Abstract
Background
Type-2 diabetes mellitus (T2DM) accelerates cognitive aging and increases risk of Alzheimer’s disease. Rodent models of T2DM show altered synaptic plasticity associated with reduced learning and memory. Humans with T2DM also show cognitive deficits, including reduced learning and memory, but the relationship of these impairments to the efficacy of neuroplastic mechanisms has never been assessed.
Objective
Our primary objective was to compare mechanisms of cortical plasticity in humans with and without T2DM. Our secondary objective was to relate plasticity measures to standard measures of cognition.
Methods
A prospective cross-sectional cohort study was conducted on 21 adults with T2DM and 15 demographically-similar non-diabetic controls. Long-term potentiation-like plasticity was assessed in primary motor cortex by comparing the amplitude of motor evoked potentials (MEPs) from single-pulse transcranial magnetic stimulation before and after intermittent theta-burst stimulation (iTBS). Plasticity measures were compared between groups and related to neuropsychological scores.
Results
In T2DM, iTBS-induced modulation of MEPs was significantly less than controls, even after controlling for potential confounds. Furthermore, in T2DM, modulation of MEPs 10-min post-iTBS was significantly correlated with Rey Auditory Verbal Learning Task (RAVLT) performance.
Conclusion
Humans with T2DM show abnormal cortico-motor plasticity that is correlated with reduced verbal learning. Since iTBS after-effects and the RAVLT are both NMDA receptor-dependent measures, their relationship in T2DM may reflect brain-wide alterations in the efficacy of NMDA receptors. These findings offer novel mechanistic insights into the brain consequences of T2DM and provide a reliable means to monitor brain health and evaluate the efficacy of clinical interventions.
Keywords: Cognitive aging, neuroplasticity, transcranial magnetic stimulation, type 2 diabetes mellitus, verbal learning
INTRODUCTION
The brain is a target organ in type-2 diabetes mellitus (T2DM) [1]. T2DM affects the central nervous system through neuronal toxicity of hyper- and hypoglycemia episodes, microvascular insults, impaired glucose, and insulin transfer and resistance [2, 3]. Presumably as a consequence of this damage, T2DM accelerates cognitive decline [4] and increases risk of dementia [5, 6]. Cognitive dysfunction in T2DM has been linked to inflammation and altered vasoreactivity [7]. Even in the absence of vascular complications, T2DM can alter synaptic plasticity in the mouse hippocampus resulting in cognitive deficits [8], and mice with T2DM are less likely to recover from stroke due to impaired neuroplastic mechanisms [9]. To our knowledge, no study has directly assessed the mechanisms of brain plasticity or their behavioral significance in humans with T2DM.
Cortical reactivity and plasticity can be measured noninvasively in the human motor cortex using transcranial magnetic stimulation (TMS; Fig. 1). Operational definitions of reactivity and plasticity can be found in the Materials and Methods; collectively they refer to the process of comparing the motor responses to individual TMS pulses at baseline with those obtained after a repetitive TMS intervention such as theta-burst stimulation (TBS) [10]. TMS-TBS measures have identified age-related changes in plasticity across the lifespan in healthy individuals [11] and revealed altered neuroplastic mechanisms in autism spectrum disorders [12], traumatic brain injury [13], and Alzheimer’s disease (AD) [14].
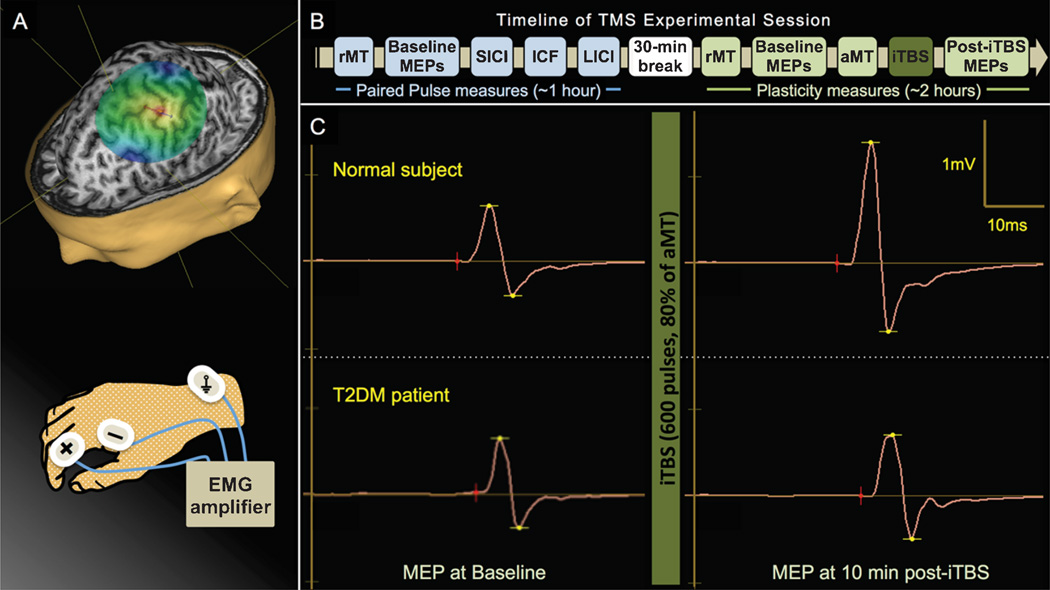
Fig. 1.
Cortical reactivity and plasticity can be measured noninvasively in the human motor cortex using TMS. Reactivity refers to the average amplitude of MEPs elicited by single-pulse TMS, while plasticity is defined as the change in reactivity induced by iTBS. A) MR-guided TMS was applied to the left primary motor cortex and resulting MEPs were recorded from the right FDI muscle by surface EMG. B). The present study assessed TMS-iTBS measures of plasticity as well as paired pulse TMS measures of cortical inhibition and facilitation. After determining resting motor threshold (rMT), 50 single (unconditioned) monophasic TMS pulses were delivered, followed by three sets of 50 pulse-pairs to assess short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and long-interval intracortical inhibition (LICI). After a break, rMT was reassessed and three sets of 30 biphasic pulses were delivered to measure baseline cortico-motor reactivity. The active motor threshold (aMT) was assessed and iTBS was applied. Cortico-motor reactivity was reassessed in six blocks of 30 pulses at 5, 10, 20, 30, 40, and 50 min post-iTBS. C) Example MEP traces from a single control subject (top) and T2DM patient (bottom) recorded at baseline (left) and 10 min after iTBS (right).
Intermittent TBS (iTBS), which assesses NMDA receptor (NMDAR)-dependent [15] long-term potentiation (LTP)-like plasticity [16], was used to directly investigate whether the mechanisms of brain plasticity are abnormal in T2DM. As the motor system is not specifically affected in T2DM, altered cortico-motor plasticity measures should reflect brain-wide declines in the efficacy of neuroplastic mechanisms. Further, if global changes in brain plasticity are driving deficits in cognitive performance, we measures obtained in the motor cortex should be associated with neuropsychological performance, especially on measures of learning and memory that are also NMDAR-dependent [17].
MATERIALS AND METHODS
Human participants
In a prospective observational cohort study, adults (50–80 y) with and without T2DM were recruited through the Joslin Diabetes Center or responded to flyers posted around Beth Israel Deaconess Medical Center. 83 adults were enrolled, including individuals with well-controlled hypertension and hypercholesterolemia, but excluding significant heart disease (heart attack or stroke). 17 were subsequently excluded for a Mini-Mental State Examination (MMSE) score <27, Geriatric Depression Scale (GDS) score >10, resting tremor, or receiving medications contraindicated for TMS [18]. Seven controls were excluded for indications of prediabetes: glycosylated hemoglobin (HbA1c) >5.6% or fasting glucose >100 mg/dL. Two T2DM patients were excluded for HbA1c >10%, indicating uncontrolled T2DM. From saliva-based genotyping, 11 individuals with an APOE-ε4 or BDNF-Met allele were excluded as these polymorphisms have been shown to alter TBS-based measures of plasticity [19, 20]. A further 10 participants were excluded or withdrew consent for various reasons, including inability to fit in the scanner, discomfort sitting, pending surgery, or failure to show up for study visits. The final cohort consisted of 21 adults with T2DM and 15 demographically-similar controls (Table 1). Most T2DM patients controlled their diabetes with Metformin and the median time since diagnosis was 10 years (range: 2–18 years).
Table 1.
Demographic and study data
| Controls (n = 15) Mean ± SE |
T2DM (n = 21) Mean ± SE |
Pairwise comparison Df† |
|t ratio| | punadjusted | Lower CL | Upper CL | padjusted* | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (y) | 63.93±2.2 | 66.90±1.8 | 34 | 1.05 | 0.299 | −2.76 | 8.70 | 0.994 |
| Education (y) | 15.60±0.6 | 15.24±0.6 | 34 | 0.44 | 0.660 | −2.02 | 1.29 | 1.000 |
| # Male (%) | 7 (46.7) | 12 (57.1) |
N = 36, df = 1, p = 0.736; Fisher’s Exact Test, padjusted = 1.000 |
|||||
| # Dextral (%) | 15 (100) | 18 (85.7) |
N = 36, df = 2, p = 0.500; Fisher’s Exact Test, padjusted = 1.000 |
|||||
| # Caucasian (%) | 14 (93.3) | 17 (81.0) |
N = 36, df = 3, p = 0.242; Fisher’s Exact Test, padjusted = 0.994 |
|||||
| Health Indices | ||||||||
| Hemoglobin A1c (%)a | 5.43±0.1 | 7.50±0.4 | 12.6 | 5.19 | <0.001 | 1.20 | 2.93 | 0.001 |
| Fasting glucose (mg/dL)b | 85.50±4.1 | 144.50±12.0 | 13.1 | 4.65 | <0.001 | 31.59 | 86.41 | 0.003 |
| Creatinine (mg/dL)c | 0.83±0.1 | 0.87±0.1 | 14 | 0.23 | 0.823 | −0.28 | 0.34 | 1.000 |
| Weight (lbs.)d | 157.10±8.8 | 187.75±6.8 | 24 | 2.77 | 0.011 | 7.83 | 53.47 | 0.033 |
| Height (in.)e | 65.62±1.3 | 66.49±0.8 | 23 | 0.62 | 0.543 | −2.04 | 3.77 | 1.000 |
| Body mass index (lbs./in.2)e | 25.48±0.9 | 29.68±1.1 | 23.0 | 2.91 | 0.008 | 1.22 | 7.18 | 0.032 |
| Cortical Thickness (mm) | ||||||||
| Left motor cortex | 2.39±0.0 | 2.25±0.0 | 34 | 2.92 | 0.006 | −0.25 | −0.04 | 0.012 |
| Left hemisphere – mean | 2.26±0.0 | 2.21±0.0 | 34 | 1.24 | 0.223 | −0.12 | 0.03 | 0.223 |
| Neuropsychological testing | ||||||||
| Mini-mental status examination (# / 30) | 29.40±0.2 | 28.95±0.2 | 34 | 1.44 | 0.158 | −1.08 | 0.18 | 0.679 |
| Wechsler test of adult reading (age-normed) | 112.93±2.6 | 112.95±2.5 | 34 | 0.01 | 0.996 | −7.50 | 7.54 | 1.000 |
| Activities of daily living inventory (# / 78) | 74.73±0.8 | 76.48±0.4 | 34 | 1.92 | 0.063 | −0.10 | 3.58 | 0.377 |
| Geriatric depression scale (# / 15) | 0.60±0.3 | 1.29±0.3 | 34 | 1.53 | 0.136 | −0.23 | 1.60 | 0.679 |
| Digit Symbol Substitution Test (# / 90) | 55.20±2.7 | 44.67±2.1 | 34 | 3.11 | 0.004 | −17.41 | −3.65 | 0.030 |
| Trail Making Test (B-A, time in s) | 45.40±15.7 | 53.27±7.1 | 33 | 0.50 | 0.622 | −24.33 | 40.06 | 1.000 |
| Digit Span Backwards Test (# correct trials) | 8.47±0.6 | 6.67±0.5 | 34 | 2.29 | 0.029 | −3.40 | −0.20 | 0.200 |
| Rey auditory verbal learning test (% correct) | ||||||||
| Learning | 80.53±1.9 | 69.71±2.8 | 32.8 | 3.20 | 0.003 | −17.69 | −3.94 | 0.027 |
| Delayed recall | 74.00±5.1 | 64.76±5.7 | 34 | 1.15 | 0.259 | −25.60 | 7.12 | 0.778 |
CL, confidence level; T2DM, type-2 diabetes mellitus; iTBS, intermittent theta-burst stimulation; TMS, transcranial magnetic stimulation.
Integers reflect pooled variance, non-integers reflect unequal variance.
Significance values for each set of tests were adjusted using Holm-Bonferroni method.
Obtained from 12 T2DM and 3 controls.
Obtained from 12 T2DM and 4 controls.
Obtained from 10 T2DM and 6 controls.
Obtained from 16 T2DM and 10 controls.
Obtained from 15 T2DM and 10 controls.
The local Institutional Review Board approved the study. All participants provided written informed consent prior to enrollment according to the Declaration of Helsinki and received monetary compensation upon completion.
Neuropsychological testing
A 30-item MMSE, 50-item Wechsler Test of Adult Reading (W-TAR), 15-item GDS, and 78-point activities of daily living (ADL) inventory were administered to characterize general neurocognitive status in the two groups. Additional tests were chosen to assess cognitive domains previously shown [21] to be impaired in T2DM: psychomotor processing speed was assessed with the Digit Symbol Substitution Test (DSST; number of correct substitutions in 90 sec); executive function was measured using the Trail Making Test (difference in time to complete Parts A & B); working memory was assessed with the Digit Span Backwards task (number of correctly-completed trials); and verbal learning and memory was assessed with a 10-item Rey Auditory Verbal Learning Test (RAVLT; percent of correctly recalled words across the five learning trials and after a 30-min delay) [22]. The RAVLT in particular was chosen as it is an NMDAR-dependent [17] measure of cognitive plasticity that is sensitive to prodromal dementia [23].
Magnetic resonance imaging
A T1-weighted anatomical magnetic resonance imaging scan was obtained in all participants on a 3T scanner (GE Healthcare, Ltd., UK) using a 3D spoiled gradient echo sequence: 162 axial-oriented slices for whole-brain coverage; 240-mm isotropic field-of-view; 0.937-mm × 0.937-mm × 1-mm native resolution; flip angle = 15°; TE/TR ≥2.9/6.9 ms; duration ≥432 s. Cortical reconstruction and automatic segmentation were performed with Freesurfer (version 6.0, http://surfer.nmr.mgh.harvard.edu/). Cortical thickness, calculated as the shortest distance between the pial and white matter surfaces [24], was measured for the primary motor cortex (precentral gyrus and central sulcus) in the left hemisphere using a subject-independent probabilistic atlas [25].
Electromyography
To measure the amplitude of TMS-induced MEPs, Ag-AgCl surface electrode-pairs (Ambu A/S, Denmark) were placed on the belly and tendon of the right first dorsal interosseous (FDI) and a ground on the right ulnar styloid process (Fig. 1A).
Transcranial magnetic stimulation
All parameters used in the study conformed to current recommended guidelines for the safe application of TMS endorsed by the International Federation of Clinical Neurophysiology (IFCN) [18]. Following IFCN guidelines [26], resting motor threshold (rMT) and active motor threshold (aMT) were measured individually and used to set the intensity of subsequent stimulation. MEP trials were randomly jittered (5000–6000 ms) to avoid train effects. A Navigated Brain Stimulation system (Nexstim Plc, Finland) was used to identify the hand region of the primary motor cortex and ensure consistent targeting throughout the experimental session (Fig. 1A). We operationally define reactivity as the average amplitude of motor evoked potentials (MEPs) elicited by suprathresh-old single-pulse TMS, and plasticity as the change in reactivity induced by subthreshold TBS [10].
Paired-pulse TMS
Neuronavigated paired-pulse TMS was applied using a handheld monophasic figure-of-eight focal coil (Nexstim Plc, Finland). Three protocols were utilized: short-interval intracortical inhibition (SICI; 80%-rMT conditioning pulse, 120%-rMT test pulse, 3-ms interval), intracortical facilitation (ICF; 80%-rMT conditioning pulse, 120%-rMT test pulse, 12-ms interval); and long-interval intracortical inhibition (LICI; 80%-rMT conditioning pulse, 120%-rMT test pulse, 100-ms interval) [27, 28]. A preceding block of single TMS pulses at 120% rMT provided a measure of unconditioned cortico-motor reactivity. Each block consisted of 50 trials and individual MEP amplitudes >2.5 SD from the mean were excluded. Measures of SICI, LICI, and ICF were calculated as the percent change of the conditioned MEPs from the unconditioned block.
Theta-burst TMS
Neuronavigated iTBS was applied to participants using a handheld passive-cooling fluid-filled figure-of-eight coil attached to a MagPro X100 stimulator (MagVenture A/S, Denmark). Intensity was 80% aMT. The pattern was a two-second train of biphasic bursts (three pulses at 50 Hz) repeated every 200 ms. Trains were repeated 20 times with an eight-second inter-train interval (600 pulses, 192 seconds). This protocol has been shown to potentiate cortico-motor reactivity for up to 40 minutes in healthy individuals [10, 29].
Figure 1B depicts the timeline of the TMS experimental session. Prior to iTBS, participants received three blocks of 30 pulses at 120% rMT The peak-to-peak amplitudes of all recorded MEPs (Fig. 1C) were measured and averaged for each individual as a measure of baseline cortico-motor reactivity. Cortico-motor reactivity was reassessed in blocks of 30 TMS pulses at 5, 10, 20, 30, 40, and 50 min post-iTBS. For each block, individual MEPs >2.5 SD from the mean were excluded. Plasticity was calculated as the percent change of each post-iTBS block from baseline.
Statistical analysis
Statistical analyses were performed in JMP Pro (version 12.0, http://www.jmp.com) and Stata (version 14.1, http://www.stata.com) using a normal distribution, Levene’s test for homoscedasticity, and a two-tailed 95% confidence interval (α = 0.05). Individual significance values for each set of tests were adjusted for multiple comparisons using Holm-Bonferroni correction.
Pairwise comparisons were made against the null hypotheses that demographic, health, cortical thickness, neuropsychological, and neurophysiological measures were equivalent between T2DM and controls. The proportions of gender, handedness, and racial-ethnic composition were compared using Fisher’s Exact tests, while all continuous variables were compared with Student’s t-tests.
To test the null hypothesis that the after-effects of iTBS are equivalent between groups, post-iTBS changes in MEP amplitudes were entered into a 2 (diagnosis) × 6 (time) full-factorial linear mixed-effects model. However, as the peak modulation of MEP amplitudes typically occurs immediately after iTBS [29], planned pairwise comparisons between T2DM and controls for each time-point were conducted using Student’s t tests.
To evaluate the behavioral significance of altered plasticity, correlation analyses were performed between MEP amplitudes 10-min post-iTBS (POST10; %Δ from baseline) and scores on the DSST, TMT, Digit Span, and RAVLT. POST10 was selected post hoc (see Results) as the time-point when plasticity measures were most altered in T2DM relative to controls. Correlations were thus performed separately for T2DM and controls to avoid confounding group differences in neuropsycholgical and TMS measures.
RESULTS
Table 1 details group means±standard error of continuous variables, numbers and proportions of categorical variables, and pairwise comparisons, including adjusted p-values. Unless otherwise indicated, p-values reported in the text are unadjusted.
Demographics, health, cortical thickness, and neuropsychological testing
Fisher’s Exact Tests yielded with no group differences in the proportion of gender, handedness, or ethnic composition, while student’s t tests indicated T2DM and control participants were similarly aged and educated (p’s > 0.2). These results indicate that the two groups had equivalent demographic composition.
As expected, the T2DM group had significantly worse health indices, including greater HbA1c and fasting glucose levels, weight, and body-mass index (p’s < 0.02), though the groups had similar creatinine levels (p = 0.823) and were of similar height (p = 0.543). All differences remained significant after Holm-Bonferroni correction. These results indicate that measures of blood sugar and obesity were higher in the T2DM group, while height and creatinine, a marker of kidney function, were equivalent.
Analysis of cortical thickness found that the mean thickness across the left hemisphere did not differ significantly (p = 0.223), however the left motor cortex (precentral gyrus and central sulcus) was thinner for T2DM than controls (p = 0.012).
In the neuropsychological measures, there were no significant group differences in the MMSE, W-TAR, or GDS (p’s > 0.1), though the T2DM group had slightly higher ADLs (p = 0.063). These results indicate T2DM did not differ from control participants in terms of overall neurocognitive status, premorbid IQ, functional independence, or levels of depression, respectively. Despite these similarities and the lack of subjective cognitive complaints, the T2DM group exhibited reduced psychomotor processing speed, working memory, and verbal learning. Specifically, T2DM made fewer correct substitutions on the DSST (p = 0.004), completed fewer trials on the Digit Span Backwards task (p = 0.029), and recalled fewer words on the RAVLT learning trials (p = 0.003). After applying Holm-Bonferroni correction, the DSST and RAVLT remained significant.
Measures of cortico-motor reactivity and plasticity
All participants tolerated TMS and iTBS with no complications or unexpected side effects. Student’s t tests yielded no significant differences in baseline neurophysiological measures, including motor thresholds and baseline MEP amplitudes and latencies (p’s >0.1). Similarly, there were no group differences in the paired pulse TMS measures (p’s > 0.1). These results indicate T2DM did not differ from controls in cortico-motor reactivity, the cortico-spinal response to TMS, or the efficacy of inhibitory and excitatory intracortical circuits (Supplementary Table 1 and Supplementary Figures 1–3).
Across all post-iTBS time-points, the mean±standard error percent change in MEP amplitude was 36.21±7.2 for controls and 7.22±6.0 for T2DM. This effect in controls is consistent with a recent meta-analysis of TBS in healthy subjects [29].
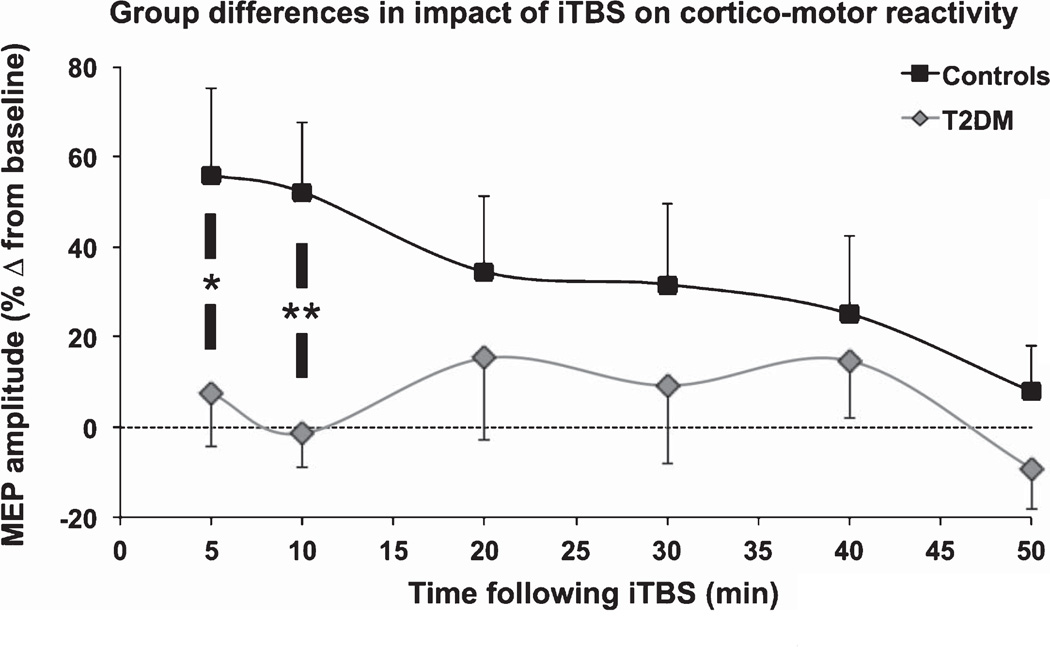
The linear mixed-effect model indicated the change in MEP amplitudes did not vary significantly (at the 0.05 level) by diagnosis, F(1,34.1) = 2.59, p = 0.117, time, F(5,167.2) = 1.97, p = 0.086, or their interaction, F(5,167.2) = 1.45, p = 0.209. However, planned t tests showed T2DM subjects had significantly less potentiation of MEP amplitudes 5-min post-iTBS (POST5; p = 0.042) and POST10 (p < 0.007) (Fig. 2), with the latter remaining significant after Holm-Bonferroni correction. From 20–50 min post-iTBS, the change in MEP amplitudes was statistically equivalent between groups (p’s > 0.3). These results indicate it is the initial impact of iTBS on cortico-motor reactivity that is selectively altered in T2DM relative to controls.
Fig. 2.
Comparison of TMS-plasticity measures by group. Mean and standard error of the percent change in MEP amplitude are shown for each post-iTBS time-point. Pairwise comparisons between controls and T2DM for each time-point were made with Student’s t tests (*p < 0.05, **p < 0.01). 5–10 min after iTBS, the change in MEP amplitudes was significantly reduced in individuals with T2DM relative to controls.
Follow-up linear regression analyses demonstrated diagnosis remained a significant predictor of POST10 plasticity (p’s < 0.02) after controlling for age, gender, BMI, HbA1c, fasting glucose, or motor cortex thickness, resting/active motor thresholds, or baseline MEP amplitude. Table 2 lists the significance of each covariate as a predictor of POST10 plasticity, as well as changes in the regression coefficient of the model, and the significance and beta coefficient of diagnosis (β1) after adding each covariate. While no covariate contributed significantly to the model (p’s>0.1), adding weight or motor cortex thickness reduced β1 by more than 10%, suggesting group differences in these factors may account for some of the observed association between T2DM and POST10 plasticity. By comparison, β1 increased by 25% after adding HbA1c, suggesting POST10 plasticity may be even more altered in T2DM once HbA1c is taken into consideration.
Table 2.
Change in regression coefficients of Diagnosis on POST10 after adding covariates
| % Δβdiagnosis | ΔPdiagnosis | Pcovariate | |||
|---|---|---|---|---|---|
| Diagnosis plus Weight | −0.17 | 0.02 | 0.03 | 0.313 | |
| Diagnosis plus M1 thickness | −0.14 | 0.02 | 0.02 | 0.378 | |
| Diagnosis plus Race | −0.09 | 0.01 | 0.01 | 0.219 | |
| Diagnosis plus BMI | −0.07 | 0.01 | 0.01 | 0.697 | |
| Diagnosis plus Age | −0.07 | 0.00 | 0.03 | 0.233 | |
| Diagnosis plus Fasting glucose | −0.06 | 0.00 | 0.00 | 0.680 | |
| Diagnosis plus Height | −0.03 | 0.00 | 0.02 | 0.409 | |
| Diagnosis plus Baseline MEP | −0.03 | 0.00 | 0.06 | 0.123 | |
| Diagnosis plus Gender | −0.02 | 0.00 | 0.01 | 0.537 | |
| Diagnosis plus RMT | −0.02 | 0.00 | 0.05 | 0.148 | |
| Diagnosis plus Creatinine | −0.01 | 0.00 | 0.01 | 0.569 | |
| Diagnosis plus AMT | 0.00 | 0.00 | 0.06 | 0.126 | |
| Diagnosis plus Handedness | 0.03 | 0.00 | 0.07 | 0.235 | |
| Diagnosis plus HbA1c | 0.25 | 0.00 | 0.06 | 0.189 |
POST10, 10-min post-iTBS; AMT, active motor threshold; RMT, resting motor threshold; MEP, motor evoked potential; BMI, body mass index; M1, primary motor cortex.
Relationship between cortico-motor plasticity and cognitive function
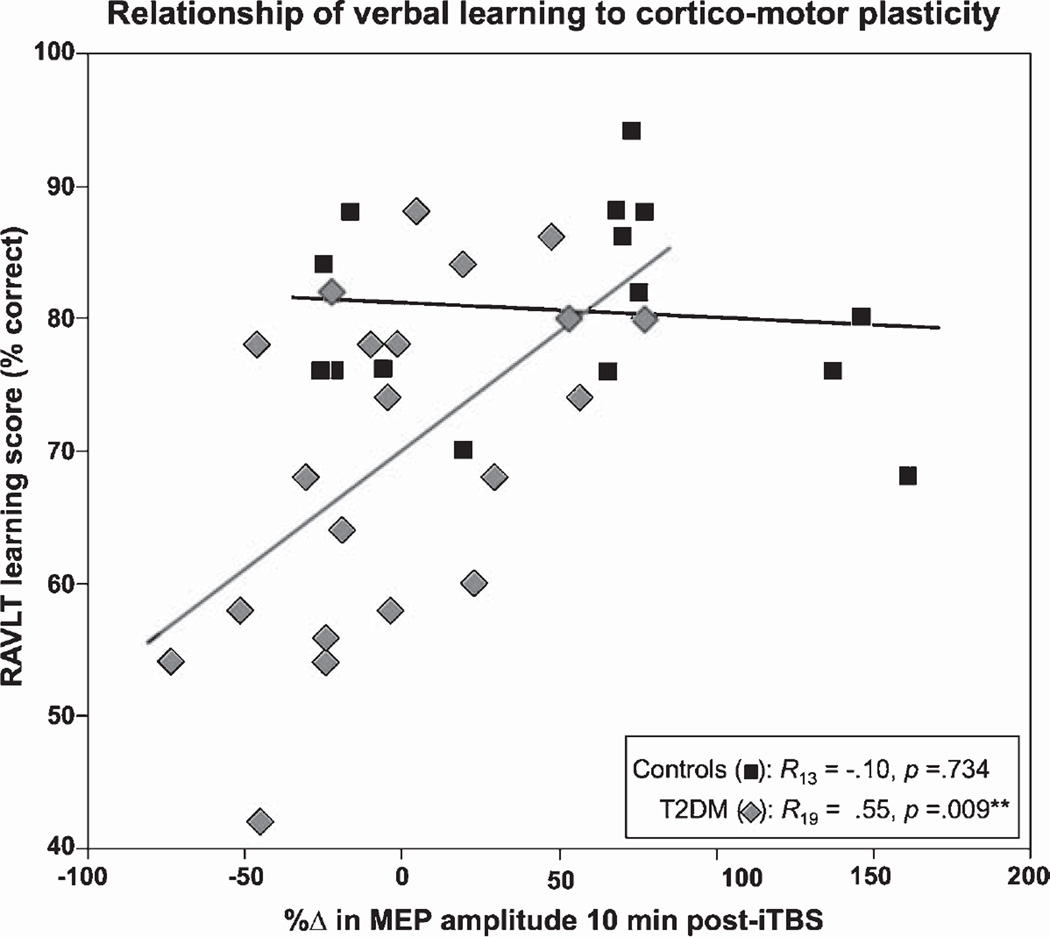
For the control group, there were no significant correlations between POST10 and any of the cognitive measures (all p’s > 0.6). In the T2DM group by comparison, there were significant positive associations between POST10 plasticity and performance on the digit span backwards task (R19 = 0.49, p = 0.025), RAVLT-learning (R19 = 0.55, p = 0.009; Fig. 3), and RAVLT-delayed recall (R19 = 0.44, p = 0.047). After Holm-Bonferroni adjustment, only the relationship between POST10 and RAVLT-learning remained significant. These results indicate that individuals with T2DM whose MEPs increased at POST10 tended to perform better on the cognitive measures than those whose MEPs remained unchanged or decreased.
Fig. 3.
Relationship between cortico-motor plasticity and verbal learning. Pearson’s correlation coefficients were calculated separately for T2DM and control groups to assess the relationship between the change in MEP amplitude 10-min post-iTBS (x-axis) and performance (y-axis) on the Rey Auditory Verbal Learning Test (RAVLT) learning trials (% correct). T2DM participants whose MEPs increased following iTBS demonstrated better verbal learning performance than those whose MEPs remained unchanged or decreased.
DISCUSSION
The present study compared older adults with and without T2DM on TMS-measures of brain plasticity assessed in the motor cortex. Our major novel finding is that individuals with T2DM, unlike their non-T2DM counterparts, did not show significant potentiation of MEP amplitudes 5–10 minutes post-iTBS. This period corresponds with the peak effect of TBS in normal individuals [10, 29] and demonstrates the highest test-retest reliability [30]. Using similar TMS measures, altered mechanisms of brain plasticity have been demonstrated in autism spectrum disorder [12], traumatic brain injury [13], and AD [14, 31, 32], and used to track changes over the lifespan in healthy adults [11]. In the future, similar plasticity measures might be obtained from higher-order association areas more directly linked to cognition using real-time integration of TMS with electroencephalography. Nonetheless the present findings suggest that TMS-based assessments of motor cortex plasticity offer a clinically relevant marker of central nervous system changes in T2DM.
It is unlikely that differences in TMS measures of brain plasticity result from a direct impact of T2DM on the motor cortex. While the motor cortex was thinner in T2DM participants, diagnosis remained a significant predictor of the impact of iTBS even after accounting for these macrostructural differences. Magnetic resonance spectroscopy has shown evidence in T2DM of abnormal metabolism in non-motor regions [33]; future studies could investigate if the motor cortex is similarly altered. T2DM has been associated with altered integrity of the cortico-spinal pathway [34]. However, a structured neurological exam or medical history review found no evidence of neuropathy in any of our T2DM participants. Moreover, motor thresholds, baseline MEP amplitudes and latencies were all equivalent, indicating that T2DM does not alter cortico-motor reactivity or the ability of TMS-induced activity to propagate along the cortico-spinal pathway and elicit a muscle contraction. Thus, alterations in the response to iTBS likely reflect T2DM-related changes to the efficacy of neuroplastic mechanisms within the cortex. Indeed, using invasive techniques to monitor brain activity, Di Lazzaro and colleagues [16] demonstrated that iTBS assesses intracortical mechanisms of plasticity. In rodents’ neocortex, iTBS has been shown to increase pyramidal cell output by reducing parvalbumin expression in fast-spiking inhibitory interneurons [35]. Importantly, the present study found no differences between T2DM and controls in any of the paired-pulse TMS measures of intracortical inhibition and facilitation. T2DM in humans does not therefore appear to alter intracortical circuits within the motor cortex, but the ability of the synapses therein to be potentiated.
Our second major findings was that reduced measures of brain plasticity in T2DM participants were associated with lower verbal learning scores on the RAVLT and fewer correct trials of the Digit Span Backwards task. These results bring human evidence of T2DM-associated cognitive impairment in line with genetic mouse models of impaired insulin signaling and insulin resistance. Mice engineered without the glucagon-like peptide 1 (GLP-1) receptor had reduced LTP in area CA1 of the hippocampus, showed impaired discrimination of learned and novel objects and performed poorly on a water maze task [36]. Similarly, reducing insulin receptor expression globally by means of β-subunit haploinsufficiency [37] or in the hippocampus using a lentiviral vector [38] severely curtailed hippocampal LTP and impaired spatial memory. What makes the present results notable is that plasticity was assessed in the motor cortex, while learning and memory are hippocampal-dependent and working memory is most closely associated with lateral prefrontal and posterior parietal cortices. While it is possible that all three systems are independent targets of T2DM-related damage, the more parsimonious explanation is that T2DM affects a common substrate. In rodents, N-methyl-D-aspartate receptors (NMDARs) are known to be crucial for induction of theta burst-driven LTP in the hippocampus [39] and iTBS after-effects in the neocortex [40] as well as for behavioral measures of working memory [41] and learning and memory [42]. Similarly, in humans, iTBS after-effects, working memory and RAVLT performance have all been shown to be NMDAR-dependent [15, 17, 43–45]. Thus, the relationship of reduced verbal learning and working memory to altered LTP-like plasticity in the present study may reflect a T2DM-associated brain-wide reduction in the density or efficacy of NMDARs. Given T2DM is associated with upregulation of the GLUT1 glucose transporter [46], and glucose provides the original source of glutamate in the brain [47, 48], chronic hyperglycemia could lead to excessive glutamate and increased risk of excitotoxicity. Any reduction in post-synaptic NMDARs to moderate this risk would consequently reduce the efficiency of LTP and alter any NMDAR-dependent measures.
Since the RAVLT in particular is sensitive to age-related cognitive decline [23] and T2DM is an important risk factor for dementia [5, 49], the present findings suggest impairments in the mechanisms underlying neuroplasticity may predicate learning and memory deficits. An alternative interpretation is that preserved brain plasticity might provide protection against cognitive decline. The relationship between plasticity and cognitive resilience deserves further investigation. Nonetheless, our results would lead to the prediction that T2DM individuals with normal TMS plasticity measures would be less likely to develop dementia. In future studies, these assessments of brain plasticity could be used to chart the progress of T2DM-related brain changes and evaluate the therapeutic efficacy of interventions.
The present findings provide neurobiological support for the epidemiological link between T2DM and AD [5, 50]. Several TMS studies have shown similar patterns of reduced LTP-like plasticity in AD patients [14, 31, 32]. In particular, Koch and colleagues have demonstrated that TMS measures of plasticity are associated with the severity of Tau neuropathology in AD [51] but independent from the age that cognitive symptoms first appear [32]. Furthermore, a 4-week treatment with a dopamine agonist was shown to rescue LTP-like plasticity in early AD [31], a finding that both provides mechanistic insight into altered cortical plasticity and offers a potential therapeutic intervention to recover it. Similarly, intranasal insulin therapy has been shown to improve cognition in healthy individuals [52], as well as patients with mild cognitive impairment/early AD [53–55] or T2DM [56]. Future studies could examine how plasticity relates to Tau levels or dopaminergic function in T2DM or investigate whether cognitive improvement following intranasal insulin administration is mediated through enhancement of LTP-like plasticity.
Several factors may limit the generalizability of our findings. While the sample size is consistent with recently-published work on diabetes and cognitive aging [7, 56, 57], it is relatively small when compared to large-scale epidemiological studies [58]. Further, we enrolled a relatively homogenous population of non-demented adults. Thus it is not possible to know if our findings extend to patients with significant comorbidities or evident dementia. Lastly, it was not possible to obtain recent HbA1c or fasting glucose levels in all participants.
Supplementary Material
Acknowledgments
This study was primarily funded by the National Institutes of Health (NIH R21 NS082870). Dr. Brem was further supported by the Young Academics Support and the Stiefel-Zangger Foundation of the University of Zurich, Switzerland, and the Swiss National Foundation (PBZHP1 147196). Dr. Cypess was further supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Pascual-Leone was further supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R01 HD069776, R01 NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
The authors thank E. Seligson and N. Atkinson (Beth Israel Deaconess Medical Center) for their assistance in data collection and A. Connor (Beth Israel Deaconess Medical Center) for assistance with evaluation of participant health and medical history. Dr. Fried had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
Footnotes
Part of the data was presented at the Annual Meeting of the Society for Neuroscience, Washington, D.C., 15–19 November 2014.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0505r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-160505.
REFERENCES
- 1.Strachan MWJ. R D Lawrence Lecture 2010. The brain as a target organ in Type 2 diabetes: Exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28:141–147. doi: 10.1111/j.1464-5491.2010.03199.x. [DOI] [PubMed] [Google Scholar]
- 2.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman APA, Kappelle LJ, Mali WPTM Study SMART Group. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- 4.Ravona-Springer R, Luo X, Schmeidler J, Wysocki M, Lesser G, Rapp M, Dahlman K, Grossman H, Haroutunian V, Schnaider Beeri M. Diabetes is associated with increased rate of cognitive decline in questionably demented elderly. Dement Geriatr Cogn Disord. 2010;29:68–74. doi: 10.1159/000265552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, Boeve BF, Tangalos EG, Ivnik RJ, Mielke MM, Petersen RC. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10:18–26. doi: 10.1016/j.jalz.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GEHM, Biessels GJ. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015;14:329–340. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 7.Chung C-C, Pimentel D, Jor’dan AJ, Hao Y, Milberg W, Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–458. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, Wong C, Brown CE. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci. 2012;32:5132–5143. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, Bashir S, Vernet M, Peña-Gómez C, Pascual-Leone A. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberman L, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A. Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. Eur J Neurosci. 2012;36:2782–2788. doi: 10.1111/j.1460-9568.2012.08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremblay S, Vernet M, Bashir S, Pascual-Leone A, Théoret H. Theta burst stimulation to characterize changes in brain plasticity following mild traumatic brain injury: A proof-of-principle study. Restor Neurol Neurosci. 2015;33:611–620. doi: 10.3233/RNN-140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch G, Di Lorenzo F, Bonní S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis. 2012;31:593–599. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peña-Gomez C, Solé-Padullés C, Clemente IC, Junqué C, Bargalló N, Bosch B, Molinuevo JL, Valls-Solé J, Pascual-Leone A, Bartrés-Faz D. APOE status modulates the changes in network connectivity induced by brain stimulation in non-demented elders. PloS One. 2012;7:e51833. doi: 10.1371/journal.pone.0051833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang WH, Bang OY, Shin Y-I, Lee A, Pascual-Leone A, Kim Y-H. BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain Stimulat. 2014;7:553–558. doi: 10.1016/j.brs.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40:785–787. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Calero MD, Navarro E. Relationship between plasticity, mild cognitive impairment and cognitive decline. Arch Clin Neuropsychol. 2004;19:653–660. doi: 10.1016/j.acn.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Salat D, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale A, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 25.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 28.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wischnewski M, Schutter DJLG. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulat. 2015;8:685–692. doi: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Vernet M, Bashir S, Yoo W-K, Oberman L, Mizrahi I, Ifert-Miller F, Beck CJ, Pascual-Leone A. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–326. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch G, Di Lorenzo F, Bonní S, Giacobbe V, Bozzali M, Caltagirone C, Martorana A. Dopaminergic modulation of cortical plasticity in Alzheimer’s disease patients. Neuropsychopharmacol. 2014;39:2654–2661. doi: 10.1038/npp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Lorenzo F, Ponzo V, Bonní S, Motta C, Negrão Serra PC, Bozzali M, Caltagirone C, Martorana A, Koch G. LTP-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol. 2016;80:202–210. doi: 10.1002/ana.24695. [DOI] [PubMed] [Google Scholar]
- 33.Santhakumari R, Reddy IY, Archana R. Effect of type 2 diabetes mellitus on brain metabolites by using proton magnetic resonance spectroscopy-a systematic review. Int J Pharma Bio Sci. 2014;5:1118–1123. [PMC free article] [PubMed] [Google Scholar]
- 34.Kucera P, Goldenberg Z, Varsik P, Buranova D, Traubner P. Spinal cord lesions in diabetes mellitus. Somatosensory and motor evoked potentials and spinal conduction time in diabetes mellitus. Neuro Endocrinol Lett. 2005;26:143–147. [PubMed] [Google Scholar]
- 35.Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–1203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205:265–271. doi: 10.1016/j.bbr.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 37.Nistico R, Cavallucci V, Piccinin S, Macri S, Pignatelli M, Mehdawy B, Blandini F, Laviola G, Lauro D, Mercuri NB, D’Amelio M. Insulin receptor β-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. Neuromolecular Med. 2012;14:262–269. doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- 38.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–3936. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson J, Lynch G. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal θ-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- 40.Labedi A, Benali A, Mix A, Neubacher U, Funke K. Modulation of inhibitory activity markers by intermittent theta-burst stimulation in rat cortex is NMDA-receptor dependent. Brain Stimulat. 2014;7:394–400. doi: 10.1016/j.brs.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 41.MacQueen DA, Dalrymple SR, Drobes DJ, Diamond DM. Influence of pharmacological manipulations of NMDA and cholinergic receptors on working versus reference memory in a dual component odor span task. Learn Mem. 2016;23:270–277. doi: 10.1101/lm.041251.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter-Levin G, Canevari L, Bliss TV. Long-term potentiation and glutamate release in the dentate gyrus: Links to spatial learning. Behav Brain Res. 1995;66:37–40. doi: 10.1016/0166-4328(94)00121-u. [DOI] [PubMed] [Google Scholar]
- 43.Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, Lahti AC. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology (Berl.) 2005;183:265–274. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- 44.Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, Gueorguieva R, He G, Leung H-C, Ramani R, Anticevic A, Suckow RF, Morgan PT, Krystal JH. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin R, Dor-Abarbanel AE, Edelman S, Durrant AR, Hashimoto K, Javitt DC, Heresco-Levy U. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: Initial findings. J Psychiatr Res. 2015;61:188–195. doi: 10.1016/j.jpsychires.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Kumagai AK, Vinores SA, Pardridge WM. Pathological upregulation of inner blood-retinal barrier Glut1 glucose transporter expression in diabetes mellitus. Brain Res. 1996;706:313–317. doi: 10.1016/0006-8993(95)01335-0. [DOI] [PubMed] [Google Scholar]
- 47.Bradford HF, Thomas AJ. Metabolism of glucose and glutamate by synaptosomes from mammalian cerebral cortex. J Neurochem. 1969;16:1495–1504. doi: 10.1111/j.1471-4159.1969.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 48.He L, Xu Z, Yao K, Wu G, Yin Y, Nyachoti CM, Kim SW. The physiological basis and nutritional function of alpha-ketoglutarate. Curr Protein Pept Sci. 2015;16:576–581. doi: 10.2174/1389203716666150630140157. [DOI] [PubMed] [Google Scholar]
- 49.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 50.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 51.Koch G, Esposito Z, Kusayanagi H, Monteleone F, Codeća C, Di Lorenzo F, Caltagirone C, Bernardi G, Martorana A. CSF tau levels influence cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis. 2011;26:181–186. doi: 10.3233/JAD-2011-110116. [DOI] [PubMed] [Google Scholar]
- 52.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, Craft S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 56.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2015;64:1025–1034. doi: 10.2337/db14-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma F, Wu T, Miao R, Xiao YY, Zhang W, Huang G. Conversion of mild cognitive impairment to dementia among subjects with diabetes: A population-based study of incidence and risk factors with five years of follow-up. J Alzheimers Dis. 2014;43:1441–1449. doi: 10.3233/JAD-141566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.