Abstract
The marijuana plant Cannabis sativa and its derivatives, cannabinoids, have grown increasingly popular as a potential therapy for inflammatory bowel disease (IBD). Studies have shown that modulation of the endocannabinoid system, which regulates various functions in the body and has been shown to play a key role in the pathogenesis of IBD, has a therapeutic effect in mouse colitis. Epidemiologic data and human therapy studies reveal a possible role for cannabinoids in the symptomatic treatment of IBD, although it has yet to be determined in human populations whether cannabinoids have therapeutic anti-inflammatory effects in IBD or are simply masking its many debilitating symptoms. Large, double-blind, randomized, placebo-controlled trials using serial inflammatory markers, biopsy findings, and endoscopic disease severity to demonstrate objective improvement in IBD are necessary before cannabis can be empirically accepted and recommended as an IBD treatment option. Questions concerning its safety profile and adverse effects prompt the need for further research, particularly in regard to dosing and route of administration to maximize benefits and limit potential harms. Cannabis use should be reserved for symptomatic control in patients with severe IBD refractory to the currently available standard-of-care and complementary and alternative medicines.
Keywords: Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, cannabis
The use of cannabis, commonly referred to as marijuana, is increasingly popular; in North America, roughly 10.7% of people ages 15 to 64 years reported cannabis use in 2009.1 In the United States, cannabis is a Schedule I substance and its use for recreational or medicinal means is illegal according to federal law. However, contrary to federal policy, individual state laws have allowed for medical use of marijuana in 24 states and recreational use in 4 states; additionally, use of marijuana is now decriminalized in 21 states.2 Given the evolving policies regarding the medical use of cannabis, physicians are increasingly prompted with questions about its therapeutic role for a variety of disorders.
In the United States, cannabis use is legalized state-to-state for the medical treatment of several chronic, debilitating disorders, including cancer, HIV/AIDS, multiple sclerosis, chronic pain, nausea, hepatitis C virus, posttraumatic stress disorder, amyotrophic lateral sclerosis, cachexia, glaucoma, and epilepsy.3,4 Data on the efficacy of cannabis use for the treatment of many of these conditions are often scarce and inconsistent, yet medical use of cannabis is increasing as patients choose complementary and alternative medicine (CAM) over more conventional, proven therapies.5
The plant Cannabis sativa has been used in medicinal practice for thousands of years.6 The pharmacologically active constituents of the plant are termed cannabinoids, of which at least 70 are known today. Phytocannabinoids (cannabinoids derived from the plant), synthetic cannabinoids (artificial compounds with cannabinomimetic effects), and endocannabinoids (endogenous compounds with cannabinomimetic effects) act together on the endocannabinoid system (ECS), which regulates various functions in the body.7
Among the phytocannabinoids, delta-9-tetra-hydrocannabinol (THC) is thought to be the most pharmacologically active, with various central and peripheral effects. THC is also considered the most active psychotropic agent among the phytocannabinoids and is largely the most studied. Other phytocannabinoids include cannabidiol, cannabigerol, and cannabichromene, all mostly devoid of central effects.8 Formulations related to these compounds include nabilone (Cesamet, Meda Pharmaceuticals), dronabinol, and nabiximols. Nabilone is approved by the US Food and Drug Administration (FDA) for chemotherapy-induced nausea and vomiting unresponsive to typical antiemetics, and dronabinol is FDA-approved for chemotherapy-induced nausea and vomiting and AIDS-associated anorexia. Nabiximols is approved outside of the United States for patients with cancer-associated pain, neuropathic pain, and spasticity in association with multiple sclerosis.3
Inflammatory bowel disease (IBD) is a chronic inflammatory condition comprised of ulcerative colitis (UC) and Crohn’s disease (CD) and characterized by relapsing and remitting episodes of inflammation primarily involving the gastrointestinal tract. The pathophysiology of IBD has yet to be fully established and appears to involve an inappropriate inflammatory response with a dysregulated immune system in the appropriate environmental and genetic background. Conventional therapies aimed at induction and remission of IBD mainly work through immune suppression and consist of aminosalicylates, antibiotics, corticosteroids, immunomodulators, and biologic therapies. Given the limited therapy options and known adverse side effects with chronic use, physicians often manage patients with disease refractory to conventional methods, prompting surgical resection of the diseased bowel.9 Patients are commonly attracted to the use of CAM for management of their IBD, and physicians should be familiar with these various therapies in order to advise patients on safe use.5
Anecdotal reports have suggested a therapeutic role for cannabis in the treatment of IBD for hundreds of years. A case report from 1990 describes patients with IBD maintaining remission of disease via cannabis use.10 The use of medical marijuana preceded the discovery of the ECS, prompting further research of cannabis as a treatment option for IBD. As the therapeutic use of cannabis gains more attention in the press, there is growing recognition of a fraction of IBD patients who are using cannabis for symptomatic control of their IBD, reportedly with successful management of abdominal pain, joint pain, cramping, diarrhea, poor appetite, weight loss, and nausea.11,12 Physicians are often unaware of the therapeutic role and adverse effects of marijuana use amid concerns of federal prosecution and the changing political status of the drug, yet its use cannot be ignored.3 This article reviews the ECS and its role in gastrointestinal physiology, population studies regarding the use of medical cannabis in IBD patients and its perceived effectiveness, results and potential pitfalls of therapy trials in the use of cannabis for treatment of IBD, and general safety concerns regarding acute and chronic cannabis use.
The Endocannabinoid System and Its Role in Gastrointestinal Physiology
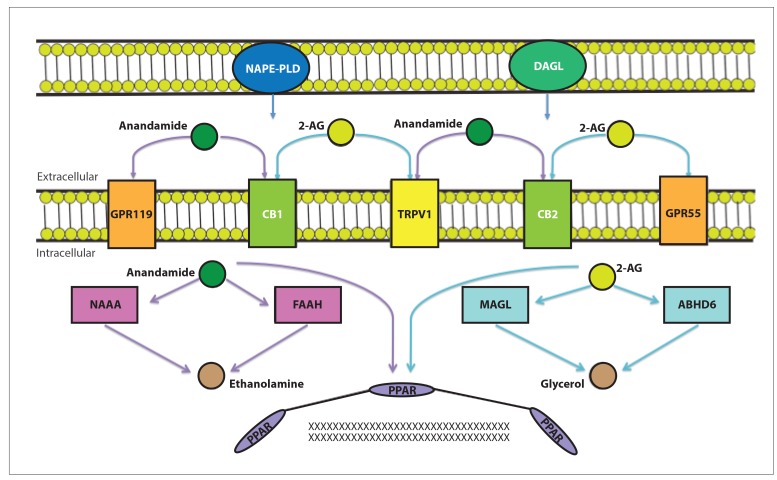
The ECS consists of endogenous cannabinoids, the receptors on which they act, and the enzymes involved in their biosynthesis and degradation (Figure 1). The 2 primary endocannabinoids are N-arachidonoylethanolamine, or anandamide, and 2-arachidonoylglycerol (2-AG). These ligands are synthesized from cellular membrane phospholipids and bind to presynaptic receptors, namely the G protein—coupled receptors cannabinoid 1 and 2 receptors (CB1 and CB2). Anandamide acts as a partial agonist of CB1 and CB2, with a slightly higher affinity to CB2; 2-AG binds to both receptors equally well with greater potency. 2-AG is found in higher levels in the gastrointestinal tract.8
Figure 1.
Anandamide and 2-arachidonoylglycerol (2-AG) are formed via phospholipid precursors by the enzymes N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAGL). These active lipids interact with membrane and intracellular receptors, including the G protein–coupled receptors 119 and 55 (GPR119 and GPR55), the cannabinoid 1 and 2 receptors (CB1 and CB2), the transient receptor potential vanilloid subtype 1 receptor (TRPV1), and the peroxisome proliferator-activated receptors (PPARs), among others. Anandamide is hydrolyzed intracellularly by N-acylethanolamine-hydrolyzing acid amidase (NAAA) and fatty acid amide hydrolase (FAAH), and 2-AG is hydrolyzed intracellularly by monoacylglycerol lipase (MAGL) and alpha/beta-hydrolase domain 6 (ABHD6).8
The phytocannabinoids THC and cannabidiol act via similar pathways as anandamide and 2-AG. THC is a partial agonist of both CB1 and CB2, also acting on noncannabinoid receptors. The actions of THC on CB1 make it largely responsible for the psychoactive effects of cannabis use. Cannabidiol binds to both CB1 and CB2 with poor affinity and primarily exerts its effects via additional pathways.8
The ECS is found in all vertebrates and humans and is distributed among organs and tissues. CB1 is mostly expressed in neurons of the central, peripheral, and enteric nervous systems, while CB2 is found mainly in immune cells. In the gastrointestinal system, CB1 and CB2 are found in all layers of intestinal sections, including the myenteric and submucosal plexi and the epithelium.13,14 Numerous mouse models have demonstrated a relationship between the ECS, intact gastrointestinal physiology, and regulation of gut inflammation (Figure 2).13-20 Expression of cannabinoid receptors is most abundant on B cells, followed by natural killer cells, monocytes, neutrophils, and CD8 and CD4 leukocytes.13 Overall, endocannabinoids acting on CB2 result in attenuation of inflammatory response, yet other data suggest that cannabinoids have proinflammatory effects and that their immunomodulatory effect is based on the frequency of cannabis use, the dose administered, the specific type of cannabinoid used, and the cells on which they act.13
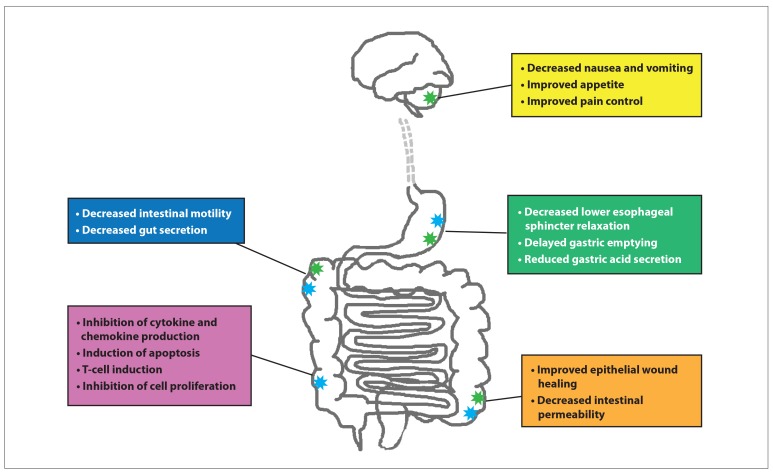
Figure 2.
Natural and synthetic cannabinoids act primarily via cannabinoid 1 receptors (CB1; green stars) and cannabinoid 2 receptors (CB2; blue stars) located in the central, peripheral, and enteric nervous systems. Cannabinoids predominately mediate inhibitory pathways in the gastrointestinal tract through reduction of vagal cholinergic tone. CB2 modulate inflammation, whereas CB1 control central functions, including pain control, satiety, nausea, and vomiting. The distribution and concentration of the endocannabinoid system in specific tissues demonstrate the physiology of cannabinoids in the gastrointestinal tract and offer possible drug targets for the management of inflammatory bowel disease. The majority of the above functions are demonstrated in mouse models; it is yet unclear if all effects mirror those seen in humans.13-20
Alteration of the Endocannabinoid System in Inflammatory Bowel Disease
The role of the ECS in gut homeostasis and its ability to modulate inflammatory responses demonstrate its part in preserving gastrointestinal function. Alterations of the ECS may predispose patients to pathologic disorders, including IBD. This has been demonstrated in both murine models8,20,21 (Table 1) and human models, described below.
Table 1.
Results of Modulation of the Endocannabinoid System in Murine Models With Inflammatory Bowel Disease
| Study | Results |
|---|---|
| Massa et al20 |
|
| Engel et al21 |
|
| Alhouayek and Muccioli8 |
|
CB1, cannabinoid 1 receptor; CB2, cannabinoid 2 receptor; DNBS, dinitrobenzene sulfonic acid; FAAH, fatty acid amide hydrolase; TNBS, trinitrobenzene sulfonic acid.
Di Sabatino and colleagues described modulation of the ECS in 2011 using endoscopic biopsy specimens from 41 patients with CD and 33 patients with UC.22 Biopsies were analyzed for endocannabinoid levels, expression of cannabinoid receptors, and activity of enzymes involved in endocannabinoid synthesis and degradation. Levels of anandamide were significantly decreased in inflamed IBD mucosa, which correlated with a decrease in expression of N-acyl-phosphatidylethanolamine-phospholipase D (NAPE-PLD) and an increase in expression of fatty acid amide hydrolase (FAAH). CB1 was also found to have increased expression in inflamed areas of both CD and UC; however, CB2 levels seemed to be unaltered.22
Marquéz and colleagues studied expression of the ECS in 24 patients with UC vs rectal samples from control patients after colonic resection for colorectal tumors.23 Results showed increases of CB2 and the diacylglycerol lipase (DAGL) and monoacylglycerol lipase (MAGL) enzymes in mild to moderate pancolitis. Severe pancolitis showed a decrease in expression of NAPE-PLD. In quiescent colitis, patients treated with aminosalicylates and corticosteroids experienced decreases in expressions of CB1, CB2, and DAGL, whereas NAPE-PLD levels rose. In patients with acute pancolitis, lamina propria immune cells showed increased amounts of MAGL and FAAH; however, this level of expression dropped after appropriate treatment.23
These studies show different levels of elements of the ECS in murine and human IBD models. Further delineation of mechanism of action is needed to determine whether these results are pathologic or reactive effects to inflammation. However, cannabinoids appear to have a clear role in gut pathology and offer a potential target for drug intervention in the treatment of IBD.
Increased Cannabis Use in Patients With Inflammatory Bowel Disease
A significant proportion of patients with IBD use CAM for additional management of symptoms. Motives for using CAM include ineffectiveness of current therapies, fewer side effects, and a sense of gaining control over the disease.5,24,25 As public awareness of medical cannabis use increases, population studies have reinforced the use of medical cannabis for symptom relief in IBD patients (Table 2) 5,12,24,26,27
Table 2.
Population Studies Evaluating Cannabis Use in Patients With IBD
| Study | Number of Subjects | Evet Used Cannabis (%) | Actively Used Cannabis (%) | Used Cannabis for IBD (%) | Used Cannabis for Abdominal Pain Relief (%) | Used Cannabis for Diarrhea Relief (%) | Weight Gain or Improved Appetite (%) |
|---|---|---|---|---|---|---|---|
| García-Planella et al5 | 214 | N/A | 10 | N/A | N/A | N/A | N/A |
| Lal et al24 | 291 | 49 | 14.4 | 43.9 | 94.4 | 33.3 | 74.1 |
| Ravikoff Allegretti et al26 | 292 | 51.3 | 12.3 | 32 | 89.5 | 41.6 | 72.9 |
| Storr et al12 | 319 | 44.2 | N/A | 17.6 | 83.9 | 28.6 | N/A |
| Weiss and Friedenberg27 | 2,084,895 | 67.3 | 15.7 | N/A | N/A | N/A | N/A |
IBD, inflammatory bowel disease.
In 2007, García-Planella and colleagues surveyed 214 patients with IBD in Spain and found that nearly 10% of patients actively used cannabis or its derivatives.5 In 2011, Lal and colleagues polled 291 patients with IBD at a tertiary care center in Ontario, Canada.24 UC patients reported a 50.5% lifetime and 11.6% active use of cannabis, and CD patients reported a 48.1% lifetime and 15.9% current use of cannabis. Interestingly, 33% and 50% of UC and CD lifetime users, respectively, reported use of medical cannabis specifically for symptom relief of IBD. A notable proportion of patients found symptomatic relief of abdominal pain, diarrhea, and poor appetite. Patients with a history of abdominal surgery, chronic analgesic use, CAM use, and a lower Short Inflammatory Bowel Disease Questionnaire score were more likely to use cannabis for symptom relief. Forty-seven percent of patients overall reported using CAM for IBD management. More than 50% of patients expressed interest in participating in a clinical trial of cannabis for IBD.24
Ravikoff Allegretti and colleagues performed the first survey regarding patterns of cannabis use in the US population.26 A total of 292 patients (a significant proportion of whom were using standard-of-care therapies) at a specialized IBD center were enrolled in a prospective cohort survey study. A 94% response rate showed a 12.3% rate of active marijuana use among IBD patients, higher than the rate of use among the general population. Thirty-two percent of lifetime users reported using marijuana for control of IBD symptoms. Multivariate analysis revealed that age and chronic abdominal pain were associated with marijuana use. A substantial proportion of patients perceived cannabis as effective for relief of abdominal pain, poor appetite, and nausea, and less successful for relief of diarrhea. The authors discuss whether these results suggest a central-mediated mechanism for cannabis relief rather than an improvement in mucosal inflammation. Similar to the study by Lal and colleagues,24 nearly half of nonusers expressed interest in cannabis use if medically legal.26
A 2014 Canadian population study noted a worse disease prognosis in patients with CD using cannabis.12 In a study of 319 patients with IBD, 17.6% reported lifetime use of cannabis for IBD, especially among patients with self-reported severe IBD, patients recently hospitalized, and patients with surgical history. Ninety-one percent of patients indicated improvement of IBD symptoms with cannabis use; 83.9% reported improved abdominal pain, 76.8% indicated improved abdominal cramping, 48.2% had improved joint pain, and 28.6% reported improved diarrhea.12 Patients also believed that cannabis improved their general well-being, stress level, and sense of control over IBD. Surprisingly, 35.7% of patients believed that cannabis worked better than corticosteroids, and nearly 43% reported fewer side effects with cannabis use compared with corticosteroids. In addition, 82.1% of users planned to continue using cannabis for their IBD and 87.5% would recommend cannabis to other patients for management of IBD. When asked why they used cannabis for IBD, 46.4% of patients said they heard that cannabis would help, followed by being frustrated with their disease, wanting to try a different approach, and feeling that medications prescribed by doctors did not help. Only 39% of patients discussed their use of cannabis with their physician, and 82% of physicians were indifferent or not supportive of marijuana use for IBD management. Overall, 64.3% of nonusers felt that cannabis should be legalized for medical use.12 However, in patients with CD, regression analysis linked prolonged cannabis use to an increased history of surgery (odds ratio, 5.03). Storr and colleagues acknowledged that it was not possible to associate the time of cannabis use with surgeries, making any association between temporal relationships or causality from their methods impossible.12 Research has suggested that cannabis use may be associated with an increased risk for surgery based on prior studies showing increased rates of liver fibrosis with marijuana use.28 Similar effects could be responsible for the fibrostenotic sequelae complicating CD and requiring surgical intervention. Furthermore, cannabis use may mask ongoing inflammation. Because of improved symptom control, patients may perceive their disease to be in remission and thus not present to physicians for routine care, resulting in adverse consequences in a young population.12
The first large population-based survey, which was conducted by Weiss and Friedenberg using the National Health and Nutrition Examination Survey in 2015, reviewed more than 2 million IBD patients vs age- and sex-matched controls in regard to patterns of cannabis use.27 Results showed that patients with IBD had a higher incidence of having used marijuana or its resin form hashish vs the matched control subjects (67.3% vs 60.0%). Patients with IBD were more likely to use a higher amount of marijuana or hashish per day, but were less likely to use marijuana or hashish every month for a year. Multivariable logistic regression analyses identified IBD, male sex, and age over 40 years as predictors of marijuana or hashish use. Patients with IBD tended to score higher on the Median Depression Score, were more likely to have alcohol-use patterns concerning for dependence and abuse, had a higher prevalence of smoking, and had higher levels of C-reactive protein (CRP).27 Results of this survey mirror those of previous smaller studies, allowing for more defined generalizations of marijuana use and its perceived benefits among IBD patients.
The aforementioned studies share several themes. Cannabis use is common among patients with IBD and often specifically for symptomatic relief. Patients report substantial therapeutic effects of cannabis in the management of abdominal pain, nausea, and diarrhea, and a significant number of patients are interested in using cannabis for management of their IBD. Additionally, patients infrequently report use of cannabis to their physicians, emphasizing the need to question patients on use. Lastly, most studied patients received treatment at specialized or dedicated IBD tertiary care centers, suggesting poor control of abdominal pain, nausea, and diarrhea in patients with severe IBD despite use of the most up-to-date therapies. Cannabis seems to be of symptomatic benefit to patients often refractory to conventional medicines; however, none of the above studies delineate whether this is a central subjective effect masking active disease or an actual treatment of inflammation.
Symptomatic Improvement With Cannabis Use in Patients With Inflammatory Bowel Disease
Following the promising results of cannabinoids in murine models of colitis (Table 3),29-32 Naftali and colleagues in 2011 presented the first study examining the response of patients with CD to cannabis use (Table 4).33 The authors conducted a retrospective, observational study of 30 CD patients in Israel who were legally using cannabis due to a lack of response to conventional treatments and chronic intractable pain. Disease activity before and after cannabis use was estimated using the Harvey-Bradshaw index for CD, and patients assessed their general medical well-being before and after use. Patients’ hospital records were obtained to monitor disease activity, rate of hospital admission, use of additional drugs, and need for surgical intervention.33 All 30 patients rated their general medical well-being as improved after cannabis use via a visual analog scale. Twenty-one patients had a notable improvement after treatment with cannabis use, and the average Harvey-Bradshaw index for all patients improved from 14 to 4.7 (P<.001). Only 2 patients required surgery during a period of 3 years of cannabis use, a rate that Naftali and colleagues claimed is a significant improvement for the normal operative rate in patients with CD.33 The mean number of bowel movements decreased from 8 to 5. Whereas 26 patients required corticosteroid therapy prior to cannabis use, only 4 patients were still maintained on corticosteroids after cannabis use, suggesting a possible corticosteroidsparing effect of cannabis. There was also a substantial drop in use of aminosalicylates, thiopurines, methotrexate, and tumor necrosis factor antagonists. The authors cited these data as objective benefits of cannabis use and advocated for more placebo-controlled studies for further evaluation of therapeutic effects of cannabis use.33
Table 3.
Results of Murine Colitis Models Treated With Cannabinoids
| Study | Results |
|---|---|
| Borrelli et al29 | In DNBS-induced colitis, cannabidiol reduced colon injury, decreased expression of inflammatory markers and inducible nitric oxide synthase, and decreased reactive oxygen species production. |
| Jamontt et al30 | TNBS-induced colitis treated with THC, cannabidiol, THC combined with cannabidiol, and sulphasalazine compared with controls showed decreased inflammation and functional disturbances after treatment with THC and cannabidiol. THC alone or with cannabidiol improved the function of cholinergic motor neurons, results not seen with sulphasalazine use. |
| Cluny et al31 | A peripherally restricted CB1/CB2 agonist was ineffective in dextran sodium sulfate—induced colitis and did not significantly reduce colitis in a TNBS-colitis model. |
| D’Argenio et al32 | Use of VDM11, an inhibitor of fatty acid amide hydrolase, increases anandamide tone, which improves TNBS- and DNBS-induced rat colitis. |
CB1, cannabinoid 1 receptor; CB2, cannabinoid 2 receptor; DNBS, dinitrobenzene sulfonic acid; THC, tetrahydrocannabinol; TNBS, trinitrobenzene sulfonic acid; VDM11, N-(-4-hydroxy-2-methylphenyl) arachidonoyl amide.
Table 4.
Therapy Studies Evaluating Clinical Response in Patients With IBD
| Study | Study Design | Subjects | Treatment | Outcomes |
|---|---|---|---|---|
| Naftali et al33 | Retrospective, observational | 30 patients with Crohn’s disease | Retrospective inhalational or oral cannabis use | Significant clinical response but need for other drugs and surgery with cannabis |
| Lahat et al34 | Prospective, observational without controls | 13 patients with IBD | 50 g of cannabis cigarette per month (3 months total) | Significant improvement in quality of life, disease activity, and weight gain |
| Naftali et al35 | Prospective, randomized, double-blind, placebo-controlled | 21 patients with Crohn’s disease | Cannabis sativa cigarette (23% THC, 0.5% cannabidiol) | Significant clinical response with cannabis but no objective decrease in inflammation |
IBD, inflammatory bowel disease; THC, tetrahydrocannabinol.
The first prospective, observational, single-arm trial was published by Lahat and colleagues.34 Thirteen patients with longstanding IBD refractory to conventional therapies and on a stable IBD medical regimen prior to inclusion were provided a total dose of 50 g of processed cannabis plant in the form of prepared cigarettes. Patients were instructed to use inhaled cannabis whenever they felt pain for a total of 3 months. Patients completed 2 quality-of-life questionnaires (the 36-Item Short Form Health Survey [SF-36] and the EuroQol 5 dimensions questionnaire [EQ-5D]), and physicians measured patient body weights and calculated Harvey-Bradshaw indexes and partial Mayo scores (excluding mucosal endoscopic appearance) before and after cannabis treatment. All patients used the entire amount of inhaled cannabis supplied each month; no cannabis usage was reported prior to study initiation. Using the SF-36, patients reported a statistically significant improvement in 12 of 14 daily activities and a notable improvement in pain after 3 months of treatment. Patients noted improvement in health perception, social functioning, ability to work, and depression. They had an average weight gain of 4.3 kg during treatment (P=.00002) and an average increase in body mass index of 1.4 (P=.002). The average Harvey-Bradshaw index was reduced from 11.36 to 2.68 (P=.001); reductions were mainly seen in general well-being and abdominal pain. The average number of daily liquid stools decreased from 5.54 to 3.18. Owing to a limited number of patients with UC, statistical analysis was unable to be performed on this subset. Lahat and colleagues were able to provide CRP levels for only 6 patients before and during treatment, and this trended toward a decrease in CRP levels during treatment with cannabis.34The authors concluded that cannabis use improves quality of life in patients with IBD, results in a statistically significant increase in patient weight and body mass index, and improves clinical disease activity index in patients with CD, and postulated that such effects were related to the analgesic, anti-inflammatory, antimotility, and additional effects of cannabinoids.34
After performing retrospective research,33 Naftali and colleagues completed the first prospective, randomized, double-blind, placebo-controlled trial by evaluating 21 patients with CD refractory to aminosalicylates, corticosteroids, immunomodulators, or biologic agents.35 The primary objective of the study was induction of remission of CD as defined by a Crohn’s Disease Activity Index (CDAI) score of less than 150 after 8 weeks of treatment. Secondary objectives were rate of response, defined by the authors as a 100-point decrease in the CDAI score, reduction of at least 0.5 mg in CRP levels, or improvement in quality of life by at least 50 points as measured by the SF-36. Patients in the treatment group were instructed to smoke 2 marijuana cigarettes containing 115 mg of THC, whereas patients in the placebo group smoked placebo cannabis flowers extracted of all THC content for a total of 8 weeks of treatment. Patients were on stable doses of medications prior to the initiation of treatment and had an average CDAI score of greater than 200. Previous cannabis use was an excluding factor. Patients were evaluated at 0, 2, 8, and 10 weeks, and evaluated parameters included CDAI score, CRP levels, and the SF-36. The primary objective was not met, as 5 of 11 patients in the treatment group achieved remission compared with 1 of 10 patients in the placebo group (P=.43). The authors suggested that the primary objective may not have been reached due to low sample size. Following 8 weeks of treatment, the secondary objective response rate via reduction of the CDAI score by 100 points was reached in 90% (10/11) of patients in the treatment group, from an average of 330 to 152, and in 40% (4/10) of patients in the placebo group, from an average of 373 to 306 (P=.028). Two weeks after cannabis treatment was stopped, the mean CDAI score in treatment and placebo groups was 331 and 280, respectively. Naftali and colleagues noted that 3 corticosteroid-dependent patients in the treatment group stopped corticosteroid use during the study and that at the end of the study, no patients in the treatment group required corticosteroids.35 They also noted that 2 patients in the treatment group using opiates for management of chronic pain stopped opiate use during the study. A significant increase in quality of life via the SF-36 was observed in the treatment group compared with the placebo group. Levels of CRP did not show any significant changes after treatment with cannabis. Endoscopic inflammation was not assessed. Naftali and colleagues reported that THC-rich cannabis produced significant clinical, corticosteroid-free benefits in patients with active CD compared with placebo and advocated for further trials to be conducted with a larger sample size.35 Given that their patients had longstanding CD with high rates of nonresponse or intolerance to biologic agents, the authors claimed such findings as impressive, yet recognized that further data are necessary and that the current role of cannabis in IBD should only be for compassionate management.35
Flaws in Human Studies
Findings from human studies have resulted in an increase in publicity regarding the efficacy of cannabis use in IBD therapy; however, the flaws of these studies are rarely mentioned.11,19,36 The population studies discussed in this article lack objective parameters showing improvement in IBD activity with cannabis use. For example, the large, population-based survey by Weiss and Friedenberg provides CRP levels for patients, but not for the full duration of the study period.27 The other studies lack measurements of sedimentation rate, fecal calprotectin levels, endoscopic inflammation, or histologic evidence of active disease. Although each of these studies reports improved levels of abdominal pain, nausea, and appetite, significant prior data have shown that cannabis use via central effects can be responsible for such benefits; the fact that fewer patients reported relief of diarrhea argues that cannabis may not have a role in mediating inflammation and instead masks active disease with symptomatic improvement and overexaggerates treatment effect, as suggested by Storr and colleagues.12 The majority of these trials occurred in specialized IBD centers with a largely white, homogeneous population that does not match the typical demographic seen in IBD patients today. Patients presented with severe, complex forms of IBD and represented a potential referral bias, demonstrating that cannabis use may be limited only to refractory cases.
Data have shown that cannabis use is often underreported among users; therefore, its use may be even higher in the general IBD population.5 However, there is still a significant effect of a recall bias, as patients whose IBD symptoms improved are more likely to search for causal events (such as cannabis use) as potential triggers.
Human trials share many of the same weaknesses as population studies, such as a small sample size of patients, a short period of study, and a limited or absent follow-up period. The retrospective trial by Naftali and colleagues studied only 30 patients, 26 of whom were male, relying on subjective measures of well-being and the Harvey-Bradshaw index to demonstrate treatment efficacy, with a clear recall bias.33 Subjective reported values of the Harvey-Bradshaw index include sense of well-being, abdominal pain, and liquid stools, and the authors only provided scores for bowel movements; objective data were limited.33 Patients also used cannabis via different routes, in different doses, and in unstandardized preparations without any reporting of additional CAM or recreational drug use.
Naftali and colleagues’ subsequent placebo-controlled trial35 generated significant media attention regarding the therapeutic use of cannabis in IBD; however, the study was met with an equal amount of criticism in the scientific community.37-39 Critics claimed the trial was underpowered, with only 21 subjects studied over 8 weeks with a 2-week follow-up. The authors measured disease activity using the CDAI, an accepted score system for disease activity in literature, although without specific variable results. The CDAI, similar to the Harvey-Bradshaw index, has subjective parameters, including stool pattern, abdominal pain, and general well-being; a patient with poorly controlled irritable bowel syndrome could appear as a poorly controlled IBD patient via CDAI measurement, as these parameters are the main drivers of the score.37 Two weeks after cannabis treatment was stopped, the mean CDAI score in the treatment group increased. Naftali and colleagues argued that these results demonstrate a therapeutic role of cannabis; however, it may be that subjects were experiencing central effects of cannabis treatment, ameliorating symptoms during the study rather than actual treatment of inflammation, or were experiencing withdrawal symptoms after completion (although the authors noted that patients denied having withdrawal symptoms after discontinuation of cannabis). Importantly, there were no significant changes in CRP levels during the study; thus, the only parameter of objective treatment efficacy was inconclusive. Endoscopic studies to correlate treatment effect were not performed. While the study attempted to be double-blinded, the authors mentioned that the psychotropic effects of the drug made blinding difficult; at the end of the study, all participants except 2 in the placebo group were able to correctly differentiate whether they had received cannabis or placebo. Critics also noted that patients in remission, defined by a CDAI score of less than 150, can still have significant inflammation on endoscopy. Vu and colleagues suggested that although the authors tried to standardize treatment via distribution of similar quantities of cannabis, the lack of testing of blood levels of cannabis is an additional flaw and hypothesized that unreported additional drug use such as alcohol may affect intrinsic THC levels.39 The studies by Naftali and colleagues33,35 were supported, and researchers were employed, by the Tikun Olam Organization, the largest and foremost supplier of medical cannabis in Israel, which openly advocates for use of medical marijuana in many medical conditions and whose website contains data regarding the beneficial effect of medical cannabis.
The prospective trial by Lahat and colleagues was observational rather than a blinded, placebo-controlled study and enrolled only 13 patients for a brief period of 3 months without subsequent follow-up.34 The authors relied on subjective health questionnaires and health indexes (SF-36, EQ-5D, and the Harvey-Bradshaw index), and were unable to provide endoscopic data, with only limited use of CRP measurements. This trial lacked use of a placebo control, and it is therefore impossible to rule out a placebo effect, as prior data have shown can be quite significant in therapy trials for IBD. Further, Lahat and colleagues were unable to standardize the actual cannabis used in the trial or demonstrate cannabinoid levels.34 Although the weight of the drug consumed was equivalent, the actual active levels of cannabinoids in the product were not measured, resulting in an absence of data of a possible dose-effect of cannabis use.
Critics share concern that cannabis may simply be masking symptoms without affecting intestinal inflammation. Larger, standardized, placebo-controlled, and blinded trials showing objective improvement in disease are needed. Further demonstration of a low adverse-effect profile prior to the widespread use of cannabis for IBD is also advised.37
Concerns Regarding Acute and Chronic Cannabis Use
The safety profile of cannabis is not well established, and use is associated with psychosocial disease and acknowledged physiologic effects. Whereas cannabis use in the United States is illegal by federal law, its legality for medical or recreational use varies by state law, allowing for poor regulation in its preparation, potency, ratio of contents, and route of usage, with variations in requirements for product labeling and testing.3 Furthermore, Storr and colleagues reported that 36% of patients with IBD who did not use cannabis were worried about side effects of its use.12
Many of the psychotropic effects of cannabis are seen in centrally acting cannabinoids, namely THC. Adverse effects of acute use include anxiety, panic, psychosis, tachycardia, and increased appetite with dry mouth.3 Long-term use also raises concerns regarding development of dependence, tolerance, and withdrawal upon discontinuation. Symptoms of withdrawal include increased irritability, sleep disturbance, anorexia, and depression, yet it is estimated that only approximately 10% of cannabis users ever develop dependency, which is comparatively less than what is seen in tobacco, alcohol, cocaine, or heroin use. No deaths have been solely attributed to marijuana.15,40
Chronic use of marijuana has been responsible for an increased risk of motor vehicle crashes, development of amotivational syndrome, altered adolescent neuropsychological development, cannabis hyperemesis syndrome, gynecomastia, impaired immune function, and decreased fertility.41-43 Diffusion-weighted magnetic resonance imaging of the brain showed impaired axonal connectivity among chronic cannabis users, although subsequent analyses have reported mixed results by linking cannabis with cognitive decline.44-46 In a systematic review of cannabinoid adverse effects, Wang and colleagues reported nearly 5000 adverse events, approximately 97% of which were not considered to be serious.43 Among the nearly 150 serious events were vomiting, urinary tract infection, and relapse of treated conditions.
Physiologic studies of cannabis have demonstrated impairment of lung function and development of bronchial inflammation with chronic use. However, this effect is inconclusive in subsequent studies.47,48 Cannabis use has not been associated with development of cancer, although it has been implicated in cardiovascular disease.49,50 A recent study by Williams and colleagues revealed that the proinflammatory effects of THC enhance expression of tissue factors with resultant elevated procoagulant activity.51 This finding suggests that cannabis use could potentiate coagulopathies, especially in individuals with chronic immune activation (eg, IBD patients known to have an increased risk of thrombotic events).
Naftali and colleagues also raise concern of the ideal preparation, drug content, and route of cannabis use if medically legalized.41 In one study, 45% of patients not using cannabis for IBD treatment declined use because they did not want to smoke drugs.12 The issue of smoking is of further concern in IBD patients given the detrimental role that smoking cigarettes has shown to have in patients with CD, and thus it would be paramount that any preparations of cannabis lacked both tobacco and nicotine.52 However, the bioavailability of cannabis is significantly decreased when ingested orally as opposed to inhaled, with significant differences in time to effect, time to peak, and time to elimination, leading to difficulty in regulating a therapeutic dosage.3,41 Furthermore, once THC enters the bloodstream, it is lipophilic and quickly absorbed in fat tissues, which raises concern of a lasting effect from slow elimination.41
In the study by Lal and colleagues, nearly one-third of patients reported significant side effects ranging from feelings of euphoria and heightened awareness to dry mouth, paranoia, palpitations, anxiety, and memory loss.24 More than 75% of cannabis users in the population study by Storr and colleagues experienced side effects of increased appetite, anxiety, dry mouth, and drowsiness, all largely rated as mild.12 However, other studies did not report significant side effects or did not include adverse events as a studied parameter.5,24,26,27
Recent data by Gubatan and colleagues linked cannabis abuse as an independent risk factor to emergency department visits in gastroenterology patients.53 Although it was not possible to establish a temporal relationship of cannabis use to emergency department visits or determine that cannabis use has detrimental effects on the primary gastrointestinal disorder in studied patients, it is important for providers to acknowledge cannabis abuse as a probable marker of disease severity.
Storr and colleagues raised the possibility that cannabis use may result in worsening severity of IBD by promotion of fibrostenosis with increased rates of surgery.12 While significant research has been published regarding cannabis therapy for IBD over the last decade, equal attention has also been focused on its role in liver disease. Studies suggest cannabis as having a proinflammatory effect on chronic liver disease, resulting in worsening rates of fibrosis.28,54 Antagonism of the ECS has been proposed as a potential treatment target for chronic hepatitis.55 However, cannabis has also been theorized to have a protective effect on cardiac fibrosis and has been implicated as protective to end-organ dysfunction in other models.56,57 It is critical for further studies to not only demonstrate the role of cannabis on inflammation in IBD patients, but also to ensure the lack of progression in the rate of complications.
It is important for future studies to establish a drug preparation that is readily orally bioavailable, demonstrates additive central and peripheral dose effects with a predictive time to effect, and optimizes the risk-to-benefit ratio in a standardized form of production.
Conclusion
A significant portion of IBD patients, particularly those with severe disease, use cannabis to relieve symptoms of pain, nausea, and appetite and to improve their overall mood. The significant morbidity seen in patients with severe disease emphasizes the limited number of conventional therapies for symptomatic control of IBD, a disorder still poorly understood. Patients with IBD have increased rates of psychiatric disease, pain, and malnutrition, and thus the use of adjunctive therapies or CAM to treat poorly controlled symptoms may improve patient morbidity. However, cannabis use, as discussed above, raises concerns of legality, side effects, and preparation, and its use in human trials has failed to provide objective evidence of therapeutic efficacy on endoscopy, biopsy, and inflammatory marker levels.58 Concerns regarding the possible profibrotic effects of cannabis need further study, as such possible side effects could have consequences in patients with stricturing disease.
The safety profile of cannabis is still not established despite acknowledged detrimental effects. However, current options for IBD management, including corticosteroids, immunomodulators, and biologic agents, carry risks for long-term side effects such as malignancy and infection.8 Large, randomized, double-blind, placebo- and standard-of-care—controlled trials using standardized, oral preparations of cannabis with long-term follow-up and safety profiles are justified prior to acceptance of medical cannabis as a therapeutic drug.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Substance Abuse and Mental Health Services Administration. Results From the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- 2.Legal issues. NORML. [Accessed October 10, 2016]. www.norml.org/legal
- 3.Gerich ME, Isfort RW, Brimhall B, Siegel CA. Medical marijuana for digestive disorders: high time to prescribe? Am J Gastroenterol. 2015;110(2):208–214. doi: 10.1038/ajg.2014.245. [DOI] [PubMed] [Google Scholar]
- 4.Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10:10. doi: 10.1186/s13722-015-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Planella E, Marín L, Domènech E, et al. Use of complementary and alternative medicine and drug abuse in patients with inflammatory bowel disease [in Spanish] Med Clin (Bare). 2007;128(2):45–48. doi: 10.1157/13097468. [DOI] [PubMed] [Google Scholar]
- 6.Russo EB, Jiang HE, Li X, et al. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J Exp Bot. 2008;59(15):4171–4182. doi: 10.1093/jxb/ern260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 8.Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med. 2012;18(10):615–625. doi: 10.1016/j.molmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, Folan RD, Kelley ML., Jr Ulcerative colitis and marijuana. Ann Intern Med. 1990;112(6):471. doi: 10.7326/0003-4819-76-3-112-6-471_1. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein M. Medical marijuana: showdown at the cannabis corral. Nature. 2015;525(7570):S15–S17. doi: 10.1038/525S15a. [DOI] [PubMed] [Google Scholar]
- 12.Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20(3):472–480. doi: 10.1097/01.MIB.0000440982.79036.d6. [DOI] [PubMed] [Google Scholar]
- 13.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev. 2016;15(6):513–528. doi: 10.1016/j.autrev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med (Berl). 2005;83(12):944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 15.Bashashati M, McCallum RW. Cannabis in gastrointestinal disorders. Pract Gastroenterol. 2014;12(4):36–46. [Google Scholar]
- 16.Izzo AA, Fezza F, Capasso R, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134(3):563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153(2):263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129(2):437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Schicho R, Storr M. Cannabis finds its way into treatment of Crohn’s disease. Pharmacology. 2014;93(1-2):1–3. doi: 10.1159/000356512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113(8):1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J Physiol Pharmacol. 2010;61(1):89–97. [PubMed] [Google Scholar]
- 22.Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011;4(5):574–583. doi: 10.1038/mi.2011.18. [DOI] [PubMed] [Google Scholar]
- 23.Marquez L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4(9):e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23(10):891–896. doi: 10.1097/MEG.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- 25.Rawsthorne P, Shanahan F, Cronin NC, et al. An international survey of the use and attitudes regarding alternative medicine by patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94(5):1298–1303. doi: 10.1111/j.1572-0241.1999.01080.x. [DOI] [PubMed] [Google Scholar]
- 26.Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(13):2809–2814. doi: 10.1097/01.MIB.0000435851.94391.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend. 2015;156:84–89. doi: 10.1016/j.drugalcdep.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Ishida JH, Peters MG, Jin C, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6(1):69–75. doi: 10.1016/j.cgh.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87(11):1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 30.Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of deltatetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol. 2010;160(3):712–723. doi: 10.1111/j.1476-5381.2010.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cluny NL, Keenan CM, Duncan M, Fox A, Lutz B, Sharkey KA. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther. 2010;334(3):973–980. doi: 10.1124/jpet.110.169946. [DOI] [PubMed] [Google Scholar]
- 32.D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20(3):568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- 33.Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13(8):455–458. [PubMed] [Google Scholar]
- 34.Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion. 2012;85(1):1–8. doi: 10.1159/000332079. [DOI] [PubMed] [Google Scholar]
- 35.Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276–1280.e1. doi: 10.1016/j.cgh.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Schicho R, Storr M. IBD: patients with IBD find symptom relief in the Cannabis field. Nat Rev Gastroenterol Hepatol. 2014;11(3):142–143. doi: 10.1038/nrgastro.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herfarth HH, Long MD, Isaacs KL. If your physician cannot help, try cannabis: how trial design may lead to hazardous conclusions. Clin Gastroenterol Hepatol. 2014;12(5):897–898. doi: 10.1016/j.cgh.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Lahiff C, Cheifetz AS. The holistic effects of cannabis in Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(5):898. doi: 10.1016/j.cgh.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Vu MP, Melmed GY, Targan SR. Weeding out the facts: the reality about cannabis and Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(5):898–899. doi: 10.1016/j.cgh.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Anthony JC, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 41.Naftali T, Mechulam R, Lev LB, Konikoff FM. Cannabis for inflammatory bowel disease. Dig Dis. 2014;32(4):468–474. doi: 10.1159/000358155. [DOI] [PubMed] [Google Scholar]
- 42.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabi-noids: a systematic review. CMAJ. 2008;178(13):1669–1678. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zalesky A, Solowij N, Yücel M, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–2255. doi: 10.1093/brain/aws136. (pt 7) [DOI] [PubMed] [Google Scholar]
- 45.Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol. 2012;20(5):420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- 46.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):e2657–e2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MHS, Hancox RJ. Effects of smoking cannabis on lung function. Expert Rev Respir Med. 2011;5(4):537–546. doi: 10.1586/ers.11.40. [DOI] [PubMed] [Google Scholar]
- 48.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307(2):173–181. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowles DW, O’Bryant CL, Camidge DR, Jimeno A. The intersection between cannabis and cancer in the United States. Crit Rev Oncol Hematol. 2012;83(1):1–10. doi: 10.1016/j.critrevonc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol. 2002;42(11) suppl:64S–70S. doi: 10.1002/j.1552-4604.2002.tb06005.x. [DOI] [PubMed] [Google Scholar]
- 51.Williams JC, Klein TW, Goldberger BA, Sleasman JW, Mackman N, Goode-now MM. A(9)-tetrahydrocannabinol (THC) enhances lipopolysaccharide-stim-ulated tissue factor in human monocytes and monocyte-derived microvesicles. J Inflamm (Lond). 2015:12–39. doi: 10.1186/s12950-015-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cosnes J, Carbonnel F, Carrat F, Beaugerie L, Cattan S, Gendre J. Effects of current and former cigarette smoking on the clinical course of Crohn’s disease. Aliment Pharmacol Ther. 1999;13(11):1403–1411. doi: 10.1046/j.1365-2036.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 53.Gubatan J, Staller K, Barshop K, Kuo B. Cannabis abuse is increasing and associated with increased emergency department utilization in gastroenterology patients. Dig Dis Sci. 2016;61(7):1844–1852. doi: 10.1007/s10620-016-4090-9. [DOI] [PubMed] [Google Scholar]
- 54.Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 55.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12(6):671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 56.Brunet L, Moodie EE, Rollet K, et al. Canadian Co-infection Cohort Investigators. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis. 2013;57(5):663–670. doi: 10.1093/cid/cit378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajesh M, Mukhopadhyay P, Bátkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56(25):2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volz MS, Siegmund B, Häuser W. Efficacy, tolerability, and safety of cannabinoids in gastroenterology: a systematic review [in German] Schmerz. 2016;30(1):37–46. doi: 10.1007/s00482-015-0087-0. [DOI] [PubMed] [Google Scholar]