Abstract
Objective
Sirtuins (Sirt) are a class of deacetylase enzymes that play an important role in cell proliferation. Sirt2 activation produces O-acetylated-ADPribose (OAADPr) which can act as a ligand for transient receptor potential cation channel, M2 (TRPM2). We tested the hypothesis that Sirt2 is activated following global cerebral ischemia and contributes to neuronal injury through activation of TRPM2.
Methods
Adult male and female mice (8–12 weeks old) C57Bl/6 and TRPM2 knock-out mice were subjected to 8 min of cardiac arrest followed by cardiopulmonary resuscitation (CA/CPR). The Sirt2 inhibitor AGK-2 was administered intravenously 30 min after resuscitation. Hippocampal CA1 injury was analyzed at 3 days after CA/CPR. Acute Sirt2 activity was analyzed at 3 and 24 h after CA/CPR. Long-term hippocampal function was assessed using slice electrophysiology 7 days after CA/CPR.
Results
AGK-2 significantly reduced CA1 injury in WT but not TRPM2 knock-out males and had no effect on CA1 injury in females. Elevated Sirt2 activity was observed in hippocampal tissue from males at 24 h after cardiac arrest and was reduced by AGK-2. In contrast, Sirt2 activity in females was increased at 3 but not 24 h. Finally, we observed long-term benefit of AGK-2 on hippocampal function, with a protection of long-term potentiation at CA1 synapses at 7 and 30 days after ischemia.
Conclusions
In summary, we observed a male specific activation of Sirt2 that contributes to neuronal injury and functional deficits after ischemia specifically in males. These results are consistent with a role of Sirt2 in activating TRPM2 following global ischemia in a sex specific manner. These results support the growing body of literature showing that oxidative stress mechanisms predominate in males and converge on TRPM2 activation as a mediator of cell death.
Keywords: TRPM2, Sirtuin, Cerebral ischemia, Cardiac arrest
1. Introduction
Brain injury caused by cerebral ischemia following stroke or cardiac arrest is a leading cause of long-term disability. Stroke and cardiac arrest are remarkably sexually dimorphic diseases, with higher rates observed in men compared to women (Koton et al., 2013; Go et al., 2014). Animal studies recapitulate this sexual dimorphism, with increased ischemic sensitivity observed in male animals (Alkayed et al., 1998; Herson et al., 2013; Zuo et al., 2013). Recent evidence demonstrates both hormone-dependent and hormone-independent factors contributing to the relative ischemic protection observed in female animals (Liu et al., 2010; Hall, 2012; Manwani et al., 2015). It is now recognized that the mechanisms of neuronal injury can differ between males and females and this is an important consideration in preclinical studies of neuroprotection. Cell death in males after in vivo or in vitro ischemia is predominantly mediated by excessive oxidative stress and subsequent over-activation of poly(ADP)ribose polymerase (PARP), while female cell death appears to recruit caspase-dependent apoptosis (McCullough et al., 2005; Lang and McCullough, 2008; Liu et al., 2009; Yuan et al., 2009; Vagnerova et al., 2010; Liu et al., 2011). Relevant to the male-specific PARP-mediated cell death pathway, we have recently demonstrated that the calcium-permeable ion channel transient receptor potential channels, M2 (TRPM2) contributes to male-specific injury following cerebral ischemia in a PARP-dependent manner (Shimizu et al., 2013). Inhibition or knock-down of TRPM2 is protective in males, but not females using in vitro and in vivo models of cerebral ischemia (Jia et al., 2011; Verma et al., 2012; Nakayama et al., 2013; Quillinan et al., 2014). A distinguishing property of TRPM2 channels is that they are gated by ADP ribose (ADPr), likely generated following the activation of PARP (Fonfria et al., 2004). In addition to ADPr, TRPM2 channels can be directly gated by the binding of O-acetyl-ADPr (Grubisha et al., 2006), generated by the histone deacetylase silent information regulator 2 (sirtuin 2, Sirt2).
Sirtuins are a family of protein/histone deacetylases that play a role in gene silencing, DNA repair and longevity. Sirt2 is a cytosolic protein that is expressed in many cell types and tissues throughout the body including the central nervous system. Expression of Sirt2 is observed in neurons as well as oligodendrocytes, microglia and astrocytes (Krey et al., 2015). Sirt2 has been demonstrated to contribute to neurodegeneration in models of Parkinson’s and Huntington’s disease (Donmez, 2013). However, the mechanism of Sirt2 mediated neurodegeneration in these animal models remains unclear. Sirtuins are NAD+-dependent deacetylases whose activation results in the production of O-acetylated-ADPribose (OAADPr) (Tanner et al., 2000), which can bind the C-terminus of TRPM2 channels and produce TRPM2 activation with similar kinetics and efficacy to ADPr (Grubisha et al., 2006). In this study we tested whether Sirt2 activation is a mediator of CA1 injury following cardiac arrest through activation of TRPM2. We identify Sirt2 as a male specific cell death pathway that when inhibited reduced neuronal injury and improved synaptic function.
2. Methods
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Colorado, School of Medicine and were performed according to the guidelines from the National Institutes of Health. Adult male and female (8–12 weeks old; 20–25 g) C57Bl/6 (Charles River laboratories, Portage, MI) and TRPM2 knockout (TRPM2 KO; kindly provided by Y Mori) (Yamamoto et al., 2008) mice were used. Wildtype age-matched mice of the same genetic background were used for comparison in experiments using TRPM2 KOs. Mice were individually housed and allowed free access to food and water. All drug treatments and analyses were performed in a blinded and randomized manner.
2.1. Cardiac arrest model
Cardiac arrest and cardiopulmonary resuscitation were performed as previously described (Kofler et al., 2004; Neigh et al., 2004; Deng et al., 2014) with slight modifications to head and body temperature. Mice were anesthetized initially with 3% isoflurane and maintained with 1.5–2% isoflurane in O2 enriched air via facemask (Isotec 5, Ohmeda, Reno, NV). Head and body temperatures were monitored using tympanic and rectal probes connected to separate automatic temperature controllers (Doric Instruments, San Diego, CA) to maintain 37 ± 0.2 °C during surgery using a head coil, heating pad and a heating lamp. For drug administration, a PE-10 catheter was inserted into the right jugular vein and flushed with heparinized 0.9% saline solution. Needle electrodes were placed subcutaneously on the chest for EKG monitoring (Medical Data Electronics, Arleta, CA) and mice were endotracheally intubated and connected to a mini ventilator (Harvard Apparatus, Holliston, MA). To induce cardiac arrest, 50 μl of 0.5 M KCl was administered via the jugular vein. Cardiac arrest was confirmed by a flat line EKG and the endotracheal tube was then disconnected from the ventilator. During CA, a tympanic temperature of 37.5 °C and rectal temperature of 35 °C was maintained. Following 8 min of cardiac arrest, resuscitation was performed by mechanical ventilation (200 breaths/min), injections of epinephrine (16 μg/ml in 0.9% saline; maximal dose 1 ml) and simultaneous chest compressions (~300 compressions/min). As soon as there was a return of spontaneous circulation (ROSC), compressions were terminated. When spontaneous breathing reached a rate of 30 breaths/min, mice were weaned off of mechanical ventilation. Temperature probes and catheters were then removed, and the skin wounds were closed. Administration of the Sirt2 specific inhibitor (greater than 10-fold selectivity for Sirt2 over Sirt1 and Sirt3), AGK2, 10 μg/kg (Tocris, Bristol, UK) or saline vehicle was performed intravenously 30 min after resuscitation. After administration of drug, endotracheal tube and jugular catheter were removed. This time of administration was chosen as it represents a relevant therapeutic window. Mice were returned to their home cages that were placed on a heated water blanket (35 °C) for recovery and received soft chow and free access to water.
2.2. Histology
At 3 days after CA/CPR mice were transcardially perfused with 4% paraformaldehyde (PFA) and post-fixed in PFA at 4 °C overnight. Brains were paraffin embedded and coronal sections containing hippocampus (6 μm at 100 μm intervals) were cut and stained with hematoxylin and eosin (H&E). Staining was visualized with a bright field microscope and analysis of cell morphology was performed bilaterally on 3 sections containing anterior hippocampus. Injured CA1 neurons were identified by hypereosinophilic cytoplasm and pyknotic nuclei and were presented as a percent of total CA1 neurons (% ischemic neurons ± SEM).
2.3. Sirt2 activity assay
Activity of Sirt2 was measured on whole hippocampi isolated 3 and 24 h after sham or CA/CPR surgery in order to assess ischemia induced activation during the acute phase of injury. Sirt2 direct fluorescent screening assay kit (Cayman Chemical Company, Ann Arbor, MI) was performed according to manufacturer’s instructions with slight modifications. Briefly, hippocampi were homogenized and centrifuged at 1000 g for 10 min to remove nuclei and cellular debris. Hippocampal lysates were added to wells containing assay buffer (acetylated substrate and NAD+) and incubated at 37° for 45 min. Stop-solution containing nicotinamide was added to the wells and fluorescence was measured using a plate reader set to excite at 360 nm and measure emission at 460 nm. Fluorescence measurements were quantified relative to protein concentration and data were normalized to sham values.
2.4. Long-term potentiation (LTP)
Extracellular recording of field excitatory postsynaptic potentials (fEPSPs) was performed in acute hippocampal sections as previously described (Orfila et al., 2014). One week after CA/CPR or sham surgery mice were deeply anesthetized with isoflurane (5%) and transcardially perfused with ice-cold artificial cerebral spinal fluid (ACSF). Brains were rapidly removed and horizontal hippocampal sections (300 μm) were cut on a vibratome (VT1200S, Leica) in ice-cold ACSF. ACSF contained (in mM): 126 NaCl, 2.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 21.4 NaHCO3, and 11 D-glucose, bubbled with 95% O2/5% CO2 to maintain pH of 7.4. Slices were equilibrated at room temperature for at least one hour prior to being transferred to an interface chamber continuously perfusing ACSF (1.5 mL/min at 32°). An extracellular recording electrode filled with NaCl (1 M) was placed in the dendritic layer of the CA1 region (stratum radiatum) and a bipolar stimulating electrode was placed in the Schaffer collateral pathway. A 20-min stable baseline of fEPSPs (0.05 Hz) were recorded and a theta-burst stimulation (TBS: 4 trains of 10 pulses@100 Hz) was used to induce LTP. fEPSPs were recorded for 1 h after TBS and fEPSP slope was analyzed. Data was reduced into 1 min averages and LTP was calculated as a % of baseline during the last 10 min of recording.
2.5. Drugs
AGK2, a selective Sirt2 inhibitor (IC50 = 3.5 μM; > 10 fold selectivity over Sirt1 and Sirt3) was obtained from Tocris. Chemicals used for electrophysiology were obtained from Fisher Scientific.
2.6. Statistics
Statistical comparisons of drug and sex effects were analyzed using two-way ANOVA. LTP comparisons were made using one-way ANOVA.
3. Results
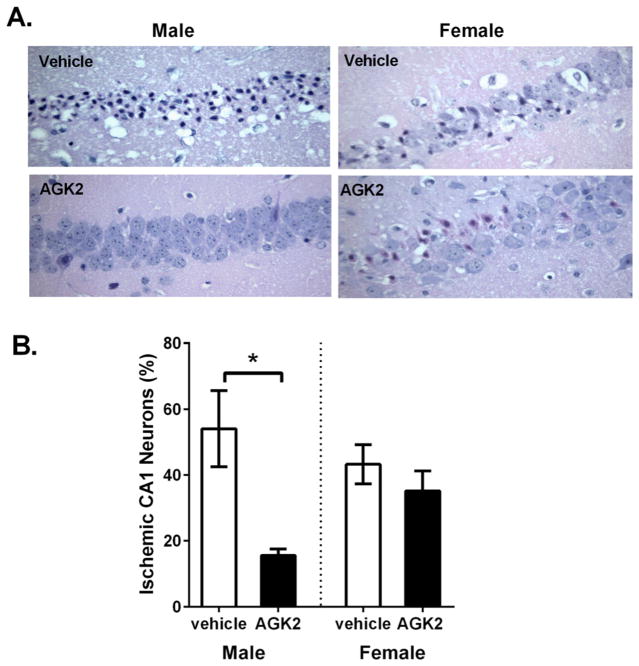
Male and female mice were subjected to 8 min CA/CPR. Immediate asystole was observed in all mice following KCl injection. Some differences in survival and ischemic time were observed in TRPM2 KO mice (Table 1). CA/CPR resulted in delayed neuronal cell death in the CA1 region of the hippocampus, as previously described (Kofler et al., 2004; Allen et al., 2011; Quillinan et al., 2015). We tested the ability of the Sirt2 inhibitor AGK to protect neurons against cerebral ischemia. Male and female mice were treated with 10 μg/kg intravenous AGK 30 min after resuscitation and CA1 injury was analyzed at 3 days after CA/CPR in H&E stained sections (6 μm). Injured CA1 neurons were identified by their hypereosinophilic cytoplasm and pyknotic nucleus (Fig. 1A). CA1 injury was 54.1 ± 11.5% (n = 8) and 43.3 ± 6% (n = 5) in saline treated males and females, respectively. AGK2 resulted in a significant reduction in CA1 injury in males to 15.6 ± 1.9% (n = 7, p = 0.0044 compared to male vehicle-treated) while having no effect in females (35.2 ± 6.1%, n = 13, p = 0.455 compared to female vehicle-treated) (Fig. 1B). These results demonstrate a role of Sirt2 activation as a sexually dimorphic mediator of neuronal injury following global cerebral ischemia.
Table 1.
Physiological parameters.
| Age (weeks) | Body weight (g) | Resuscitation time (sec) | Epinephrine (μg/g) | Survival (%) | ||
|---|---|---|---|---|---|---|
| WT Male | Vehicle | 9.7 ± 0.5 | 22.8 ± 0.5 | 87 ± 4 | 0.39 ± 0.02 | 80 |
| AGK2 | 8.8 ± 0.2 | 22.9 ± 0.7 | 95.7 ± 7 | 0.4 ± 0.03 | 75 | |
| WT Female | Vehicle | 15.8 ± 0.6 | 22.4 ± 0.5 | 93.4 ± 9.3 | 0.4 ± 0.04 | 87.5 |
| AGK2 | 14.3 ± 0.5 | 21.2 ± 0.2 | 92 ± 8.2 | 0.41 ± 0.02 | 93.3 | |
| TRPM2 Male | Vehicle | 8.1 ± 0.3 | 27.9 ± 0.5 | 126.2 ± 11.4 | 0.44 ± 0.02 | 57 |
| AGK2 | 7.6 ± 0.4 | 26.8 ± 0.5 | 159 ± 26.5 | 0.57 ± 0.02 | 63.3 | |
| TRPM2 Female | Vehicle | 9.8 ± 0.3 | 22.4 ± 0.5 | 97.8 ± 6.1 | 0.41 ± 0.03 | 90 |
Fig. 1.
The SIRT2 inhibitor AGK2 is neuroprotective in males but not females. A) Representative images of H&E stained sections of CA1 pyramidal neurons from male and female vehicle or AGK2 treated mice subjected to 8 min CA and recovered for 3 days. Injured neurons were identified by pyknotic nuclei and hypereosinophilic cytoplasm. B) Injured and non-injured cells were quantified and are presented as % ischemic neurons (mean ± SEM). AGK2 administration reduced % ischemic neurons in males but not females (* indicates p < 0.05).
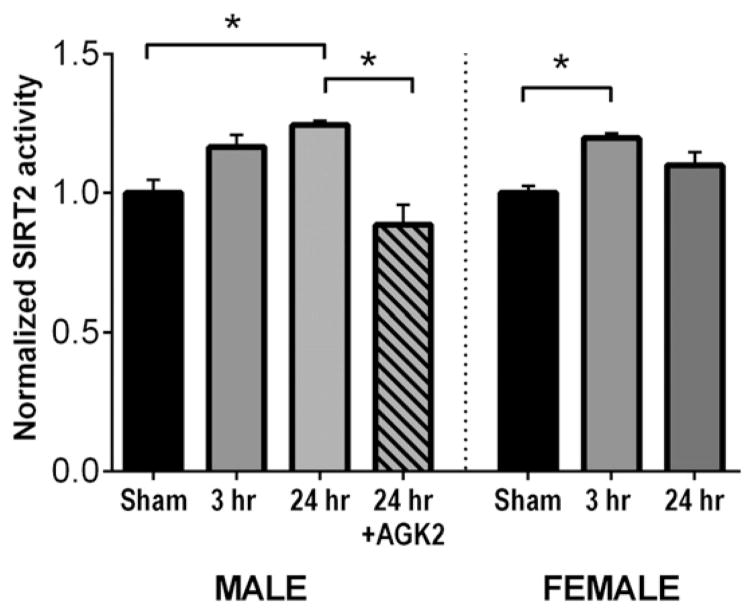
In order to characterize the ischemia-induced activation status of Sirt2, a fluorescent Sirt2 activity assay that measures substrate deacetylation was performed to determine whether there is differential activation of this enzyme in males and females following cerebral ischemia. Hippocampi isolated from male and female mice 3 or 24 h after CA/CPR (or sham) surgery and Sirt2 activity was measured in protein lysates. Fig. 2 demonstrates that in male mice subjected to CA/CPR, Sirt2 activity levels were similar to sham controls (n = 12) at 3 h (112 ± 4% sham, n = 6, p = 0.13 compared to sham) and elevated at 24 h after CA/CPR and (120 ± 1%, n = 9, p = 0.007 compared to sham). Female mice showed a transient increase in Sirt2 activity at 3 h (121 ± 4% of sham, n = 7, p = 0.007 compared to sham) that returned to sham levels at 24 h after CA/CPR (107 ± 5%, n = 5, p = 0.44 compared to sham). The increase in males observed at 24 h was blocked by AGK2 (87 ± 7%, n = 8, p = 0.0002 compared to 24 h).
Fig. 2.
Different time course of SIRT2 activation in males and females. Fluorescent measurements obtained on hippocampal lysates using a SIRT2 activity assay were normalized to sham control values. One-way ANOVA was performed separately on male and female groups (* indicates p < 0.05).
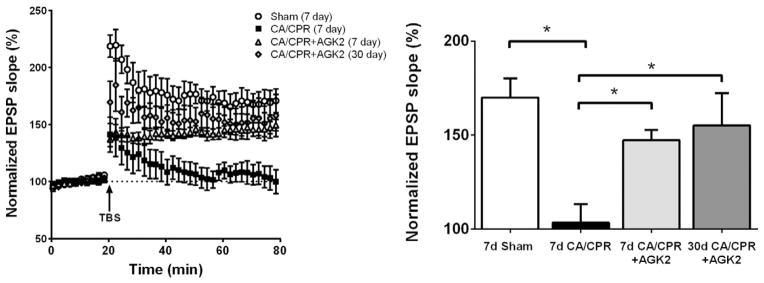
We have previously demonstrated that CA/CPR-induced functional deficits are associated with long lasting impairments in hippocampal synaptic plasticity (Allen et al., 2011; Orfila et al., 2014). Therefore, in order to assess the functional benefit of AGK2 neuroprotection, male mice subjected to CA/CPR were administered AGK2 at 30 min after CA/CPR and hippocampal synaptic function was assessed in acute slices at 7 or 30 days after CA/CPR. Long-term potentiation (LTP) is a cellular substrate of learning and memory (Shapiro, 2001; Abraham et al., 2002; Pastalkova et al., 2006; Gruart and Delgado-Garcia, 2007). To measure synaptic plasticity (LTP), extracellular field EPSPs (fEPSPs) were recorded in the CA1 region in response to Schaffer collateral stimulation (0.05 Hz). In sham animals, a brief theta burst stimulation (40 pulses) resulted in an increase in fEPSP slope to 170 ± 10.3% of baseline (n = 6) (Fig. 3). LTP impairments were observed in vehicle-treated males at 7 days after CA/CPR, with no significant increase in fEPSP following TBS (103.4 ± 9.7% of baseline, n = 6, p = 0.003 compared to sham). In contrast, fEPSP potentiated to 147.3 ± 5.4% of baseline (n = 4, p = 0.5 compared to sham) in male mice administered AGK2. Preservation of synaptic function with AGK2 was observed up to 30 days after CA/CPR (155.2 ± 17.1% of baseline, n = 5, p = 0.61 compared to sham). These results demonstrate that CA/CPR-induced ischemia reduces LTP and that acute treatment with the Sirt2 inhibitor AGK2 provides sustained functional protection.
Fig. 3.
AGK2 neuroprotection provides long-term benefit to synaptic function in males. Long-term potentiation was induced with theta-burst stimulation (TBS) of Schaffer collaterals. fEPSPs are presented as % of baseline slope. One-way ANOVA was performed with Newman–Keuls post-hoc analysis (* indicates p < 0.05).
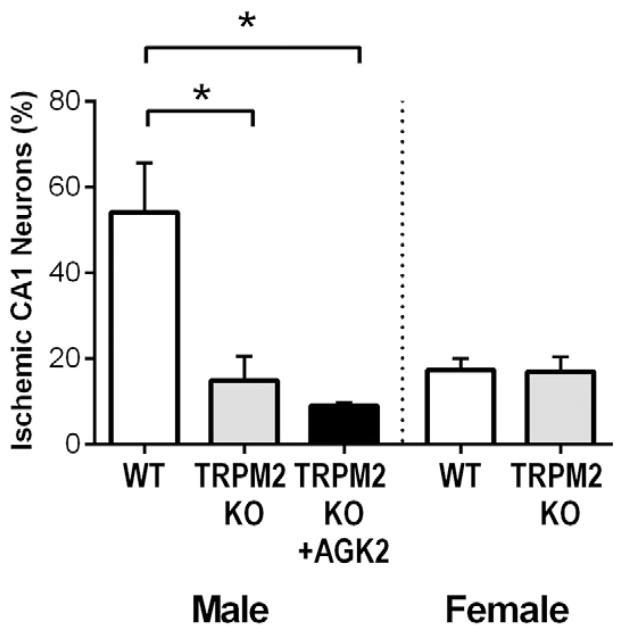
To determine if the neuroprotective effect of AGK2 observed in males is through reduced activation of TRPM2, mice lacking TRPM2 (TRPM2 KO) were administered AGK2. Male TRPM2 KO mice exhibited significantly less CA1 injury (14.8 ± 5.7%, n = 11) compared to and WT littermate controls (54.1 ± 11.5%, n = 8, p = 0.0007) (Fig. 4). In contrast, CA1 injury in TRPM2 KO females (16.9 ± 3.6%, n = 9) was not different from WT littermates (17 ± 2.9%, n = 9, p = 0.93). These data are consisted with our previously published results using a TRPM2 inhibitor following CA/CPR and TRPM2 KO mice following experimental stroke (Shimizu et al., 2013). Importantly, AGK2 administered to TRPM2 KO mice showed a similar injury (9.0 ± 0.7%, n = 5) to TRPM2 KO mice treated with vehicle (p = 0.85) suggesting there is no further benefit of Sirt2 inhibition in mice lacking TRPM2 expression.
Fig. 4.
No benefit of AGK2 in TRPM2 KO males. CA/CPR was performed on male and female TRPM2 KO and WT mice. Male TRPM2 KO mice were administered AGK2. CA1 injury was analyzed in H&E stained sections at 3 days after CA/CPR. Injury in males was compared using one-way ANOVA and in females using Student’s t-test (* indicates p < 0.05).
4. Discussion
The results of this study demonstrate that Sirt2 is a mediator of ischemic injury in males and that administration of the Sirt2 inhibitor AGK2 protects against CA1 injury and functional impairments in males, but not females. Male-specific protection is also observed in TRPM2 knockouts and no further benefit of AGK2 was observed in TRPM2 KO males. These results are consistent with Sirt2 serving as an upstream mediator of TRPM2 activation and subsequent CA1 injury. Sex differences in AGK2 neuroprotection may be due to differences in kinetics of Sirt2 activation observed following CA/CPR, with males showing a more prolonged activation than females.
Sirtuins have an important role in regulating cell survival and degeneration. Sirt1 activation upregulates pro-survival pathways and Sirt1 inhibition is hypothesized to provide benefit as a cancer therapy. In contrast, Sirt2 plays an opposing role in activating cell death mechanisms and inhibitors of this isoform have potential as a therapy for neurodegenerative diseases. Indeed, Sirt2 inhibition has been demonstrated to provide benefit against neurodegeneration in cellular and in vivo models of Huntington’s and Parkinson’s disease, but not amyotrophic lateral sclerosis or ischemic stroke (Outeiro et al., 2007; Chopra et al., 2012; Donmez, 2013; Donmez and Outeiro, 2013; Chen et al., 2015). In vitro studies have identified tubulin deacetylation and alterations in cholesterol biosynthesis as possible effectors of Sirt2 however conflicting results have been obtained in vivo (Luthi-Carter et al., 2010; Bobrowska et al., 2012). The lack of neuroprotection in a model of ischemic stroke with pharmacological or genetic inhibition of Sirt2 is in direct contrast to our observations with the protection observed with AGK2 in our mouse CA/CPR model (Chen et al., 2015). Interestingly, while Sirt2 knockout mice did not display histological benefit following ischemic stroke, they had improved functional outcome compared to wild-type mice (Krey et al., 2015). Oxidative stress is a common injury mechanisms in many neurodegenerative disease, particularly those resulting from cerebral ischemia and TRPM2 is a well-established mediator of oxidative cell death (Belrose et al., 2012). Our results suggest that there may be differences in signaling pathways engaged following focal versus global ischemia that converge on TRPM2 activation to cause neuronal injury. We have previously shown that PARP-1 activation as the pathway likely to mediate TRPM2 activation following ischemic stroke, while our results in global ischemia suggest Sirt2 as a pathway upstream of TRPM2 (Shimizu et al., 2013). It remains to be determined whether there is differential activation of Sirt2 and PARP-1 in focal and global ischemia.
Using a deacetylase assay we observed an increase in Sirt2 activity in both males and females at 3 h. At 24 h however, Sirt2 activity in females was returned to control values while the males showed sustained activation. While it is possible that other deacetylase enzymes contribute to the increased activity following cardiac arrest, the activity assay uses NAD+ as a co-substrate and nicotinamide as an inhibitor, features that are unique to sirtuins among the HDAC classes. Of the sirtuins that display deacetylase activity, Sirt1 is the most likely family member that would contribute to increased activity in our assay since other isoforms are localized to the nucleus. While activation of Sirt1 following cardiac arrest is possible, the reduction in males was prevented by the inhibitor AGK2, giving us confidence in the specificity of the assay for Sirt2. The mechanism by which activation of Sirt2 remains sustained in males, but not females is unclear. There is evidence in ischemic stroke that cell death pathways in males and females are fundamentally different, with oxidative stress pathways being dominant in males, and caspase dependent cell death playing a larger role in females (Yuan et al., 2009; Liu et al., 2011). Our data suggest the transient activation of Sirt2 in females is not sufficient to produce activation of TRPM2 and cause cell death in female neurons, likely accounting for sex differences observed in AGK2 neuroprotection. Differential activation of an oxidative stress activated enzyme that is upstream of TRPM2 is similar to results obtained with PARP-1 activation in males and females following stroke. Sex specific activation of cell death pathways observed following stroke (caspase and PARP-1) remains to be demonstrated in global cerebral ischemia, however our current and previous results with TRPM2 inhibition and knockdown support a divergence in signaling mechanisms. Further studies are needed to elucidate the mechanisms of sex differences in TRPM2 activation following cerebral ischemia.
Neuroprotection by endogenous female sex steroids has been demonstrated in many animal models of cerebral ischemia (Lang and McCullough, 2008; Vagnerova et al., 2008; Herson et al., 2009; Liu et al., 2010). The mechanism of neuroprotection by female sex steroids is primarily through inhibition of caspase dependent cell death, which is the dominant form of cell death in females (Liu et al., 2009; Liu et al., 2011). Our results obtained with TRPM2 KO demonstrating neuroprotection in male but not female mice are consistent with our previously published observations that activation of TRPM2-mediated cell death following cerebral ischemia is male specific (Jia et al., 2011; Verma et al., 2012; Nakayama et al., 2013; Shimizu et al., 2013; Quillinan et al., 2014). These results demonstrate for the first time male specificity of TRPM2 using genetic knockouts in global ischemia, and are consistent male specific neuroprotection with administration of AGK2 following CA/CPR. Our results with no added benefit of AGK2 in TRPM2 knockout also supports our hypothesis that Sirt2 acts as an upstream mediator of TRPM2 activation. It is possible that the lack of AGK2 benefit observed in TRPM2 KO males is the result of a floor effect and that no further reduction in neuronal injury is possible. It is also possible that AGK2 neuroprotection occurs independent of TRPM2 channel activation. In vitro studies have identified acetylated tubulin as a substrate for Sirt2 enzymatic activity (Outeiro et al., 2007; Harting and Knöll, 2010). There is also in vitro data to suggest that Sirt2 regulates cholesterol biosynthesis and that inhibition of Sirt2 reduces expression of enzymes involved in cholesterol biosynthesis (Luthi-Carter et al., 2010). However, studies performed in Sirt2 knockout mice shows a lack of effect of Sirt2 ablation on tubulin acetylation or brain cholesterol biosynthesis in WT mice or in a model of Huntington’s disease (Bobrowska et al., 2012). Therefore, the male specificity of AGK neuroprotection combined with TRPM KO data support our conclusion that TRPM2 acts downstream of Sirt2. Sirt2 and TRPM2 are both expressed in a variety of cell types, therefore it remains to be determined whether the neuroprotective effects of Sirt2 inhibition with AGK2 are through direct inhibition within neurons or in other cell types that contribute to neuronal injury such as microglia or astrocytes.
In summary, Sirt2 inhibition protects against ischemic injury in males, likely through reduced TRPM2 activation. These results add to the growing body of literature implicating male-specific activation of oxidative stress mechanisms that engage TRPM2 ion channel to produce neuronal death in response to ischemic injury.
References
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Allen D, Nakayama S, Kuroiwa M, Nakano T, Palmateer J, Kosaka Y, Ballesteros C, Watanabe M, Bond CT, Luján R, Maylie J, Adelman JP, Herson PS. SK2 channels are neuroprotective for ischemia-induced neuronal cell death. J Cereb Blood Flow Metab. 2011;31:2302–2312. doi: 10.1038/jcbfm.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belrose JC, Xie Y, Gierszewski LJ, MacDonald JF, Jackson MF. Loss of glutathi-one homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol Brain. 2012;5:11. doi: 10.1186/1756-6606-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington’s disease phenotypes in vivo. PLoS One. 2012;7:e34805. doi: 10.1371/journal.pone.0034805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H, Silverman RB, Ayata C, Maxwell MM, Steegborn C, Schwarzschild MA, Outeiro TF, Kazantsev AG. The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS One. 2015;10:e0116919. doi: 10.1371/journal.pone.0116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, Ferrante RJ, Hersch S, Kazantsev AG. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Rep. 2012;2:1492–1497. doi: 10.1016/j.celrep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, Traystman RJ. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J Neurosci Methods. 2014;222:34–41. doi: 10.1016/j.jneumeth.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G. Sirtuins as possible targets in neurodegenerative diseases. Curr Drug Targets. 2013;14:644–647. doi: 10.2174/1389450111314060004. [DOI] [PubMed] [Google Scholar]
- Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Gruart A, Delgado-Garcia JM. Activity-dependent changes of the hippocampal CA3–CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav. 2007;6(Suppl 1):24–31. doi: 10.1111/j.1601-183X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL, Scharenberg AM, Denu JM. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem 281. 2006:14,057–14,065. doi: 10.1074/jbc.M513741200. [DOI] [PubMed] [Google Scholar]
- Hall RLRED. Gender Differences in Acute CNS Trauma and Stroke: Neuroprotective Effects of Estrogen and Progesterone. 2012:1–32. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Harting K, Knöll B. SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol. 2010;89:262–269. doi: 10.1016/j.ejcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Palmateer J, Hurn PD. Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res. 2013;4:413–419. doi: 10.1007/s12975-012-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, Herson PS. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab. 2011;31:2160–2168. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries A, Martin L, Hurn P, Traystman R. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Koton S, Telman G, Kimiagar I, Tanne D. Gender differences in characteristics, management and outcome at discharge and three months after stroke in a national acute stroke registry. Int J Cardiol. 2013;168:4081–4084. doi: 10.1016/j.ijcard.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Krey L, Luhder F, Kusch K, Czech-Zechmeister B, Konnecke B, Outeiro TF, Trendelenburg G. Knockout of silent information regulator 2 (SIRT2) preserves neurological function after experimental stroke in mice. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35:127–143. [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke. 2011;42:1090–1096. doi: 10.1161/STROKEAHA.110.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J Mol Neurosci. 2013;51:92–98. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Kofler J, Meyers JL, Bergdall V, Perle KM, Traystman RJ, DeVries AC. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. J Cereb Blood Flow Metab. 2004;24:372–382. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfila J, Shimizu K, Garske A, Deng G, Maylie J, Traystman R, Quillinan N, Adelman J, Herson PS. Increasing SK Channel Activity Reverses Ischemia-Induced Impairment of LTP. European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Grewal H, Klawitter J, Herson PS. Sex Steroids Do Not Modulate TRPM2-Mediated Injury in Females following Middle Cerebral Artery Occlusion. 2014 doi: 10.1523/ENEURO.0022-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillinan N, Grewal H, Deng G, Shimizu K, Yonchek JC, Strnad F, Traystman RJ, Herson PS. Region-specific role for GluN2B-containing NMDA receptors in injury to Purkinje cells and CA1 neurons following global cerebral ischemia. Neuroscience. 2015;284:555–565. doi: 10.1016/j.neuroscience.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch Neurol. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ, Herson PS. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab. 2013;33:1549–1555. doi: 10.1038/jcbfm.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A. 2000;97(14):178–14. 182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, Herson PS. Poly (ADP-ribose) polymerase-1 initiated neuronal cell death pathway—do androgens matter? Neuroscience. 2010;166:476–481. doi: 10.1016/j.neuroscience.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Quillinan N, Yang Y, Nakayama S, Cheng J, Kelley MH, Herson PS. TRPM2 channel activation following in vitro ischemia contributes to male hippocampal cell death. Neurosci Lett. 2012;530:41–46. doi: 10.1016/j.neulet.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Zhang W, Chen N. Sexual dimorphism in cerebral ischemia injury. Eur J Pharmacol. 2013;711:73–79. doi: 10.1016/j.ejphar.2013.04.024. [DOI] [PubMed] [Google Scholar]