G&H What is volumetric laser endomicroscopy, and how does it work?
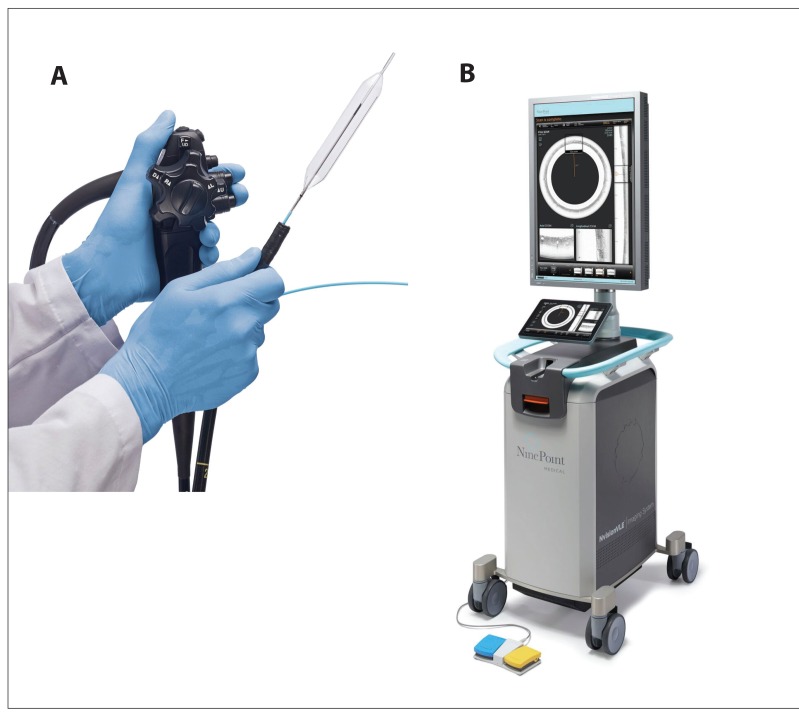
HW Volumetric laser endomicroscopy (VLE) is a new endoscopic imaging technology (NvisionVLE Imaging System, NinePoint Medical) utilizing advanced optical coherence tomography with near infrared light and balloon-centered imaging probes that produce scans of 6-cm segments of the esophagus, with surface and subsurface image depth greater than 3 mm with 7-pm axial resolution. These high-speed scans allow real-time diagnosis of surface and subsurface abnormalities as well as guide endoscopic treatment (Figure 1).
G&H What are the common indications for the use of VLE?
HW VLE is mainly indicated for 3 esophageal conditions, the most common being Barrett esophagus. VLE has been used to conduct surveillance of high-risk, treatment-naive Barrett esophagus patients; to guide the selection of treatments (ranging from radiofrequency ablation and cryotherapy to endoscopic mucosal resection) in Barrett esophagus patients in whom precancerous or cancerous cells have already been detected; and to scan surface and subsurface tissues for signs of recurrence or persistence of disease posttreatment that would require further treatment (Figure 2).
G&H How significant is the learning curve associated with VLE?
HW Formal studies are ongoing and have yet to be published in full form. However, early experience suggests that the learning curve for VLE appears to be much shorter than that of other technologies, including narrow-band imaging and confocal laser endomicroscopy. The image console and user interface with its large display enhances image analysis for the detection of VLE abnormalities that have been associated with cancerous and precancerous changes.
G&H How effective is VLE for the detection or diagnosis of dysplasia?
HW Although VLE is a fairly new device, several studies have been published recently that address this specific question. Dr Jacques Bergman and colleagues at the Academic Medical Center in Amsterdam, The Netherlands investigated the use of a computer-aided algorithm to analyze VLE scans. The results of the study, presented on October 18, 2016 at the United European Gastroenterology Week meeting in Vienna, Austria, demonstrated a 90% sensitivity and 93% specificity for the detection of dysplasia in Barrett esophagus using this computer-aided algorithm. This study was conducted using 60 VLE images (30 dysplastic, 30 nondysplastic) that had been matched to histology results. From this dataset, a set of features was extracted by a computer and used to automatically classify the images. While the results are encouraging, it is still the early stages for computer analysis of VLE images, and larger studies will be needed to verify these findings.
G&H Can, or should, VLE be used in conjunction with other procedures?
HW Absolutely. One of the main areas of work thus far has been using VLE to guide treatment of Barrett esophagus. Therefore, some of its most important clinical utility and value will likely come from being used in conjunction with other treatments. To that end, a laser-guided device has been developed and is now commercially available (NinePoint Medical). This laser places a mark on the mucosal surface that corresponds to the abnormal surface and subsurface areas identified on the VLE scans to facilitate precise diagnosis and guide endoscopic treatment.
G&H What advantages and disadvantages are associated with VLE compared with other endoscopic techniques?
HW The main advantages of VLE are that it provides contiguous, comprehensive microscopic imaging of 6 cm of the esophagus; high-resolution cross-sectional imaging (ie, a penetration depth of 3 mm with an axial resolution of 7 um); an acquisition time of 90 seconds for a full scan; and high targeting accuracy with laser marking. A disadvantage is that the use of this device may relate to an increase in capital equipment costs. However, it seems reasonable that real-time, high-speed imaging with laser-guided image targeting will facilitate endoscopic diagnosis and treatment cost savings by reducing the need for subsequent endoscopic procedures.
G&H What were the design and key findings of your study on the use of VLE?
HW My colleagues and I conducted a safety and feasibility study to determine whether this new imaging modality was robust, safe, and could effectively acquire image scans in our initial 100 patients. The design of the study was, in some ways, a challenge. We evaluated the feasibility of performing examinations with VLE and the safety of the procedure to ensure that no untoward problems occurred; however, given the design of the study, we were not able to provide more clinically relevant information. Given the potential of this imaging technology, physicians have understandably wanted more information about the accuracy of the diagnosis of Barrett esophagus and whether subsquamous glands can be detected. We are now seeing the presentation and publication of numerous subsequent studies demonstrating the imaging capabilities of this device.
G&H How safe is VLE compared with other imaging or endoscopic techniques?
HW Based on the results of our study, we found that VLE is a safe endoscopic imaging technique. Other endoscopic imaging techniques such as confocal laser en-domicroscopy require infusion of a contrast that is given intravenously, which leads to the possibility of an adverse reaction to the contrast. VLE does not require the injection of external agents, making it at least as safe as, and likely safer than, most other imaging techniques. Intervention-type techniques such as endoscopic mucosal resection have a small but significant risk of bleeding and perforation that is not present with VLE. My colleagues and I did not find serious adverse events in any of the 100 patients included in our study. A couple of technical failures of the imaging probe occurred, and these issues have been sorted out in the latest versions of the VLE system. Overall, VLE is a safe technique in patients who can safely undergo sedated endoscopy and who do not have any contraindication to having a balloon inflated in their esophagus (eg, altered esophageal anatomy or tight narrowing such as an anastomotic stricture).
G&H Has cost-effectiveness analysis been applied to this procedure?
HW The device is still too new for a cost-effectiveness analysis. So much information is coming from early studies that there are more questions than answers at this point. Cost-effectiveness of VLE, compared with endoscopic ultrasound or other imaging technologies, is going to be an important topic over the next several years.
G&H Is VLE able to identify buried Barrett glands after mucosal ablation?
HW Studies by Dr Anne-Fré Swager and colleagues and Dr Waku Hatta and colleagues suggest that it can, with a subsurface scanning accuracy that is unmatched. Endoscopic ultrasound and other imaging techniques are not accurate enough to supply the type of information that would allow physicians to detect subsurface or subsqua-mous glands. Results of the study by Dr Hatta and colleagues demonstrated 80% sensitivity and 100% specificity in detecting subsquamous Barrett esophagus in 14 patients with concomitant Barrett esophagus adenocarcinoma. Dr Swager and colleagues demonstrated the use of VLE in 17 patients who had achieved complete eradication of intestinal metaplasia. Even in the patient population that had negative endoscopic findings and negative biopsies, VLE detected a case of subsquamous Barrett esophagus. Both studies had excellent histologic correlation.
G&H What is the association between proton pump inhibitor use and malignancy arising from buried Barrett glands? If the likelihood of malignancy is low, does it reduce the importance of this finding?
HW Proton pump inhibitors are associated with squamous overgrowth of Barrett mucosa in many patients. Clinicians are uncertain as to whether this is an important risk factor for cancer development. In the past, there has not been a tool for reliable imaging of subsquamous Barrett esophagus. Early experience suggests that VLE will likely be a valuable imaging modality in this setting, and there are many centers actively studying this question presently.
G&H What are the priorities of research in this field?
HW The research in this field is going in several different directions. Work is ongoing in developing a computer-aided feature detection system and computer-aided image analysis, and studies are being performed to assess and refine the Barrett esophagus dysplasia criteria. In addition, Dr Gary Tearney has developed a VLE-imaging device on a tethered capsule to be swallowed and used to detect Barrett disease and precancerous changes in the esophagus without requiring patients to undergo an expensive endoscopic procedure. This VLE application is intended for use in primary care settings as a method to screen and identify Barrett esophagus patients with cancer that would otherwise not be detected.
VLE has also been used to stage early squamous cell carcinoma, a type of esophageal cancer that has become much less common in the United States over the past 100 years. However, as squamous cell carcinoma remains prevalent in some areas in Asia, Africa, and certain parts of the world, studies have been conducted in this patient population using VLE.
The third condition for which VLE is indicated is achalasia. VLE is used to guide peroral endoscopic my-otomy, an endoscopic treatment that cuts the muscle layers of the esophagus in order to help it drain better and to aid food transit from the esophagus into the stomach.
A smaller version of the imaging probe, used without the balloon-centering device, has recently been developed for use in much smaller tubular lumens, such as the common bile duct and pancreatic duct. Future applications of this device include the detection of cancerous and precancerous conditions in the pancreaticobiliary system.
Currently, VLE scans are reviewed manually based on an enhanced diagnostic algorithm developed by Dr Cadman Leggett and colleagues at the Mayo Clinic in Rochester, Minnesota. This diagnostic algorithm, which is a refinement of earlier work conducted by Dr Gary Tearney and colleagues at Massachusetts General Hospital, produces a sensitivity and specificity of 86% and 88%, respectively, for the detection of Barrett esophagus dysplasia. This study was published in the May 2016 issue of Gastrointestinal Endoscopy.
A low likelihood of malignancy in Barrett esophagus patients with subsquamous glands does not reduce the importance of the ability to detect buried Barrett glands. It is sobering to have a patient whose esophageal lining has returned to the normal flat, white-pink squamous mucosa only to then show signs of a subtle mucosal irregularity that is found to be subsurface cancer. It is unknown how common that scenario is, but it is a serious concern. A relatively small problem does not mean it is not important, and now there may be an imaging capability to help find these patients so they can get treated before the cancer spreads.
As mentioned previously, a smaller version of the imaging probe without a centering balloon has been developed for use in small lumens such as the common bile duct and pancreatic duct. Future work will study the clinical utility of these new applications of this imaging technology.
Because VLE is a relatively young technology and we are in the early stages of understanding its use, there are currently many unanswered questions. However, led by my colleagues at the Mayo Clinic, this technology has been adopted around the world at other centers, and subsequent studies are being conducted to find answers.
Figure 1.
Volumetric laser endomicroscopy with a balloon-centered, high-speed optical imaging probe (A) and image console with user interface (B). The imaging probes are compatible with endoscope channels 2.8 mm or larger and are available in balloon sizes of 14 mm,17 mm, and 20 mm. The 7-μm high-resolution imaging probes utilize near infrared light for surface and subsurface imaging depths of greater than 3 mm. The probe scans a 6-cm length of the esophagus with real-time, high-speed data acquisition and image reconstruction on the image console and user interface, with the large screen display for correlation between the scanned images and gut anatomy to facilitate tissue diagnosis and guide endoscopic treatment.
Figure 2.
A volumetric laser endomicroscopy image demonstrates the layered structure of normal squamous epithelium compared with the characteristic undulating surface of gastric cardia (without layers and featuring a pattern of higher surface signal intensity) and regular surface of nondysplastic Barrett esophagus (lacking the layered morphology of squamous mucosa and featuring an increased surface signal intensity).
Biography

Footnotes
The Mayo Clinic has received support for the research of Dr Wolfsen, funded in part by NinePoint Medical, Mauna Kea Technologies, EXACT Sciences Corporation, Olympus America, Boston Scientific, and Pinnacle Biologics. Dr Wolfsen is a member of the clinical advisory board for NinePoint Medical.
Suggested Reading
- Hatta W, Uno K, Koike T, et al. Feasibility of optical coherence tomography for the evaluation of Barrett’s mucosa buried underneath esophageal squamous epithelium. Dig Endosc. 2016;28(4):427–433. doi: 10.1111/den.12576. [DOI] [PubMed] [Google Scholar]
- Leggett CL, Gorospe EC, Chan DK, et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest Endosc. 2016;83(5):880–888.e2. doi: 10.1016/j.gie.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MJ, Gora MJ, Lauwers GY, et al. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest Endosc. 2014;79(6):886–896. doi: 10.1016/j.gie.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swager A, Van der Sommen D, Zinger S, et al. Feasibility of a computer algorithm for detection of early Barrett’s neoplasia using volumetric laser endomicroscopy. Paper presented at United European Gastroenterology Week; October 18, 2016; Vienna, Austria. [Google Scholar]
- Swager AF, Boerwinkel DF, de Bruin DM, et al. Detection of buried Barrett’s glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointest Endosc. 2016;83(1):80–88. doi: 10.1016/j.gie.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Trindade AJ, George BJ, Berkowitz J, Sejpal DV, McKinley MJ. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett’s esophagus. Endosc Int Open. 2016;4(3):E318–E322. doi: 10.1055/s-0042-101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsen HC, Sharma P, Wallace MB, Leggett C, Tearney G, Wang KK. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett’s esophagus (with videos) Gastrointest Endosc. 2015;82(4):631–640. doi: 10.1016/j.gie.2015.03.1968. [DOI] [PubMed] [Google Scholar]