Abstract
Background
Current biomarkers in ulcerative colitis (UC) are limited by their performance, cost and limited availability in daily practice. This study examined alterations in the leukocyte profiles as biomarkers of UC activity including the effects of age, gender and medications.
Methods
Case control study that included 110 UC subjects, 75 subjects with C. difficile infection, and 75 non-inflammatory bowel disease (IBD) subjects, randomly selected from a single institution IBD database. Mean values of neutrophils (N), lymphocytes (L), monocytes (M) and their ratios were compared between groups. ROC curve analyses assessed the performance of each biomarker in discriminating disease states. Subgroup analyses examined leukocytes profiles with endoscopic activity.
Results
Elevated monocyte counts and decreased L/M values significantly differed between subjects with active UC and UC in remission and performed better than other leukocyte profiles. A monocyte count of 483 and L/M ratio of 3.1 were 60% sensitive and had a specificity of 61% and 53% respectively for active UC. Monocyte count > 860 and L/M value < 1.6 had a 75% positive predictive value for UC activity. Those markers also correlated with endoscopically active disease. L/M and N/L values performed best at differentiating active UC from non-IBD controls, while N/L and neutrophil values performed best at differentiating from C. diff controls.
Conclusion
Monocytosis and a low L/M ratio might be effective, readily available and low cost biomarkers to identify disease activity in UC patients. N/L values were more effective at distinguishing active UC patients from patients without IBD and those with C. diff infection.
Keywords: Ulcerative colitis, peripheral blood leukocytes, biomarkers
Introduction
Biomarkers in inflammatory bowel disease (IBD) can aid in the diagnosis and monitoring of disease activity in clinical practice. However given the suboptimal performance of currently available biomarkers, endoscopy remains the gold standard for diagnosis and disease activity monitoring. Thus, an important research focus has been the discovery of new biomarkers.1,2,3,4,5
Numerous disease biomarkers have been studied in ulcerative colitis (UC) such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), with sensitivities and specificities ranging between 50–60%6,7. On the other hand, fecal calprotectin and lactoferrin are more specific and sensitive but expensive and still not commonly used in clinical practice due to the need for on demand specimen collection.6, 7, 8, 9 Thus easily attainable, efficacious and cost effective biomarkers are still lacking for optimal UC management.
Leukocytes or white blood cell differentials are routinely checked during clinical visits and have been evaluated as biomarkers in inflammatory diseases. Neutrophils and peripheral blood mononuclear cells (PMBCs) were found to correlate with disease activity and predict disease severity in rheumatoid arthritis (RA) and acute coronary syndrome. 10, 11, 12 Alterations of leukocyte numbers, in particular monocytes, were recognized early on in IBD13,14,15. Indeed monocytes, macrophages and neutrophils are uniquely involved in the pathophysiology of IBD.16 Recent interest in the need for cost effective biomarkers in IBD have reinvigorated the examination of leukocyte patterns for this purpose, but only a few studies have reported their potential to serve as biomarkers in IBD. Absolute neutrophil and lymphocyte counts and their ratios have been recently reported to correlate with disease activity in UC.17, 18 However, other cell types and ratios were not examined, and the effects of medications and infections on those parameters and their correlation with endoscopic disease activity are unknown.
We hypothesized that neutrophil and PBMC signatures could correlate with UC disease activity and also differentiate it from Clostridium difficile (C. diff) infection and non-inflammatory controls. The objective of this study was to examine the association of neutrophil, lymphocyte and monocyte values with quiescent and active UC, while considering the effects of medications and common infections, in order to identify patterns that could serve as effective biomarkers in UC.
Materials and Methods
Patients
This retrospective case control study was approved by the institutional review board of the University of Iowa. A retrospective analysis of electronic medical records for patients 18–70 years old was performed to identify subjects with UC, C. diff infection and non-inflammatory controls followed at our institution between January 2001 and September 2012. Patients followed in the IBD program at the University of Iowa have standardized comprehensive disease specific information recorded at each visit. Subjects were randomly selected with the following exclusion criteria: concomitant infection (positive urine culture, blood cultures, infiltrates on CXR, documented skin infection…), UC patients with positive stool PCR for C. diff toxin 30 days before or after the visit, malignancy or chemotherapy, leukemia, lymphoma, autoimmune disease and acquired or congenital immunodeficiency. Subjects without known IBD and with a diagnosis of C. diff infection based on positive toxin A or B PCR in the stool and subjects without known gastrointestinal disease meeting the same exclusion criteria were consecutively selected as controls.
Chart review
A systemic chart review of the electronic medical records of UC patients included in the study was performed and data was collected from clinic notes, progress notes, discharge summaries, laboratory studies, endoscopic and pathology reports. Each patient with UC had data recorded for one or more clinical visits and this included: gender, age at diagnosis, age at visit, disease localization, detailed medication list (5-aminosalicylate, oral corticosteroids, immunosuppressant and biologics), partial clinical Mayo UC score19,20,21 short inflammatory bowel disease questionnaire (SIBDQ) score 22,23, laboratory studies including ESR (mm/hr), CRP (mg/dl), total white blood cell count (K/mm3) with automated leukocyte differential detected by flow cytometry (neutrophil, lymphocytes and monocytes counts in mm3), and endoscopic disease activity and extent. ESR, CRP, disease activity scores, and colonoscopy reports were not available for all UC visits. The following ratios of leukocyte fractions were calculated: neutrophils to lymphocyte ratio (N/L), lymphocyte to monocyte ratio (L/M) and neutrophil to monocyte ratio (N/M).
Clinical assessment
The assessment in the note from the clinic visit was examined to see if the physician determined the UC to be clinically active or in clinical remission. The following terms were present to define impression of disease activity: exacerbation, active, mildly, moderately, severely active, quiescent, inactive, and remission. Notes that did not indicate a clear clinical impression were not included in the analysis. A visit was considered to be in the “UC active” group if the provider reported clinically active disease plus one of the following objective indicator of disease activity: 1) CRP> 0.5 mg/dL, 2) ESR > 20 mm/hr, 3) partial Mayo UC score >2.5, 4) active disease on colonoscopy or 5) escalation in treatment of active disease. Controls were also divided into two groups one with C. diff infection and one with no inflammatory or gastrointestinal disease called non-IBD controls. For controls, age, gender and total white blood cell count (K/mm3) with automated leukocyte differential detected by flow cytometry (neutrophil, lymphocytes and monocytes counts in mm3) were collected. Leukocyte fractions were also calculated. In adults the mean monocyte count is reported at 400 K/mm3 and monocytosis is considered 800 K/mm3 or greater 24. The upper limit of our laboratory for monocytosis is defined at greater than 860 K/mm3.
Subgroup analyses
To investigate the leukocyte pattern correlation with endoscopic activity those subjects that had a colonoscopy and a differential within a week of the procedure were analyzed separately. Endoscopic findings were divided into quiescent and active disease based on both endoscopic and histopathologic activity.
Statistical Analysis
Log-transformed leukocyte cell counts and ratios were modeled as dependent variables using generalized linear regression techniques in SAS (version 9.3, Cary, NC). Proc GENMOD, which applied the generalized estimating equations (GEE) method for fitting the model to account for correlation of multiple data values from the same subject, was used to examine the association of endoscopic activity with leukocyte cell counts (or ratios). This was done by comparing leukocyte cell counts (or ratios) of UC active with UC remission, non-IBD controls, and non-IBD C. diff controls, with p-values based on Dunnett’s test to account for multiple tests. The comparison of UC active versus the two control groups was used for identifying diagnostic biomarkers, and the comparison between UC active and UC remission was used for assessing markers of disease activity. Since there may be effects of age, gender, and medication use on leukocyte cell counts and ratios, this analysis was also performed including these variables as covariates. Logistic regression models fitted by the GEE method were used to construct ROC curves to assess the performance of each biomarker in discriminating between disease states. As the biomarker should strongly detect active ulcerative colitis from other sets of patients, separate ROC-curves were constructed for discriminating active UC subjects from UC subjects in remission, those with C. diff and from non-IBD controls.
Results
Subject characteristics
A total of 175 patients diagnosed with ulcerative colitis were identified, of which 65 patients did not meet the inclusion and exclusion criteria. A total of 110 UC patients were included as subjects for the study; 55 males and 55 females. 75 patients with C. diff infection (29 males and 46 females) and 75 patients without inflammatory or gastrointestinal disease (33 males and 42 females) were included as controls. There were no significant differences in gender and age between the UC group and the control groups (p>0.05). In the UC group, each subject had data collected for one or more clinical visit. A total of 297 visits were collected and based on the criteria described above, divided into 147 “UC remission” and 150 “UC active” visits. Patient characteristics per visit are reported in table 1.
Table 1.
Patient characteristics of the ulcerative colitis cohort.
| Ulcerative colitis cohort | |
|---|---|
| Total number of visits | 297 |
|
| |
| Age at visit in years, median (IQR) | 31 (25–41) |
| [Range] | [12–70] |
|
| |
| Duration of disease, median (IQR) | 4(2–9) |
| [Range] | [0–41] |
|
| |
| Remission | 147 (49%) |
| Active Disease | 150 (51%) |
|
| |
| Colonoscopy (visits) | N=105 |
| Quiescent | 20 (19%) |
| Macroscopic | 73 (70%) |
| Microscopic | 12 (11%) |
|
| |
| Disease extent (most recent visit) | N=110 |
| Proctitis | 9 (8%) |
| Left sided | 27 (25%) |
| Extensive | 23 (21%) |
| Pancolitis | 51 (46%) |
|
| |
| Medication history | |
| Steroids | 77 (26%) |
| 5-ASA | 236 (79%) |
| Immunosuppressant | 135 (45%) |
| Biologics | 19 (6%) |
|
| |
| ESR | N=83 |
| Median (IQR) | 15 (6–28) |
| [Range] | [0–84] |
|
| |
| CRP | N=137 |
| Median (IQR) | 0.25 (0–1.0) |
| [Range] | [0–38.9] |
|
| |
| SIBDQ | N=38 |
| Median (IQR) | 52(44–62) |
| [Range] | [20–70] |
|
| |
| Partial Mayo UC score | N=39 |
| Median (IQR) | 3(1–4) |
| [Range] | [0–8] |
Leukocyte subtypes and ratios as biomarkers for diagnosis and disease activity
Neutrophil and PBMC numbers with calculated ratios were examined and compared among the groups. The results of these comparisons are summarized in Table 2. Comparison of symptomatic UC subjects with non-IBD controls showed significant difference in monocyte and lymphocyte counts. Patients with active UC had lower lymphocytes to monocytes ratio (L/M) and higher neutrophils to lymphocytes ratio (N/L) compared to non-IBD controls (p<0.0001), table 2.
Table 2.
Leukocyte subtype values and ratios compared to active ulcerative colitis.
| UC active | UC Remission | p-value* | Non-IBD | p-value* | C diff. infection | p-value* | |
|---|---|---|---|---|---|---|---|
| Monocyte count (M) | 617 [524–726] | 471 [401–554] | <0.0001 | 482 [390–595] | 0.005 | 785 [616–1002] | 0.05 |
| Lymphocyte count (L) | 1329 [1117–1581] | 1378 [1162–1633] | >0.99 | 1697 [1397–2063] | 0.004 | 1218 [960–1544] | >0.99 |
| Neutrophil count (N) | 5181 [4377–6132] | 4420 [3735–5231] | 0.02 | 4331 [3573–5251] | 0.065 | 8462 [6716–10661] | <0.0001 |
| L/M ratio | 2.13 [1.82–2.49] | 2.92 [2.44–3.50] | <0.0001 | 3.51 [2.96–4.16] | <0.0001 | 1.54 [1.22–1.93] | 0.04 |
| N/M ratio | 8.42 [7.03–10.07] | 9.71 [8.07–11.67] | 0.1 | 9.03 [7.07–11.53] | 0.8 | 10.94 [8.14–12.70] | 0.1 |
| N/L ratio | 3.94 [3.12–4.97] | 3.30 [2.54–4.29] | 0.1 | 2.56 [1.99–3.29] | <0.0001 | 7.02 [5.17–9.54] | <0.0001 |
Values are the mean [95% CI], adjusted for age, gender and medications.
Compared to UC active.
Symptomatic UC subjects compared to non-IBD C diff. controls showed significantly higher mean absolute neutrophil counts in C diff. subjects (p<0.0001), which is consistent with the differential shift seen in bacterial infections. Monocyte count was also significantly higher in C. diff subjects, (p=0.050). C. diff. controls also had a significantly lower L/M (p=0.036) and higher N/L (p<0.0001) than active UC, table 2.
Symptomatic UC subjects compared to UC subjects in clinical remission showed significantly higher absolute monocyte counts (p<0.0001) and absolute neutrophil counts (p=0.017), and lower L/M ratio (p<0.0001) compared to UC in remission, table 2. The N/L ratio did not show a significant difference between the two groups when controlling for age, gender and medications. These findings suggest that leukocyte subsets may be useful biomarkers of disease activity.
Our analyses controlled for the effects of medications, which can affect the leukocyte counts and thus the ratios as well. We specifically analyzed the effect of each IBD medication (steroids, 5ASA, immunosuppressants and biologics) on the leukocyte subsets, supplemental Table 3. Steroids significantly increased mean neutrophil counts by 74%, 95% CI (55.72 – 93.77), p < 0.0001 and in turn the N/L ratio by 85%, 95% CI (56.63 – 119.83), p < 0.0001. Immunosuppressants (azathioprine, 6MP & methotrexate) significantly decreased mean lymphocyte counts by 25%, 95% CI (−32.44 - −16.28), p < 0.0001, decreased mean monocyte counts by 16%, 95% CI (−28.96 - −1.61), p = 0.04, and decreased mean neutrophil counts by 14%, 95% CI (−24.60 - −1.61), p = 0.04. The data also suggested an increase in the mean N/L ratio and decrease in the mean L/M ratio with immunosuppressants but was not significant at the 0.05 significance level (both p=0.096). On the other hand, 5ASAs and biologics did not significantly influence the absolute counts or their ratios.
Diagnostic biomarker performance
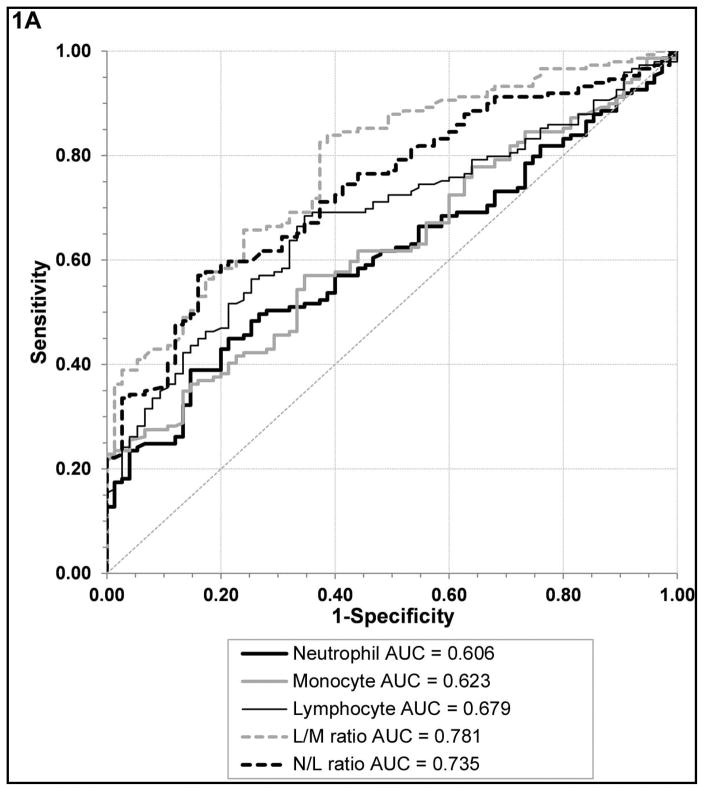
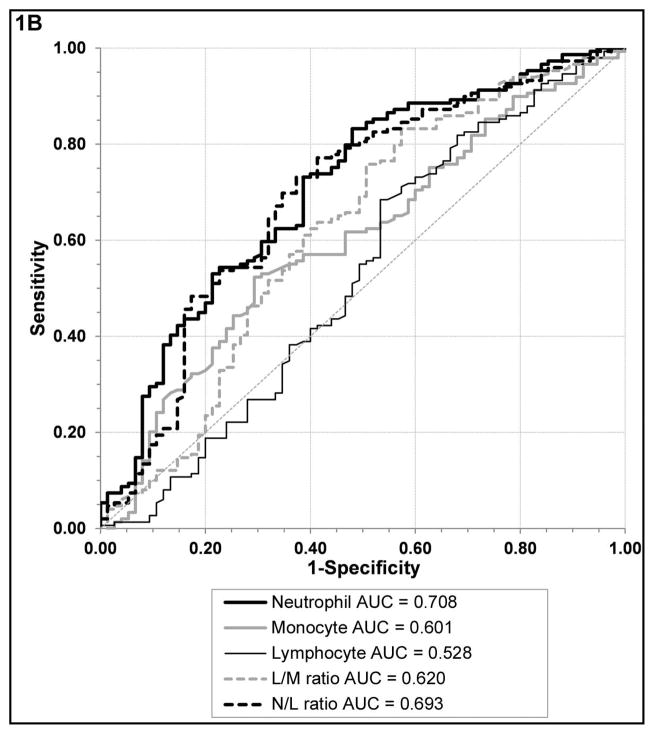
We identified several leukocyte markers that were able to differentiate active UC from non-IBD controls and C diff. infection, respectively. Receiver operator curve (ROC) analyses revealed that L/M (AUC 0.781, 95% CI [0.720–0.842]) and N/L (AUC 0.735, 95% CI [0.670–0.801]) ratios were the best biomarkers to differentiate active UC from non-IBD controls, with significantly larger AUC than absolute monocyte and neutrophil counts; figure 1A, supplemental table 1A. On the other hand, an elevated N/L (AUC 0.693, 95% CI [0.616–0.769]) and absolute neutrophil count (AUC 0.708, 95% CI [0.635–0.781]) performed similarly with significantly larger AUC than elevated absolute monocyte counts and decreased L/M for differentiating active UC from C. diff infection; figure 1B, supplemental table 1B.
Figure 1. Biomarker performance.
Receiver operator curves of the various leukocyte subtypes and ratios in UC active compared to non-IBD controls (A) and UC active compared to C diff. controls (B).
For differentiating UC active from non-IBD controls a L/M cut off value of 3.4 had a sensitivity of 70% and specificity of 64% with lower values becoming progressively more specific, a N/L cut off value of 2.6 had a sensitivity of 70% and specificity of 63% with higher values becoming progressively more specific, and a monocyte count cut off of 589 had a sensitivity of 61% and specificity of 56%. If the monocyte count was greater than 860 (upper limit of normal range in our laboratory) the specificity was greater than 79%. For differentiating UC active from C. diff infection, an absolute neutrophil count of 5655 had a sensitivity of 73% sensitive and specificity of 61%, a L/M cut off value of 3.1 had a sensitivity of 61% and specificity of 61% with higher values becoming progressively more specific, a N/L cut off value of 3.1 had a sensitivity of 70% and specificity of 65% with lower values becoming progressively more specific, and the same monocyte count cut off of 589 only had a sensitivity of 71% and specificity of 38%.
Disease activity biomarker performance
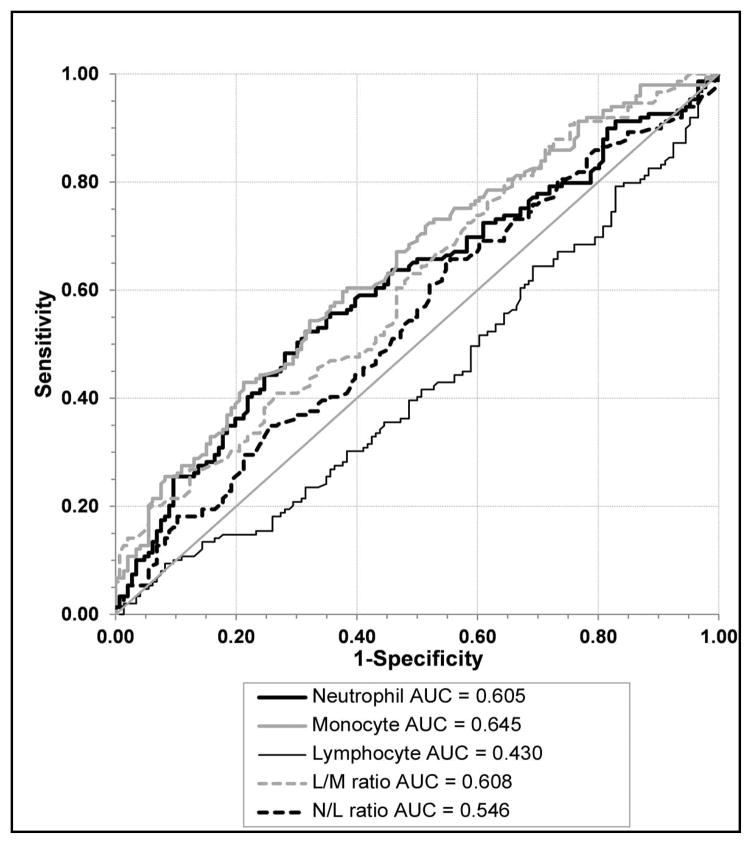
As reported in table 2, decreased L/M values and elevated monocyte and neutrophil counts significantly differentiated between active and quiescent UC, while lymphocyte and N/L values did not. ROC analyses revealed that L/M (AUC 0.608 95% CI [0.544–0.672]) and monocyte counts (AUC 0.645 95% CI [0.583–0.708]) ratios were the best biomarkers to differentiate active UC from UC in remission, only slightly better than neutrophil counts; figure 2, supplemental table 2.
Figure 2. Biomarker performance.
Receiver operator curves of the various leukocyte sub-types and ratios in UC active compared to UC remission.
For differentiating UC active from UC remission a L/M cut off value of 3.1 had a sensitivity of 60% and specificity of 53% with lower values becoming progressively more specific, a monocyte count cut off value of 483 had a sensitivity of 60% and specificity of 61% with higher values becoming progressively more specific. If the monocyte count was 860 the specificity was 92% with a 75% positive predictive value. 23% of our subjects with active UC had monocytes counts greater than 860.
Leukocyte subtypes and ratios in endoscopically active and quiescent disease
Since endoscopic disease is the gold standard for differentiating between active and quiescent disease a subgroup analysis of subjects that had concurrent colonoscopy data was performed that included 87 active and 15 quiescent colonoscopy visits. Leukocyte patterns were compared between UC subjects with active mucosal disease and quiescent colitis on colonoscopy. Elevated monocyte counts and decreased L/M values were still able to significantly distinguish UC active from UC quiescent, p = 0.0007 and 0.0002 respectively, table 3. N/L values were not significantly different between the two groups.
Table 3.
Comparison of leukocyte subtypes and ratios between endoscopically active and quiescent disease.
| Active on colonoscopy | Quiescent on colonoscopy | p-value | |
|---|---|---|---|
| Monocyte count (M) | 633 [542–740] | 511 [432–604] | 0.0007 |
| Lymphocyte count (L) | 1367 [1160–1610] | 1401 [1181–1661] | 0.623 |
| Neutrophil count (N) | 4959 [4166–5903] | 4639 [3906–5510] | 0.145 |
| L/M ratio | 2.06 [1.77–2.40] | 2.73 [2.32–3.21] | 0.0002 |
| N/M ratio | 8.11 [6.81–9.67] | 9.35 [7.83–11.16] | 0.046 |
| N/L ratio | 3.76 [2.98–4.79] | 3.40 [2.64–4.37] | 0.144 |
Values are the mean [95% CI], adjusted for age, gender, and medication
Discussion
Cost effective, noninvasive and easily accessible biomarkers that function as surrogates for active mucosal inflammation are important for the clinical management and diagnosis of ulcerative colitis. Fecal calprotectin is our best clinically available biomarker of disease activity in UC patients, but is limited in routine practice by cost, sample processing time, and ability to collect stool samples25. The use of peripheral blood leukocyte subtypes as biomarkers of inflammatory disorders, including IBD, have been recently described10,11,12,13,14,17,18,26. Complete blood counts with differential are almost universally monitored at clinic visit and thus routinely available. We examined the association of neutrophil and PBMC values as a marker of disease activity in ulcerative colitis while taking into account the potential effects that medications and infections could have on these outcomes. Our analysis discovered a significant association between elevated monocyte counts in patients with clinically active UC (617/mm3), compared to those with quiescent UC (471/mm3) and without IBD (482/mm3); as well as decreased L/M ratio in patients with clinically active UC (2.1), compared to those with quiescent UC (2.9) and without IBD (3.5). These associations remained significant for endoscopically active disease with elevated monocyte counts of 633/mm3 and decreased L/M ratio of 2.1 compared to those with mucosal healing (511/mm3 and L/M 2.7, respectively). Neutrophil counts were also elevated in patients with active UC compared to quiescent UC and non-inflammatory controls (5181/mm3 vs. 4420 vs. 4331, respectively). However monocyte counts and LMR performed better than neutrophil counts in distinguishing active disease from other disease status.
Two recent studies identified the N/L ratio as the most effective marker of active UC, but this study excluded patients with immunosuppressive medications, potentially included patients with concurrent autoimmune diseases, and used less rigorous criteria to identify an active flare of UC17,18. Our analysis showed that the N/L ratio was effective at differentiating active UC from non-inflammatory controls, but not from quiescent UC; nor was it effective at differentiating endoscopically active disease. These studies did not report other leukocyte subtypes or endoscopically active disease.
Monocytes are a subset of circulating white blood cells that differentiate into macrophages and dendritic cells in tissues, and play a role in innate immunity. During infectious and non-infectious inflammatory disorders, monocytes are generated in the bone marrow and recruited to the inflamed tissues through the release of pro-inflammatory cytokines, chemokines and/or other microbial or pathogen-associated molecular patterns such as LPS. There they differentiate into tissue resident macrophages and dendritic cells. Persistent activation of monocytes and defective innate immune responses are known to be involved in the development of IBD26. Thus, monocyte counts are expected to be elevated during active inflammation and infections27. Indeed, monocytosis was reported 34 years ago in ulcerative colitis compared to normal controls and found to correlate with the severity of the disease.13 Thus it is possible that immunosuppressive medications and different stages of UC activity would influence monocyte counts and other leukocyte subtypes. In particular, Azathioprine or 6MP can significantly lower lymphocyte, monocyte and neutrophil counts, but not significantly the N/L and L/M ratios. While steroids can significantly increase neutrophil counts and subsequently the N/L ration. Thus, we accounted for these potential confounders by controlling for these medications during the statistical analysis. Our results still revealed an association of elevated monocyte counts with disease activity, suggesting that these biomarkers could be used regardless of concurrent immunosuppressive medications.
In addition to these confounders, infections will also influence leukocyte subtype patterns, and active infections such as C difficile colitis must also be considered during UC flares and stool testing is routinely performed in clinical practice. Therefore we also sought to examine the effect that C. difficile infection had on these same leukocyte subtypes patterns. Elevated monocyte counts were also seen in C. diff infections and were not significantly different from active UC patients (785/mm3 vs. 617/mm3, respectively); however L/M ratio values were significantly lower in C. diff infection compared to active UC (1.5 vs. 2.1, respectively). The strongest association with C. diff infection were elevated neutrophil counts (8462/mm3) and N/L values (7.0) that significantly differentiated C. diff from active UC (5181/mm3 and 3.9, respectively). Thus our analysis suggests that monocyte counts and L/M ratios would not be well suited to discriminate concurrent C. diff infection during an active UC flare, but elevated neutrophil counts, and N/L ratio, might prompt the clinician to make sure that C. diff was ruled out. However, we did not examine UC patients with concurrent C diff. infection due to low patient numbers; consequently we cannot generalize this finding to differentiate C. difficile in active UC patients. Future studies should consider subjects with clinically active UC symptoms and concurrent C. diff infections to better assess this biomarker association.
Through this retrospective study we identified signals that suggest monocyte counts and L/M ratios can serve as diagnostic biomarkers for disease state and disease activity. Variations in these values can be the first indicator to the provider that there may be ongoing gastrointestinal inflammation, which could then be proactively addressed. When using these markers to be vigilant for preclinical disease activity to prevent flares, the clinician is looking for a highly sensitive marker. We found that a L/M value of 3.1 and monocyte count of 483 can predict active disease with sensitivity and specificity of 60% and 53%, and 60% and 61%, respectively. However, it is accepted that a lack of clinical symptoms do not correlate with endoscopic remission. Thus a L/M value less than 1.6 and/or a monocyte value greater than 860, which carries a 75% PPV for active UC should prompt a clinician that there is continued UC activity. Alternatively, when the patient has clinically active disease that a clinician needs to differentiate from irritable bowel syndrome then a more specific marker is indicated. A monocyte count higher than 640 and a L/M value lower than 2 can predict active disease with a specificity of greater than 80%.
In current daily practice, the most commonly used non-invasive serum biomarkers to assess active disease are ESR and CRP. In terms of differentiating disease activity, a recent study showed that ESR and CRP only modestly correlated with endoscopic activity indices in UC patients and the ranges of sensitivity and specificity for detecting endoscopic remission were 50–53% and 85–87% respectively for CRP and 69–71 % and 63–66 % respectively for ESR. 28 The limited correlation of ESR and CRP with endoscopic activity was also reported by several other studies.6,29,30 In our study, we elected to use ESR and CRP in the definition of a flare because they are still the most commonly used biomarkers in clinical practice. However, given their modest correlation with endoscopic activity we analyzed a subgroup excluding ESR and CRP from the definition of active disease. The L/M ratio and monocyte counts could significantly identify active mucosal disease but we were unable to report sensitivity and specificity of the biomarkers in predicting endoscopic activity because of low patients numbers. However looking at clinical disease activity our results were found to have a similar to slightly higher sensitivities and specificities compared to ESR and CRP with the benefit of being more common, readily available and having a lower cost.
The limitations of our study were its design as a retrospective single center study and that some patients had up to three visits, but this was accounted for in our statistical analysis. Additionally, the recruitment of patients with clinically active disease using subjective assessment of chart reviews could potentially miss patients with active UC. Thus to strengthen our study we performed a subanalysis of patients with available colonoscopy data, considered the gold standard of disease activity, and the leukocyte associations correlated with the clinical findings. Additional strengths of our study were inclusion of active disease, quiescent disease, infectious colitis, non-inflammatory controls and taking into consideration the influence of immunosuppressive medications..
We report a comprehensive evaluation of neutrophil, lymphocyte and monocyte values in clinically and endoscopically active UC. Absolute monocytes counts and L/M are promising biomarkers in ulcerative colitis. They can be additive to the other tools clinicians use in daily practice to manage UC. They offer the advantage of being routinely available, rapidly obtained and lower in cost. Future work to prospectively assess these biomarkers is warranted. It would also be prudent to analyze the efficacy of these biomarkers in Crohn’s disease and their role as possible phenotypic markers.
Supplementary Material
Acknowledgments
Source of Funding: CTSA: 2 UL1 TR000442-06 for biostatistical support
Footnotes
Conflicts of Interest: None to declare for all authors.
References
- 1.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012 Apr;159(4):313–25. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirts B, von Roon AC, Tebo AE. The entire predictive value of the prometheus IBD sgi diagnostic product may be due to the three least expensive and most available components. Am J Gastroenterol. 2012 Nov;107(11):1760–1. doi: 10.1038/ajg.2012.238. [DOI] [PubMed] [Google Scholar]
- 3.Faubion WA, Jr, Fletcher JG, O’Byrne S, et al. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013 Dec;108(12):1891–900. doi: 10.1038/ajg.2013.354. [DOI] [PubMed] [Google Scholar]
- 4.Dubinsky MC, Ofman JJ, Urman M, et al. Clinical utility of serodiagnostic testing in suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2001 Mar;96(3):758–65. doi: 10.1111/j.1572-0241.2001.03618.x. [DOI] [PubMed] [Google Scholar]
- 5.Abraham BP, Sellin JH. Fecal calprotectin: controlling the cost of care. Clin Gastroenterol Hepatol. 2014 Feb;12(2):263–4. doi: 10.1016/j.cgh.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013 Feb;19(2):332–41. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009 Nov;15(11):1746–54. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 10.Lester SE, Proudman SM, Lee AT, et al. Treatment-induced stable, moderate reduction in blood cell counts correlate to disease control in early rheumatoid arthritis. Intern Med J. 2009;39:296–303. doi: 10.1111/j.1445-5994.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang G, Zhong XN, Zhong B, et al. Significance of white blood cell count and its subtypes in patients with acute coronary syndrome. Eur J Clin Invest. 2009;39:348–58. doi: 10.1111/j.1365-2362.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohshima S, Saeki Y, Mima T, et al. Long-term follow-up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti-TNF alpha antibody therapy. J Clin Immunol. 1999;19:305–13. doi: 10.1023/a:1020543625282. [DOI] [PubMed] [Google Scholar]
- 13.Mee AS, Berney J, Jewell DP. Monocytes in inflammatory bowel disease: absolute monocyte counts. J Clin Pathol. 1980 Oct;33(10):917–20. doi: 10.1136/jcp.33.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Braat H, Faber KN, et al. Monocytes and their pathophysiological role in Crohn’s disease. Cell Mol Life Sci. 2009 Jan;66(2):192–202. doi: 10.1007/s00018-008-8308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer WR, Jr, Charland C, Field CE. The subpopulations of circulating white blood cells in inflammatory bowel disease. Gastroenterology. 1976 Sep;71(3):379–84. [PubMed] [Google Scholar]
- 16.Wallace KL, Zheng LB, Kanazawa Y, et al. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014 Jan 7;20(1):6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torun S, Tunc BD, Suvak B, et al. Assessment of neutrophil lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36(5):491–497. doi: 10.1016/j.clinre.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Celikbilek M, Dogan S, Ozbakır O, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013 Jan;27(1):72–6. doi: 10.1002/jcla.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1081–1088. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 23.Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96:2921–2928. doi: 10.1111/j.1572-0241.2001.04682.x. [DOI] [PubMed] [Google Scholar]
- 24.Miale JB. Laboratory Medicine Hematology. Mosby; St. Louis: 1982. Leukocytes; p. 658. [Google Scholar]
- 25.Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008 Jan;103( 1):162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald TT, Monteleone I, Fantini MC, et al. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011 May;140(6):1768–75. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011 Oct 10;11(11):762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon JY, Park SJ, Hong SP, et al. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014 Apr;59(4):829–37. doi: 10.1007/s10620-013-2907-3. [DOI] [PubMed] [Google Scholar]
- 29.Gomes P, du Boulay C, Smith CL, et al. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986 Jan;27(1):92–5. doi: 10.1136/gut.27.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solem CA, Loftus EV, Tremaine WJ, et al. Correlation of C-reactive protein(CRP) with clinical, radiographic, and endoscopic activity in inflammatory bowel disease (IBD) Inflamm Bowel Dis. 2005;11:707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.