in seizure initiation and the development of SE in the early phases after exposure. Here, we show that pretreatment of rats with the selective M1 receptor antagonist, VU0255035 [N-(3-oxo-3-(4-(pyridine-4-yl)piperazin-1-yl)propyl)-benzo[c][1,2,5]thiadiazole-4 sulfonamide], significantly suppressed seizure severity and prevented the development of SE for about 40 minutes after exposure to paraoxon or soman, suggesting an important role of the M1 receptor in the early phases of seizure generation. In addition, in in vitro brain slices of the basolateral amygdala (a brain region that plays a key role in seizure initiation after nerve agent exposure), VU0255035 blocked the effects produced by bath application of paraoxon—namely, a brief barrage of spontaneous inhibitory postsynaptic currents, followed by a significant increase in the ratio of the total charge transferred by spontaneous excitatory postsynaptic currents over that of the inhibitory postsynaptic currents. Furthermore, paraoxon enhanced the hyperpolarization-activated cation current Ih in basolateral amygdala principal cells, which could be one of the mechanisms underlying the increased glutamatergic activity, an effect that was also blocked in the presence of VU0255035. Thus, selective M1 antagonists may be an efficacious pretreatment in contexts in which there is risk for exposure to organophosphates, as these antagonists will delay the development of SE long enough for medical assistance to arrive.
Introduction
There is ample evidence to suggest that the primary mechanism by which organophosphates (OPs), used as insecticides or in chemical warfare (nerve agents), induce prolonged, severe seizures or status epilepticus (SE) involves hyperstimulation of muscarinic acetylcholine receptors (mAChRs). Thus, phosphorylation of acetylcholinesterase by these toxins inactivates the enzyme (Sirin et al., 2012), resulting in accumulation of acetylcholine at cholinergic synapses. Although both nicotinic acetylcholine receptors and mAChRs can be expected to be activated by the elevated acetylcholine, it is primarily the activation of mAChRs that elicits the generation of seizures. Evidence for this has been provided by studies showing that mAChR antagonists prevent the induction of seizures if administered before OP exposure (Ashani and Catravas, 1981; McDonough et al., 1987; Capacio and Shih, 1991; Skovira et al., 2010) and can arrest seizures if administered shortly after exposure (McDonough et al., 1989; Anderson et al., 1997), whereas nicotinic receptor antagonists do not suppress OP-induced seizures in vivo (Shih et al., 1991; Dekundy et al., 2003) or epileptiform activity in vitro (Harrison et al., 2004). Antagonists of mAChRs are ineffective in stopping seizures if injected at delayed time points after OP exposure, because after the initial phase of SE, glutamatergic mechanisms that have come into play are primarily responsible for reinforcing and sustaining seizures (McDonough and Shih, 1997; Weissman and Raveh, 2008). It has not been clear, however, which of the mAChR subtypes are involved in the initiation of seizures, leading to and sustaining SE in the early phases postexposure.
There are five subtypes of mAChRs: M1–M5. They all are G protein coupled; M2 and M4 are preferentially coupled to the Gi class of G proteins, whereas M1, M3, and M5 are coupled to the Gq family (Wess, 2003; Kruse et al., 2014). Hamilton et al. (1997) and Bymaster et al. (2003) found that pilocarpine, a muscarinic agonist, induces SE in wild-type mice but not in mice lacking the M1 receptor, suggesting a key role for M1 receptors in the induction of SE by pilocarpine. In addition, a highly selective M1 receptor antagonist was developed recently, VU0255035 [N-(3-oxo-3-(4-(pyridine-4-yl)piperazin-1-yl)propyl)-benzo[c][1,2,5]thiadiazole-4 sulfonamide] (Weaver et al., 2009), and was tested against seizures induced by pilocarpine. Mice pretreated with VU0255035 did not develop SE, at least for 40 minutes after injection of pilocarpine (Sheffler et al., 2009). Because pilocarpine and OPs share the primary mechanism of seizure induction (muscarinic receptor hyperstimulation), it has been suggested that findings in the pilocarpine model of SE may also apply to OP-induced SE (Tetz et al., 2006; Tang et al., 2011). Whether M1 receptor activation, which is necessary for induction of seizures by pilocarpine, is also necessary for induction of seizures by OPs was recently tested in wild-type and M1-knockout mice (Kow et al., 2015) exposed to paraoxon (O,O-diethyl O-p-nitrophenyl phosphate), which is the active metabolite of the insecticide parathion (Garcia et al., 2003). No difference was found in paraoxon-induced seizure severity between the wild-type and the M1-knockout mice, leading to the conclusion that in contrast to the significant role that the M1 receptor plays in pilocarpine-induced seizures, these receptors are not involved in the generation of seizures by paraoxon exposure (Kow et al., 2015).
Delineating the mechanisms that are involved in the generation of seizures after OP exposure can result in more targeted pretreatments and early-phase, postexposure treatments that have minor or no side effects. Therefore, to shed more light on the role of the M1 receptor in seizure generation by OPs, we examined the effect of pretreatment with the selective M1 antagonist VU0255035 on seizure development and severity by exposure of rats to soman or paraoxon. We further examined the effects of paraoxon on neuronal excitability parameters in in vitro brain slices and whether M1 receptors are involved in these effects. The in vitro studies were performed in the basolateral amygdala (BLA), a seizure-prone amygdala nucleus (Aroniadou-Anderjaska et al., 2008), because there is strong evidence to suggest that the BLA plays a key role in seizure generation after nerve agent exposure (McDonough et al., 1987; Prager et al., 2013).
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in an environmentally controlled room (20–23°C, 12-hour light/dark cycle, lights on 06:00 AM), with food and water available ad libitum. The animal care and use programs at the Uniformed Services University of the Health Sciences (Bethesda, MD) and the U.S. Army Medical Research Institute of Chemical Defense (Aberdeen Proving Ground, MD) are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources and the U.S. National Research Council and were approved by the Institutional Animal Care and Use Committees of both of our institutions.
Paraoxon and Soman Exposure.
Ten-week-old male rats were administered 25 mg/kg (i.p.) of the selective M1 receptor antagonist VU0255035 (Tocris Bioscience, Ellisville, MO) diluted at 25 mg/ml in dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO), or 1 ml/kg (i.p.) DMSO as the vehicle, 15 minutes before exposure to soman (pinacoyl methylphosphonofluoridate; obtained from the Edgewood Chemical Biologic Center, Aberdeen Proving Ground, Edgewood, MD), or 30 minutes before exposure to paraoxon (paraoxon-ethyl; Sigma-Aldrich). The concentration of VU0255035 that we used is within the range that has no significant effect on cognitive functions (Sheffler et al., 2009). For the soman experiments, animals were subcutaneously injected with 1.8 × LD50 soman (198 μg/kg, diluted in cold physiologic saline). For the paraoxon experiments, animals were subcutaneously injected with 4 mg/kg paraoxon; paraoxon solutions were prepared fresh by adding 10-ml stock solution (1.274 g/ml) to 3 ml ice-cold phosphate-buffered saline in a glass vial and mixing thoroughly. Soman was used because of its high toxicity and the difficulty in counteracting soman-induced seizures, which implies that if a drug is effective in suppressing seizures induced by soman, it is likely to also be effective against other nerve agents. Paraoxon was used because it is highly potent, has similar effects to those of soman, and there is extensive literature on its use as a model of OP poisoning, including its use as a surrogate for chemical warfare nerve agents. The peripheral toxic cholinergic effects of soman and paraoxon were controlled by intramuscular injection of either 2 mg/kg atropine methylnitrate, or 2 mg/kg atropine sulfate along with 25 mg/kg pralidoxime (all three drugs from Sigma-Aldrich), administered within 1 minute after injection of the OP. Seizures were monitored behaviorally in a blind fashion by two expert observers. Seizure severity was scored for 1 hour, using a modified version of the Racine (1972) scale as we described previously (Figueiredo et al., 2011): stage 0, no behavioral response; stage 1, behavioral arrest; stage 2, oral/facial movements, chewing, head nodding; stage 3, unilateral/bilateral forelimb clonus without rearing, Straub tail, extended body posture; stage 4, bilateral forelimb clonus plus rearing; stage 5, rearing and falling; and stage 6, full tonic-clonic seizures. Behavioral seizures above stage 3 indicate SE (Abdullah and Rafiqul Islam, 2012; Rossetti et al., 2012; Apland et al., 2014; Deshpande et al., 2014).
Electrophysiology.
Male rats, aged 25–30 days, were anesthetized with isoflurane prior to decapitation for preparation of brain slices. Coronal slices (400-μm thick) containing the amygdala were cut as described previously (Aroniadou-Anderjaska et al., 2012). Recording solution [artificial cerebrospinal fluid (ACSF)] consisted of the following: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 21 mM NaHCO3, 2 mM CaCl2, 1 mM MgCl2, and 11 mM d-glucose. The ACSF was saturated with 95% O2/5% CO2 to achieve a pH near 7.4, and the osmolarity was adjusted to 325 mOsm with d-glucose. The slice chamber (0.7-ml capacity) had continuously flowing ACSF (approximately 8 ml/min) at a temperature of 31–32°C. Tight-seal (>1 GΩ), whole-cell recordings from principal neurons in the BLA were performed as described previously (Pidoplichko et al., 2014). Neurons were visualized under infrared light using Nomarski optics of an upright microscope (Axioskop 2; Zeiss, Thornwood, NY) through a ×40 water immersion objective, equipped with a CCD-100 camera (Dage-MTI, Michigan City, IN). BLA principal cells were identified by their pyramidal shape and the presence of the hyperpolarization-activated cationic current (Ih; Aroniadou-Anderjaska et al., 2012). Borosilicate glass patch electrodes had a resistance of 3.5–4.5 MΩ when filled with an internal solution of the following composition: 60 mM CsCH3SO3, 60 mM KCH3SO3, 10 mM EGTA, 10 mM HEPES, 5 mM KCl, 5 mM Mg-ATP, and 0.3 mM Na3GTP, pH 7.2, 290 mOsm. The 5 mM KCl allowed the simultaneous recordings of spontaneous inhibitory postsynaptic currents (sIPSCs) and excitatory postsynaptic currents (sEPSCs). Access resistance (15–24 MΩ) was regularly monitored during recordings, and cells were rejected if the resistance changed by >15% during the experiment. Ionic currents were amplified using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and filtered (1 kHz) with a four-pole, low-pass Bessel filter. Currents were digitally sampled (up to 2 kHz) using pClamp 10.2 software (Molecular Devices, Sunnyvale, CA) and further analyzed using the Mini Analysis Program (Synaptosoft, Fort Lee, NJ) and Origin (OriginLab, Northampton, MA). Drugs used for the in vitro experiments were paraoxon-ethyl, VU0255035, atropine sulfate, d-AP5 [d-(−)-2-amino-5-phosphonopentanoic acid, an N-methyl-d-aspartate receptor antagonist; Tocris Bioscience], SCH50911 [(2S)-(+)-5,5-dimethyl-2-morpholineacetic acid, an GABAB receptor antagonist; Tocris Bioscience], and LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid, a metabotropic glutamate group II/III receptor antagonist; Tocris Bioscience].
Statistical Analysis.
Seizure score differences between the OP-exposed and the vehicle groups were tested for statistical significance using independent-samples t tests. In vitro electrophysiological results were analyzed using paired t tests. Differences were considered statistically significant when P < 0.05. Data are presented as means ± S.E.M. Sample sizes (n) refer to the number of animals for the in vivo experiments and the number of recorded neurons for the whole-cell experiments.
Results
Effects of VU0255035 Pretreatment on Seizure Severity after Exposure to Soman.
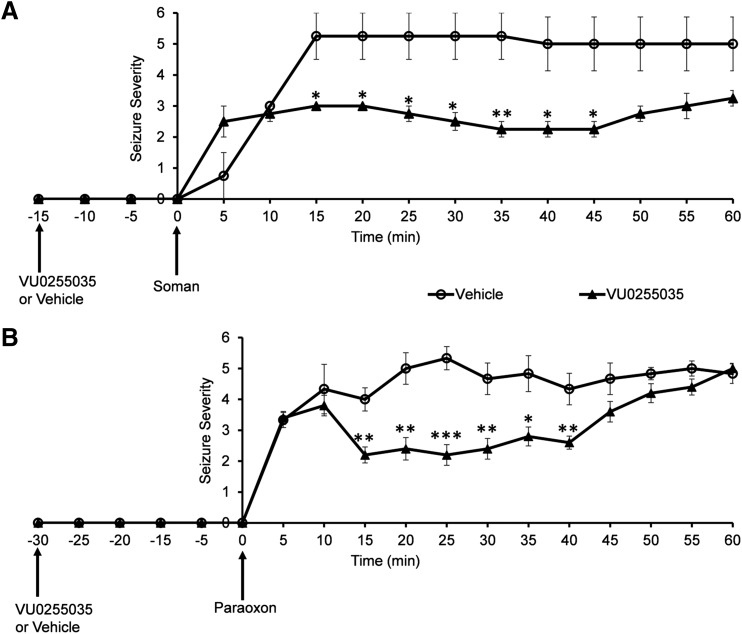
Eight rats were randomly divided into two groups: a group that was injected with VU0255035 (25 mg/kg, n = 4) and a group injected with the vehicle (DMSO; 1 ml/kg, n = 4), at 15 minutes before exposure to soman (198 μg/kg). All rats developed seizures, and there was no statistically significant difference in seizure scores between the two groups at 5 minutes (0.75 ± 0.75 for the vehicle group and 2.5 ± 0.5 for the VU0255035 group; P = 0.1) or 10 minutes (3 ± 0 for the vehicle group and 2.75 ± 0.25 for the VU0255035 group; P = 0.3) after injection of soman. However, for the next 35 minutes, Racine scale scores for the VU2055035 group were significantly lower compared with the vehicle group (Fig. 1A). At 15 minutes after soman injection, the seizure score was 5.25 ± 0.75 for the vehicle group and 2.75 ± 0.25 for the VU0255035 group (P = 0.025). At 20 minutes postexposure, seizure scores were 5.25 ± 0.75 for the vehicle group and 3 ± 0 for the VU0255035 group (P = 0.025). At 25 minutes, seizure scores were 5.25 ± 0.75 for the vehicle group and 2.75 ± 0.25 for the VU0255035 group (P = 0.020). At 30 minutes, seizure scores were 5.25 ± 0.75 for the vehicle group and 2.5 ± 0.3 for the VU0255035 group (P = 0.015). At 35 minutes after soman injection, seizure scores were 5.25 ± 0.75 for the vehicle group and 2.25 ± 0.25 for the VU0255035 group (P = 0.009). At 40 minutes, seizure scores were 5 ± 1 for the vehicle group and 2.25 ± 0.25 for the VU0255035 group (P = 0.026). At 45 minutes postexposure, seizure scores were 5 ± 1 for the vehicle group and 2.25 ± 0.25 for the VU0255035 group (P = 0.026). During the last 15 minutes of observation, there were no significant differences in the Racine scale scores between vehicle and VU0255035 groups, respectively, (Fig. 1A; at 50 minutes, 5 ± 1 and 2.75 ± 0.25, P = 0.052; 55 minutes, 5 ± 1 and 3 ± 0.41, P = 0.09; and 60 minutes, 5 ± 1 and 3.25 ± 0.25, P = 0.10). These results are summarized in Table 1.
Fig. 1.
Pretreatment with the selective M1 receptor antagonist VU0255035 reduces seizure severity after exposure to soman or paraoxon. (A) Administration of VU0255035 (25 mg/kg), 15 minutes before exposure to soman (1.8 × LD50), significantly reduced seizure severity scores from 15 minutes to 45 minutes after soman injection (n = 4 in each of the two groups). (B) Administration of VU0255035 (25 mg/kg), 30 minutes before exposure to paraoxon (4 mg/kg), significantly reduced seizure severity scores from 15 minutes to 40 minutes after paraoxon injection (n = 6 in the VU0255035 group and n = 5 in the vehicle group). *P < 0.05; **P < 0.01; ***P < 0.001.
TABLE 1.
Seizure severity after exposure to soman, in VU0255035-pretreated rats compared with vehicle-pretreated rats
Data are presented as means ± S.E.M.
| Time after Soman Injection | Behavioral Seizure Score |
P Value | |
|---|---|---|---|
| Vehicle Group | VU0255035 Group | ||
| min | |||
| 5 | 0.75 ± 0.75 | 2.5 ± 0.5 | 0.1 |
| 10 | 3 ± 0 | 2.75 ± 0.25 | 0.3 |
| 15 | 5.25 ± 0.75 | 2.75 ± 0.25 | 0.025* |
| 20 | 5.25 ± 0.75 | 3 ± 0 | 0.025* |
| 25 | 5.25 ± 0.75 | 2.75 ± 0.25 | 0.02* |
| 30 | 5.25 ± 0.75 | 2.5 ± 0.3 | 0.015* |
| 35 | 5.25 ± 0.75 | 2.25 ± 0.25 | 0.009** |
| 40 | 5 ± 1 | 2.25 ± 0.25 | 0.026* |
| 45 | 5 ± 1 | 2.25 ± 0.25 | 0.026* |
| 50 | 5 ± 1 | 2.75 ± 0.25 | 0.052 |
| 55 | 5 ± 1 | 3 ± 0.41 | 0.09 |
| 60 | 5 ± 1 | 3.25 ± 0.25 | 0.1 |
P < 0.05; **P < 0.01.
Effects of VU0255035 Pretreatment on Seizure Severity after Exposure to Paraoxon.
Next, we examined whether pretreatment with VU0255035 also decreases seizure severity after exposure to paraoxon. Because behavioral seizure scores did not differ between the VU0255035 group and the vehicle group during the first 10 minutes after soman exposure (Fig. 1A), with VU0255035 administered 15 minutes before soman injection, this time we administered VU0255035 30 minutes before paraoxon exposure, considering that perhaps 15 minutes were not sufficient for VU0255035 to take full effect. Eleven rats were randomly divided into two groups: a group that was injected with 25 mg/kg VU0255035 (n = 6) and a group injected with the vehicle (DMSO; 1 ml/kg, n = 5), at 30 minutes before exposure to paraoxon (4 mg/kg). All rats developed seizures. Again, the Racine score did not differ significantly between the vehicle group and the VU0255035 group, respectively, during the first 10 minutes after paraoxon injection (at 5 minutes, 3.33 ± 0.24 and 3.4 ± 0.21, P = 0.8; and at 10 minutes, 4.33 ± 0.8 and 3.8 ± 0.33, P = 0.5). However, for the next 30 minutes, Racine scale scores for the VU2055035 group were significantly lower compared with the vehicle group (Fig. 1B). At 15 minutes after paraoxon injection, the seizure score was 4 ± 0.37 for the vehicle group and 2.2 ± 0.26 for the VU0255035 group (P = 0.003). At 20 minutes postexposure, seizure scores were 5 ± 0.51 for the vehicle group and 2.4 ± 0.37 for the VU0255035 group (P = 0.002). At 25 minutes, seizure scores were 5.33 ± 0.37 for the vehicle group and 2.2 ± 0.33 for the VU0255035 group (P = 0.0001). At 30 minutes, seizure scores were 4.67 ± 0.51 for the vehicle group and 2.4 ± 0.33 for the VU0255035 group (P = 0.004). At 35 minutes after paraoxon injection, seizure scores were 4.83 ± 0.58 for the vehicle group and 2.8 ± 0.25 for the VU0255035 group (P = 0.010). At 40 minutes, seizure scores were 4.33 ± 0.51 for the vehicle group and 2.6 ± 0.21 for the VU0255035 group (P = 0.008). During the last 20 minutes of observation, there were no significant differences in the Racine scale scores between vehicle and VU0255035 groups, respectively (Fig. 1B; at 45 minutes, 4.67 ± 0.51 and 3.6 ± 0.33, P = 0.104; at 50 minutes, 4.83 ± 0.2 and 4.2 ± 0.31, P = 0.134; at 55 minutes, 5 ± 0.24 and 4.4 ± 0.26, P = 0.131; and at 60 minutes, 4.83 ± 0.32 and 5 ± 0.17, P = 0.635). These results are summarized in Table 2.
TABLE 2.
Seizure severity after exposure to paraoxon in VU0255035-pretreated rats compared with vehicle-pretreated rats
Data are presented as means ± S.E.M.
| Time after Paraoxon Injection | Behavioral Seizure Score | P Value | |
|---|---|---|---|
| Vehicle Group | VU0255035 Group | ||
| min | |||
| 5 | 3.33 ± 0.24 | 3.4 ± 0.21 | 0.8 |
| 10 | 4.33 ± 0.8 | 3.8 ± 0.33 | 0.5 |
| 15 | 4 ± 0.37 | 2.2 ± 0.26 | 0.003* |
| 20 | 5 ± 0.51 | 2.4 ± 0.37 | 0.002* |
| 25 | 5.33 ± 0.37 | 2.2 ± 0.33 | 0.0001** |
| 30 | 4.67 ± 0.51 | 2.4 ± 0.33 | 0.004* |
| 35 | 4.83 ± 0.58 | 2.8 ± 0.25 | 0.01*** |
| 40 | 4.33 ± 0.51 | 2.6 ± 0.21 | 0.008* |
| 45 | 4.67 ± 0.51 | 3.6 ± 0.33 | 0.104 |
| 50 | 4.83 ± 0.2 | 4.2 ± 0.31 | 0.134 |
| 55 | 5 ± 0.24 | 4.4 ± 0.26 | 0.131 |
| 60 | 4.83 ± 0.32 | 5 ± 0.17 | 0.635 |
P < 0.05; **P < 0.01; ***P < 0.001.
Effects of Paraoxon on Spontaneous Synaptic Activity in the BLA and the Role of M1 Receptors.
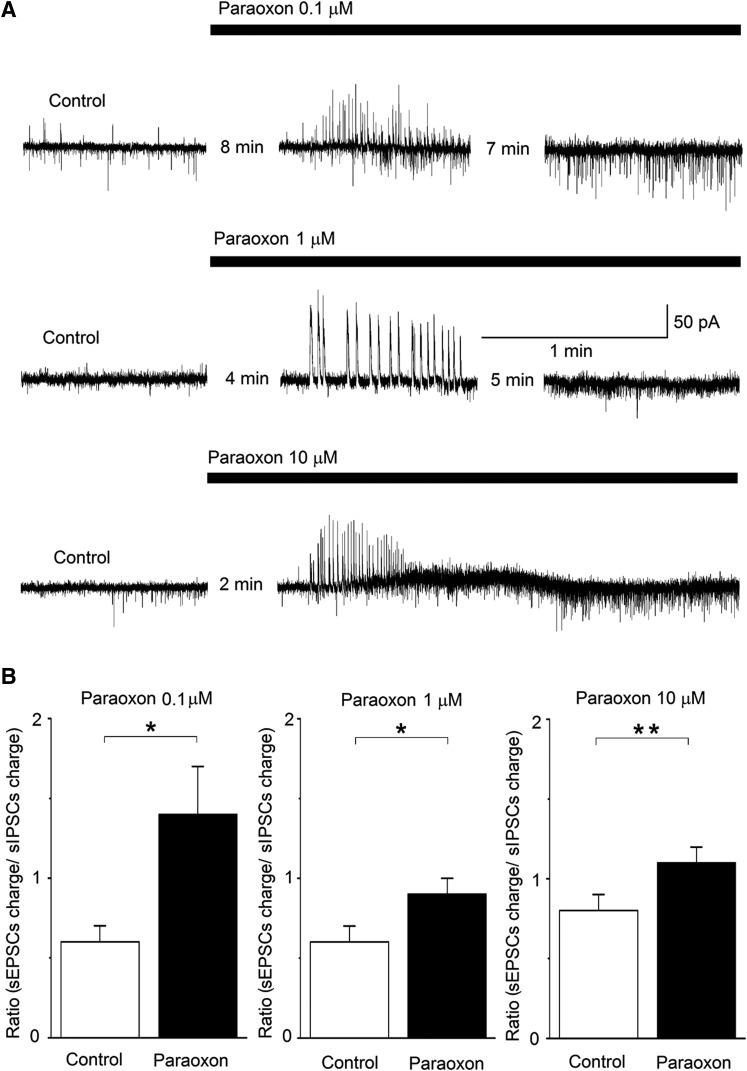
Whole-cell simultaneous recordings of sIPSCs and sEPSCs were obtained from principal BLA neurons, at Vh of −58 mV, in the presence of d-AP5 (50 μM), SCH50911 (10 μM), and LY341495 (3 μM); under these conditions, outward currents are mediated by GABAA receptors, whereas inward currents are mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors (Pidoplichko et al., 2014). Bath application of paraoxon, at three different concentrations (0.1, 1, and 10 μM), induced a transient barrage of large-amplitude sIPSCs with a duration of 1.1 ± 0.1 minutes (n = 21), which was followed by a lasting enhancement of preferentially sEPSCs (Fig. 2A). Paraoxon took effect (appearance of the barrage of sIPSCs) within 2–8 minutes after application, depending on the concentration (the lower concentration of paraoxon was slower to take effect). To quantify the relative increase of excitatory synaptic activity by paraoxon, we calculated the charge transferred by the excitatory and the inhibitory currents, during a 20-second time window, in control conditions and then again after paraoxon had taken effect. The charge transferred, in picocoulombs, was calculated as the area delimited by the excitatory or inhibitory current and the baseline. The ratio of the charge transferred by sEPSCs to the charge transferred by sIPSCs was increased significantly in the presence of paraoxon. When 0.1 μM paraoxon was applied, the sEPSC/sIPSC ratio was increased from 0.6 ± 0.1 in control conditions to 1.4 ± 0.3 at 17 minutes after paraoxon application (P < 0.05, n = 7). When 1 μM paraoxon was applied, the sEPSC/sIPSC ratio was increased from 0.6 ± 0.1 in control conditions to 0.9 ± 0.1 at 9 minutes after paraoxon application (P < 0.05, n = 4). Finally, when 10 μM paraoxon was applied, the ratio of sEPSC/sIPSC charge transfer was increased from 0.8 ± 0.1 in control conditions to 1.1 ± 0.1 at 6 minutes after paraoxon application (P < 0.01, n = 10; Fig. 2B).
Fig. 2.
Paraoxon enhances both sIPSCs and sEPSCs, with a significantly greater, lasting effect on the sEPSCs, in the BLA. Whole-cell simultaneous recordings of sIPSCs and sEPSCs were obtained from BLA principal cells (Vh = −58). (A) Representative examples of the effects of paraoxon at 0.1 μM, 1 μM, and 10 μM (traces are from three different neurons). Outward currents (upward deflections) are GABAergic and inward currents (downward deflections) are glutamatergic. The number of minutes between successive traces in a row is the time lapse from the time point paraoxon was applied, and then the interval between the two successive recordings. (B) Group data of the effects of paraoxon on the ratio of the charge transferred by sEPSCs over the charge transferred by sIPSCs. The charge transferred was calculated during a 20-second time window, in control conditions and after bath application of paraoxon. Calculations were made at 17 minutes after application of 0.1 μM paraoxon (n = 7; left), at 9 minutes after application of 1 μM paraoxon (n = 4; middle), and at 6 minutes after application of 10 μM paraoxon (n = 10; right). *P < 0.05; **P < 0.01.
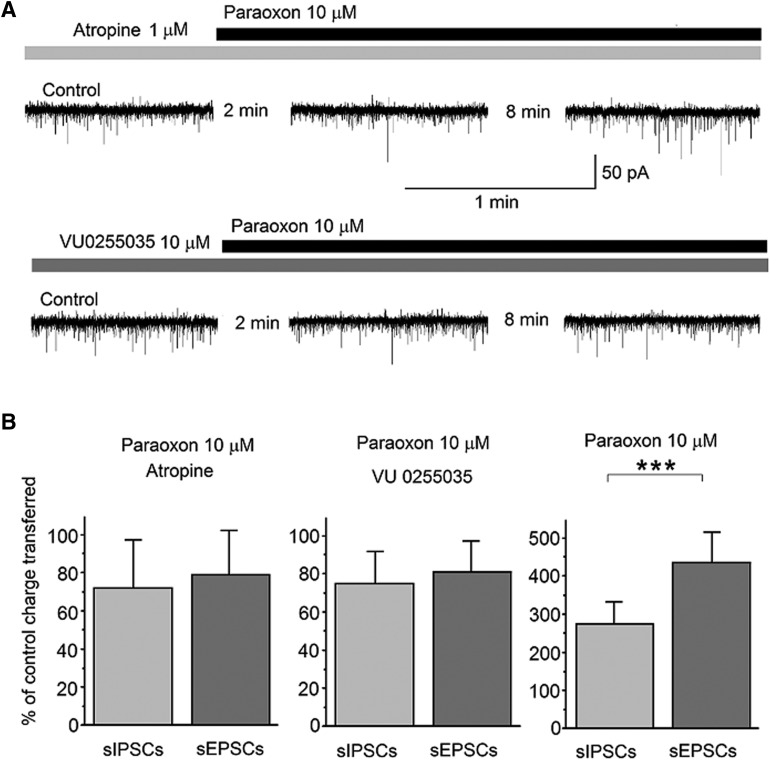
To determine whether mAChRs were involved in mediating the effects of paraoxon, first, we pretreated the slices with atropine (1 μM) for about 4 minutes, and then applied 10 μM paraoxon, while recording from principal BLA neurons. The holding potential was again −58 mV, and d-AP5 (50 μM), SCH50911 (10 μM), and LY341495 (3 μM) were present in the slice medium. Paraoxon, in the presence of atropine, had no significant effect on sIPSCs or sEPSCs (Fig. 3A, top traces). The total charge transferred by sIPSCs or sEPSCs during a 20-second time window, 10 minutes after paraoxon application, was expressed as a percentage of the total charge transferred, during 20 seconds, in control conditions (in the presence of atropine). When 1 μM atropine was present in the slice medium, the charge transferred by sIPSCs after 10 minutes in paraoxon was 72% ± 25% of the control (n = 7, P = 0.203), whereas the charge transferred by sEPSCs was 79% ± 23% of the control (n = 7, P = 0.055; Fig. 3B, left bar graph). Next, we performed similar experiments in the presence of VU0255035, to determine whether the M1 receptor was involved in mediating the effects of paraoxon. In 10 μM VU0255035, paraoxon had no significant effect on sIPSCs or sEPSCs (Fig. 3A, lower traces); the charge transferred by sIPSCs after 10 minutes in paraoxon was 75% ± 17% of the VU0255035-containing control (n = 7, P = 0.156), whereas the charge transferred by sEPSCs was 81% ± 16% of the VU0255035-containing control (n = 7, P = 0.389; Fig. 3B, middle bar graph). These results are in contrast with the effects of 10 μM paraoxon in the absence of muscarinic receptor antagonists (Fig. 2A, third row of traces). Under these conditions, the charge transferred by sIPSCs was increased by paraoxon to 275% ± 57% of the control (n = 10, P = 0.0001), and the charge transferred by sEPSCs was increased by paraoxon to 435% ± 80% of the control (n = 10, P = 0.00002; Fig. 3B, right bar graph). The difference between the increase in sIPSCs and the increase in sEPSCs was statistically significant (P < 0.001).
Fig. 3.
M1 muscarinic receptors mediate the effects of paraoxon on sIPSCs and sEPSCs in the BLA. Whole-cell simultaneous recordings of sIPSCs and sEPSCs were obtained from BLA principal cells (Vh = −58). (A) Representative traces show that 10 μM paraoxon had no significant effect when applied in the presence of atropine (1 μM, top row of traces) or VU0255035 (10 μM, second row of traces). (B) Effects of 10 μM paraoxon on sIPSCs and sEPSCs under three conditions: pretreatment with atropine (left bar graph; n = 7), pretreatment with VU0255035 (middle bar graph; n = 7), and in the absence of muscarinic receptor antagonists (right bar graph; n = 10). The total charge transferred by sIPSCs or sEPSCs during a 20-second time window, 10 minutes after paraoxon application, was expressed as a percentage of the total charge transferred, during 20 seconds, in control conditions. The total charge transferred by sIPSCs and sEPSCs was increased by paraoxon only when there was no atropine or VU0255035 in the slice medium, and the difference between the increase in sIPSCs and the increase in sEPSCs was statistically significant. ***P < 0.001.
Effects of Paraoxon on the Ih Current.
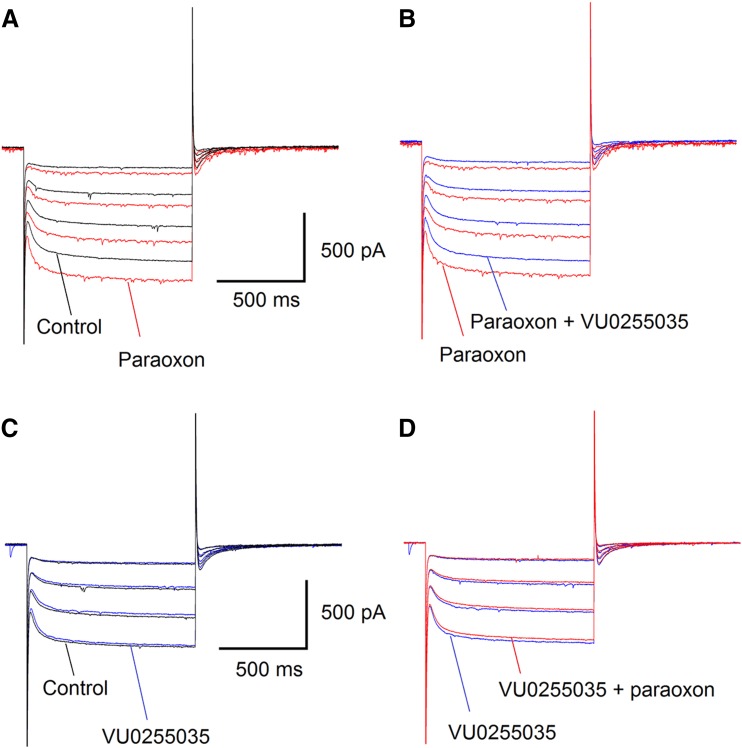
Previous studies have shown that one of the mechanisms by which M1 receptor activation depolarizes pyramidal neurons in the hippocampus is the increase of the hyperpolarization-activated, mixed Na+/K+ current (Ih; Fisahn et al., 2002). The preferential increase of spontaneous glutamatergic activity by paraoxon, in the BLA, suggests that paraoxon may depolarize principal BLA neurons. To determine whether such an effect could be mediated via enhancement of the Ih current, we evoked the Ih in principal BLA neurons by voltage steps from a Vh of −70 mV to −110 mV, in 10-mV increments. Bath application of 10 μM paraoxon had a consistent and clear enhancing effect on the Ih current (n = 4; Fig. 4A). Addition of 10 μM VU0255035 to the slice medium reversed the effect of paraoxon (Fig. 4B). Bath application of 10 μM VU0255035 alone (in the absence of paraoxon) had no effect on the Ih (Fig. 4C). When 10 μM paraoxon was added to the recording solution in the presence of 10 μM VU0255035, there was no effect on the Ih (Fig. 4D). These results suggest that the enhancement of Ih by paraoxon was mediated by M1 receptor activation.
Fig. 4.
Paraoxon enhances the hyperpolarization-activated cation current (Ih) in principal BLA neurons, via M1 muscarinic receptor activation. (A) Bath application of 10 μM paraoxon increased the Ih current elicited by hyperpolarizing steps (at 10-mV increments) from a Vh of −70 mV. The recordings shown are 3 minutes after application of paraoxon. (B) Addition of 10 μM VU0255035 to the slice medium reversed the effects of paraoxon. (A) and (B) are from the same neuron. (C) Application of 10 μM VU0255035 alone had no effect on the Ih. (D) Bath application of 10 μM paraoxon in the presence of 10 μM VU0255035 had no effect on the Ih. (C) and (D) are from the same neuron [different from (A) and (B)].
Discussion
This study demonstrated that pretreatment with the selective M1 receptor antagonist VU0255035 significantly suppresses behavioral seizures induced by either soman or paraoxon, for at least 40 minutes after exposure, preventing the development of SE during this time period. The in vitro experiments aiming to uncover some of the mechanisms involved in seizure generation by paraoxon and the involvement of M1 receptors showed that paraoxon enhances both inhibitory and excitatory spontaneous activity recorded from BLA principal cells, with a lasting greater increase in sEPSCs, such that the ratio of the charge transferred by sEPSCs over that of sIPSCs is increased; these effects of paraoxon are blocked in the presence of VU0255035. Finally, we found that paraoxon increases the Ih Na+/K+ current in BLA principal cells, an effect that is also blocked by VU0255035. The depolarization that the increase of Ih can induce may be one of the mechanisms by which paraoxon increases spontaneous excitatory activity in the BLA.
Knowledge of the specific subtypes of mAChRs that are involved in the initiation of SE after exposure to an OP can lead to the development of pretreatments or early postexposure treatments that do not have the side effects of nonselective mAChR antagonists. The role of M1 receptors, however, in seizure induction by OPs has been unclear. Previous studies have shown that pilocarpine, a muscarinic agonist, induces severe seizures in wild-type mice and in mice lacking the M2, M3, M4, or M5 receptor (Bymaster et al., 2003), but not in mice lacking the M1 receptor (Hamilton et al., 1997; Bymaster et al., 2003). In consistency with these studies, pretreatment of wild-type mice with VU0255035 decreased the severity of seizures induced by pilocarpine; the effect was significant at 35 and 40 minutes after pilocarpine injection, resulting in seizure severity below the level of SE at least for the first 40 minutes postinjection (Sheffler et al., 2009). Since both pilocarpine and OPs are known to induce seizures by mAChR hyperstimulation, it is reasonable to suggest that M1 receptors may also be important in the induction of seizures by OPs, including nerve agents (Bhattacharjee et al., 2013). However, Kow et al. (2015) found no differences between wild-type and M1 knockout mice in seizure severity scores or proportion of animals displaying at least one tonic-clonic seizure after exposure to paraoxon; it was concluded that pilocarpine and paraoxon induce seizures by different mechanisms. This study, however, in rats shows that the M1 receptor plays a central role in the severity of seizures induced by paraoxon, at least during the first 40 minutes after the exposure. This discrepancy may imply species differences in the role of the M1 receptor in OP-induced seizures.
The data in our study make it evident that during the first 10 minutes after OP injection, low-intensity seizures were initiated despite the VU0255035 pretreatment. Behavioral seizures stage 2 to 3 and slightly higher, which correspond to epileptic discharges on EEG (Abdullah and Rafiqul Islam, 2012), were observed during the first 10 minutes after soman or paraoxon injection in the VU0255035-treated rats as in the vehicle-treated rats, whereas the effects of VU0255035 pretreatment became significant after 10 minutes postexposure. Pharmacokinetic studies have shown that VU0255035 has already reached high concentrations in the brain at 30 minutes after the injection (Sheffler et al., 2009). Therefore, the absence of a significant effect of the VU0255035 pretreatment during the first 10 minutes is probably not attributable to a delay of the drug reaching the brain. Other mAChR subtypes may be involved in the generation of seizure activity at this early phase, or an increase of glutamate may be the culprit, since extracellular glutamate has been found to increase in the amygdala within the first 10 minutes after exposure to soman (Lallement et al., 1991). During the next 30 minutes (after the initial 10-minute period), the M1 antagonist in the VU0255035-treated group prevented SE (stage 3 and higher), but seizures below stage 3 were ongoing; again, other mAChR subtypes and/or glutamatergic mechanisms may be involved in sustaining low-intensity seizures during this postexposure period. After the 40-minute postexposure, the seizure-suppressing effects of VU0255035 pretreatment were no longer significant. Since VU0255035 concentrations in the brain remain relatively high for 2 hours after injection (Sheffler et al., 2009), the reduction in the seizure-suppressing effect of VU0255035 after the 40-minute period postexposure may not be attributable to the clearance of the compound from the brain but mainly reflects reduced significance of the M1 receptors, because glutamatergic mechanisms take over the primary role in sustaining and reinforcing seizures (McDonough and Shih, 1997; Weissman and Raveh, 2008). Thus, M1 receptors appear to be important during a certain time period after OP exposure (after the first 10 minutes and before 1 hour). This implies that if the effects of an M1 antagonist or the consequences of knocking out the M1 receptor (in mice) on seizure induction by pilocarpine or OPs are averaged over a period of time, instead of being evaluated every 5 minutes or during the 10–40 minutes postexposure, a significant role of the receptor may be missed.
The in vitro experiments were performed in the BLA because, in addition to the importance of other brain regions in seizure generation by OPs (Myhrer, 2007; Myhrer et al., 2007, 2010), there is strong evidence to suggest that the BLA plays a key role (Aroniadou-Anderjaska et al., 2009). Thus, when McDonough et al. (1987) microinjected nerve agents into different brain regions, they observed that convulsions were elicited only when the nerve agent was injected into the BLA. Prager et al. (2013) recently found that in the small proportion of rats who do not develop SE when exposed to high doses of soman, the activity of acetylcholinesterase is reduced in the hippocampus and the piriform cortex to a similar extent as in the rats who develop SE, but to a significantly lesser extent in the BLA; this suggests a critical role of acetylcholinesterase reduction in the BLA for SE to be induced. The importance of the amygdala in nerve agent–induced seizure generation is also highlighted by the findings that the amygdala displays the earliest increase in extracellular glutamate after exposure to soman (Lallement et al., 1991) and generates ictal-like discharges after in vitro application of soman (Apland et al., 2009). It is to be expected, therefore, that when the BLA is exposed to an OP, neuronal excitability will increase, which will be reflected in an increase of spontaneous glutamatergic activity. Indeed, in this study, paraoxon increased sEPSCs in the BLA significantly more than the increase of sIPSCs.
The in vitro effects of paraoxon were blocked in the presence of VU0255035, suggesting that both the increase in sIPSCs and that in sEPSCs were mediated via M1 receptor activation. The cellular location of these receptors where paraoxon acted remains to be determined. Cholinergic synapses in the BLA are formed by the dense cholinergic afferents arising from the basal forebrain (Ben-Ari et al., 1977; Nagai et al., 1982; Carlsen and Heimer, 1986; Muller et al., 2011) but also by an intrinsic population of small cholinergic neurons (Carlsen and Heimer, 1986). Cholinergic nerve terminals synapse on both pyramidal cells and interneurons (Carlsen and Heimer, 1986; Nitecka and Frotscher, 1989; Muller et al., 2011), but the majority of the cholinergic input is directed onto pyramidal neurons (Muller et al., 2011). From the different types of mAChRs, both the M1 and M2 subtypes, are present in the rat BLA (Cortés and Palacios, 1986; Mash and Potter, 1986; Spencer et al., 1986; Mash et al., 1988), as well as the M3 and/or M4 receptors (Smith et al., 1991), but the M1 receptors predominate (Buckley et al., 1988). Immunocytochemistry along with electron microscopy have revealed that M1 receptors in the rat BLA are located on somata, dendritic shafts, and, to a lesser extent, spines of pyramidal neurons, as well as on aspiny dendrites with morphologic features typical of interneurons (Muller et al., 2013). In addition, M1 immunoreactivity has been found on presynaptic terminals, most of which formed asymmetric synapses with dendritic spines, but some of which formed symmetrical synapses with neuronal somata and dendritic shafts (Muller et al., 2013). It appears, therefore, that M1 receptors in the rat BLA are in a position to affect the activity of both glutamatergic and GABAergic neurons, by postsynaptic or presynaptic mechanisms. The increase in GABAergic inhibitory activity by paraoxon could be due to M1 receptor–mediated direct depolarization of GABAergic interneurons, facilitation of GABA release from GABAergic presynaptic terminals, or depolarization of glutamatergic neurons or terminals synapsing onto inhibitory interneurons; presynaptic facilitation of GABA release may be the least likely of the three possibilities, because M1 receptor activation inhibits GABA release in the rat lateral amygdala (Sugita et al., 1991). The increase in sEPSCs by paraoxon could be the result of M1 receptor–mediated depolarization of BLA principal neurons via enhancement of the Ih current, as shown previously in CA3 pyramidal neurons in the hippocampus (Fisahn et al., 2002) and in BLA principal neurons in this study. In addition, M1 receptors on glutamatergic terminals may facilitate glutamate release; this remains to be demonstrated in the BLA, but in granule cells of the hippocampus, the frequency and amplitude of sEPSCs are increased by application of paraoxon via a presynaptic mechanism, and this effect is blocked by preapplication of atropine (Kozhemyakin et al., 2010).
Up to 30 mg/kg VU0255035 has been used in mice to block M1 receptors, and no effect was observed on learning as determined by contextual fear conditioning (Sheffler et al., 2009). In M1 knockout mice, Miyakawa et al. (2001) found evidence of hyperactivity under stressful conditions and no significant cognitive impairments in the Morris water maze and in contextual fear conditioning, whereas Anagnostaras et al. (2003) found enhancements, or deficits, or no effect depending on the type of memory. Taken together with the results presented here, it is suggested that selective M1 antagonists may be an efficacious pretreatment in situations in which there is risk for exposure to OPs because the antagonist may produce only minimal or no significant side effects on cognitive function, whereas, upon exposure, it will delay the development of SE long enough for medical assistance to arrive.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- d-AP5
d(−)-2-amino-5-phosphonopentanoic acid
- BLA
basolateral amygdala
- DMSO
dimethylsulfoxide
- LY341495
(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid
- mAChR
muscarinic acetylcholine receptor
- OP
organophosphate
- SCH50911
(2S)-(+)-5,5-dimethyl-2-morpholineacetic acid
- SE
status epilepticus
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- VU0255035
N-(3-oxo-3-(4-(pyridine-4-yl)piperazin-1-yl)propyl)-benzo[c][1,2,5]thiadiazole-4 sulfonamide
Authorship Contributions
Participated in research design: Braga, Miller, Aroniadou-Anderjaska, Apland.
Conducted experiments: Miller, Pidoplichko, Figueiredo, Apland, Krishnan.
Performed data analysis: Miller, Pidoplichko, Figueiredo.
Wrote or contributed to the writing of the manuscript: Aroniadou-Anderjaska, Apland, Braga.
Footnotes
This research was supported by the National Institutes of Health CounterACT Program, the National Institutes of Health Office of the Director, and the National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant 5U01NS058162-07]. The views of the authors do not purport to reflect the position or policies of the Department of Defense or the U.S. Army.
References
- Abdullah JM, Rafiqul Islam M. (2012) Telemetric EEG and the rat: a guide for neuroscientists. Malays J Med Sci 19:1–5. [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6:51–58. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ. (1997) Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol 20:115–131. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MF. (2009) Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience 159:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. (2014) The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302. J Pharmacol Exp Ther 351:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Qashu F, Braga MF. (2009) Primary brain targets of nerve agents: the role of the amygdala in comparison to the hippocampus. Neurotoxicology 30:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. (2008) Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res 78:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. (2012) Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience 221:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashani Y, Catravas GN. (1981) Seizure-induced changes in the permeability of the blood-brain barrier following administration of anticholinesterase drugs to rats. Biochem Pharmacol 30:2593–2601. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. (1977) Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res 120:435–444. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Pomponio JW, Evans SA, Pervitsky D, Gordon RK. (2013) Discovery of subtype selective muscarinic receptor antagonists as alternatives to atropine using in silico pharmacophore modeling and virtual screening methods. Bioorg Med Chem 21:2651–2662. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. (1988) Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci 8:4646–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, McKinzie DL, Felder CC, Wess J. (2003) Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res 28:437–442. [DOI] [PubMed] [Google Scholar]
- Capacio BR, Shih TM. (1991) Anticonvulsant actions of anticholinergic drugs in soman poisoning. Epilepsia 32:604–615. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. (1986) A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J Comp Neurol 244:121–136. [DOI] [PubMed] [Google Scholar]
- Cortés R, Palacios JM. (1986) Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res 362:227–238. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Kaminski RM, Turski WA. (2003) Dizocilpine improves beneficial effects of cholinergic antagonists in anticholinesterase-treated mice. Toxicol Sci 72:289–295. [DOI] [PubMed] [Google Scholar]
- Deshpande LS, Carter DS, Phillips KF, Blair RE, DeLorenzo RJ. (2014) Development of status epilepticus, sustained calcium elevations and neuronal injury in a rat survival model of lethal paraoxon intoxication. Neurotoxicology 44:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. (2011) The GluK1 (GluR5) kainate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther 336:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. (2002) Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 33:615–624. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Abu-Qare AW, Meeker-O’Connell WA, Borton AJ, Abou-Donia MB. (2003) Methyl parathion: a review of health effects. J Toxicol Environ Health B Crit Rev 6:185–210. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. (1997) Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA 94:13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PK, Sheridan RD, Green AC, Scott IR, Tattersall JE. (2004) A guinea pig hippocampal slice model of organophosphate-induced seizure activity. J Pharmacol Exp Ther 310:678–686. [DOI] [PubMed] [Google Scholar]
- Kow RL, Cheng EM, Jiang K, Le JH, Stella N, Nathanson NM. (2015) Muscarinic M1 receptor and cannabinoid CB1 receptor do not modulate paraoxon-induced seizures. Pharmacol Res Perspect 3:e00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakin M, Rajasekaran K, Kapur J. (2010) Central cholinesterase inhibition enhances glutamatergic synaptic transmission. J Neurophysiol 103:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. (2014) Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 13:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Blanchet G. (1991) Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res 563:234–240. [DOI] [PubMed] [Google Scholar]
- Mash DC, Potter LT. (1986) Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience 19:551–564. [DOI] [PubMed] [Google Scholar]
- Mash DC, White WF, Mesulam MM. (1988) Distribution of muscarinic receptor subtypes within architectonic subregions of the primate cerebral cortex. J Comp Neurol 278:265–274. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Jaax NK, Crowley RA, Mays MZ, Modrow HE. (1989) Atropine and/or diazepam therapy protects against soman-induced neural and cardiac pathology. Fundam Appl Toxicol 13:256–276. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, McLeod CG, Jr, Nipwoda MT. (1987) Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res 435:123–137. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. (1997) Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 21:559–579. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21:5239–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. (2011) Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol 519:790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, McDonald AJ. (2013) Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: ultrastructural localization and synaptic relationships to cholinergic axons. J Comp Neurol 521:1743–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrer T. (2007) Neuronal structures involved in the induction and propagation of seizures caused by nerve agents: implications for medical treatment. Toxicology 239:1–14. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Enger S, Aas P. (2007) Anticonvulsant effects of damage to structures involved in seizure induction in rats exposed to soman. Neurotoxicology 28:819–828. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Enger S, Aas P. (2010) Roles of perirhinal and posterior piriform cortices in control and generation of seizures: a microinfusion study in rats exposed to soman. Neurotoxicology 31:147–153. [DOI] [PubMed] [Google Scholar]
- Nagai T, Kimura H, Maeda T, McGeer PL, Peng F, McGeer EG. (1982) Cholinergic projections from the basal forebrain of rat to the amygdala. J Neurosci 2:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, Frotscher M. (1989) Organization and synaptic interconnections of GABAergic and cholinergic elements in the rat amygdaloid nuclei: single- and double-immunolabeling studies. J Comp Neurol 279:470–488. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. (2014) ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci 34:3130–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. (2013) Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology 38:84–90. [DOI] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Rossetti F, de Araujo Furtado M, Pak T, Bailey K, Shields M, Chanda S, Addis M, Robertson BD, Moffett M, Lumley LA, et al. (2012) Combined diazepam and HDAC inhibitor treatment protects against seizures and neuronal damage caused by soman exposure. Neurotoxicology 33:500–511. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, et al. (2009) A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Koviak TA, Capacio BR. (1991) Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev 15:349–362. [DOI] [PubMed] [Google Scholar]
- Sirin GS, Zhou Y, Lior-Hoffmann L, Wang S, Zhang Y. (2012) Aging mechanism of soman inhibited acetylcholinesterase. J Phys Chem B 116:12199–12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovira JW, McDonough JH, Shih TM. (2010) Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci 40:56–62. [DOI] [PubMed] [Google Scholar]
- Smith TD, Annis SJ, Ehlert FJ, Leslie FM. (1991) N-[3H]methylscopolamine labeling of non-M1, non-M2 muscarinic receptor binding sites in rat brain. J Pharmacol Exp Ther 256:1173–1181. [PubMed] [Google Scholar]
- Spencer DG, Jr, Horváth E, Traber J. (1986) Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res 380:59–68. [DOI] [PubMed] [Google Scholar]
- Sugita S, Uchimura N, Jiang ZG, North RA. (1991) Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci USA 88:2608–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FR, Loke WK, Ling EA. (2011) Comparison of status epilepticus models induced by pilocarpine and nerve agents - a systematic review of the underlying aetiology and adopted therapeutic approaches. Curr Med Chem 18:886–899. [DOI] [PubMed] [Google Scholar]
- Tetz LM, Rezk PE, Ratcliffe RH, Gordon RK, Steele KE, Nambiar MP. (2006) Development of a rat pilocarpine model of seizure/status epilepticus that mimics chemical warfare nerve agent exposure. Toxicol Ind Health 22:255–266. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Sheffler DJ, Lewis LM, Bridges TM, Williams R, Nalywajko NT, Kennedy JP, Mulder MM, Jadhav S, Aldrich LA, et al. (2009) Discovery and development of a potent and highly selective small molecule muscarinic acetylcholine receptor subtype I (mAChR 1 or M1) antagonist in vitro and in vivo probe. Curr Top Med Chem 9:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman BA, Raveh L. (2008) Therapy against organophosphate poisoning: the importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol 232:351–358. [DOI] [PubMed] [Google Scholar]
- Wess J. (2003) Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci 24:414–420. [DOI] [PubMed] [Google Scholar]