Abstract
Background
Cigarette smoking has been associated with higher risk of incident heart failure independent of coronary artery disease, but the impact of tobacco use on cardiac structure and function in the general population is uncertain. This study evaluated the relationship between smoking and echocardiographic measures in a large elderly cohort.
Methods and Results
We studied 4,580 participants free of overt coronary artery disease, heart failure and significant valvular disease from the fifth visit of the Atherosclerosis Risk in Communities (ARIC) Study who underwent transthoracic echocardiography. Participants were classified into 3 categories based on self-reported smoking habits: never (43.2%), former (50.5%) and current smokers (6.3%). Pack-years and years of smoking were also estimated. Compared to never smokers, current smokers had greater left ventricular (LV) mass index (80.4±1.1g/m2vs. 76.7±0.4g/m2; p<0.001), LV mass/volume ratio (1.93±0.03g/mL vs. 1.83±0.03g/mL; p<0.001), higher prevalence of LV hypertrophy (15% vs. 9%; p=0.008), and worse diastolic function, as reflected by higher E/E′ ratio (11.7±0.2 vs. 10.9±0.1; p<0.001), after adjusting for potential confounding factors. In contrast, former smokers showed similar echocardiographic features when compared to never smokers. Furthermore, estimated pack-years and years of smoking, measures of cumulative cigarette exposure, were associated with greater LV mass index, LV mass/volume ratio, and worse diastolic function (higher E/E′ ratio) in current smokers after multivariable analysis (all p<0.01).
Conclusions
Active smoking and cumulative cigarette exposure were associated with subtle alterations in LV structure and function in an elderly community-based population free of overt coronary artery disease and heart failure.
Keywords: Smoking, Echocardiography, Left ventricle, Tissue Doppler, longitudinal strain
Cigarette smoking is a major preventable cause of cardiovascular diseases, particularly of coronary artery disease (1). Epidemiological studies have suggested that active smoking is associated with incident heart failure in general populations, even after accounting for potential confounders and coronary artery disease (2, 3). Nevertheless, the mechanisms linking cigarette smoking to cardiac dysfunction are not established (4).
Analyses of the relationship between smoking and cardiac structure and function in community-based cohorts have yielded conflicting results. Although active smoking was associated with higher left ventricular (LV) mass (5, 6, 7) in some populations, neutral (8) or even inverse associations (9) were described in others. In addition, the relationship between smoking and LV systolic and diastolic function remains controversial (10, 11, 12, 13, 14). Moreover, it is uncertain whether tobacco-related effects on blood pressure (15) and arterial stiffness (7) and the coexistence of other risk factors for cardiovascular remodeling (16) might account for the association between smoking and altered cardiac structure and function independent of the development coronary artery disease.
We analyzed the cross-sectional association between smoking and echocardiographic features in a large elderly sample free of overt coronary heart disease or heart failure who attended the fifth visit of the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study population
ARIC is an ongoing, prospective observational study. Detailed study rationale, design, and procedures have been previously published (17). The original cohort included 15,792 participants aged 45 to 64 years recruited between 1987 and 1989 (visit 1), selected from 4 communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Institutional review boards from each site approved the study, and informed consent was obtained from all participants. In this study we considered the 6,538 surviving participants attending visit 5 (2011–2013). We excluded those with prevalent coronary artery disease, heart failure or moderate or severe valvular disease (n=1,187) and participants whose race was neither black nor white (n=14) or with missing echocardiographic examination (n=304) or smoking data (n=453), resulting in 4,580 individuals eligible for the present analysis.
Measurements
Smoking history
Smoking history was ascertained by means of an interviewer-administered questionnaire. At visit 5, participants were asked if they currently smoked cigarettes or whether they had done so in the past. This approach yielded 3 categories: never smokers, former smokers and current smokers. Cumulative pack-years and years of smoking were available up to visit 4 (1996-1998), while between visit 4 to visit 5 the participant smoking status (current smoker/non smoker) was ascertained by annual follow-up evaluations without assessment of intensity of smoking. As a result, the cumulative exposure to smoking up to visit 5 was estimated by assuming that smoking intensity obtained at visit 4 persisted over the interval from visit 4 to visit 5 among those who continued to smoke, allowing for the estimation of pack-years between visit 4 to visit 5 by multiplying the number of cigarettes/day at visit 4 by the number of years smoked between visits 4 and 5.
Echocardiography Protocol
The echocardiographic imaging and analysis protocol has been previously described in detail (18). All studies were acquired at visit 5 on Philips IE33 machines (Philips, Andover, MA) by trained sonographers. Analyses were performed by expert technicians and overread by echocardiographers in a central echo core laboratory. LV mass was indexed to body surface area and LV hypertrophy was defined as LV mass index >115g/m2 in men or >95g/m2 in women. Normal LV mass index or LV hypertrophy coupled with relative wall thickness ≥0.42 was defined as concentric remodeling or concentric hypertrophy, respectively, while normal LV mass index or LV hypertrophy coupled with relative wall thickness <0.42 was considered normal ventricular structure or eccentric hypertrophy, respectively (19). Left atrial volume was indexed to body surface area. Peak lateral and septal mitral annular relaxation (E′) velocities were assessed using tissue Doppler imaging. Right ventricular (RV) function was assessed using the tricuspid annular peak systolic velocity, and RV fractional area change was calculated as the percent change in cavity area. Global longitudinal strain was derived from speckle-tracking echocardiography.
Measurement of Other Baseline Covariates
Information on demographics, clinical history, anthropomorphic measures, and blood pressure was obtained at the time of echocardiography. Definitions for hypertension, diabetes mellitus, current alcohol consumption, coronary artery disease and heart failure were used as previously described in the ARIC study (20). Low-density and high-density lipoprotein cholesterol levels were measured in a centralized laboratory. Carotid-femoral pulse wave velocity was measured using a ColinVP-1000 plus system (Omron Co., Komaki, Japan) (21).
Statistical methods
Descriptive data are presented as the mean ± standard deviation for normally distributed variables and median [25th-75th percentile] for non-normally distributed variables. Categorical variables are expressed as proportions. Significant pairwise comparisons are shown only for variables in which a significant global difference was detected using one-way ANOVA or Kruskal-Wallis tests. The chi-square test was used to compare categorical variables. Echocardiographic data are presented as multivariable adjusted means with p-values estimated from linear or logistic regression across smoking categories. Multivariate model covariates were selected based on a priori knowledge. Two regression models were constructed: Model 1 included age, sex and race; Model 2 additionally adjusted for diabetes mellitus, anti-hypertensive medications, body mass index, systolic blood pressure, current alcohol consumption, heart rate and carotid-femoral pulse-wave-velocity. Linear regression analysis between echocardiographic parameters and pack-years of smoking or years of smoking was performed adjusting for Model 2 covariates and included never (0 pack-years) and current smokers. To further quantify the impact of possible bias due to selective attrition before visit 5 due to non-attendance among living cohort participants, we calculated inverse probability weights (22, 23) to estimate the likelihood of visit 5 participation among cohort participants known to be alive on December 31th, 2011. Nonattendance to visit 5 was modeled using the following variables assessed at visit 1: age, sex, race, field center, diabetes mellitus, body mass index, hypertension, systolic blood pressure, heart rate, estimated glomerular filtration rate and smoking status. The inverse of these resulting estimated probabilities were used as weights so that, in order to better represent the full ARIC population, visit 5 attendees with characteristics more similar to those who did not attend were given more weight in subsequent analyses of associations with echocardiographic analyses. Tests for interaction were performed using the likelihood ratio test for the cross-product interaction term between sex, race and measures of cardiac structure and function. Two-sided p-values <0.05 were considered significant. Analyses were performed using Stata version 13.1 (Stata Corp., College Station, Texas).
Results
Among the 4,580 ARIC visit 5 participants included in this analysis, 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers and 1,977 (43.2%) never smoked. Table 1 illustrates the characteristics of the study population according to smoking status. Never smokers were more likely to be women, were less frequently alcohol drinkers and had higher low-density and high-density lipoprotein cholesterol levels compared to the other smoking groups. Current smokers were younger, had lower average body mass index and had higher estimated glomerular filtration rate than the other smoking groups. Moreover, they were more likely to be black, women and had higher pack-years and years of smoking compared to former smokers.
Table 1. Participants characteristics according to smoking status.
| Smoking status | Never (n=1,977) | Former (n=2,316) | Current (n=287) |
|---|---|---|---|
| Male sex, n(%) | 524 (27) | 1086 (47)* | 116 (40)*† |
| Age, years | 75.9 ± 5.0 | 75.8 ± 5.1 | 74.0 ± 4.4*† |
| Black, n(%) | 457 (23) | 465 (20) | 74 (26)† |
| Body mass index, kg/m2 | 28.6 ± 5.7 | 28.7 ± 5.5 | 27.0 ± 5.6*† |
| Pack-years of smoking | ---- | 13 [3, 28] | 34 [13, 54]† |
| Years of smoking | ---- | 18 [7, 31] | 46 [28, 54]† |
| Systolic blood pressure, mmHg | 131 ± 18 | 130 ± 17 | 130 ± 18 |
| Diastolic blood pressure, mmHg | 67 ± 10 | 67 ± 10 | 66 ± 11 |
| Hypertension, n(%) | 1594 (81) | 1887 (81) | 220 (77) |
| Heart rate, b.p.m. | 63 ± 10 | 63 ± 10 | 63 ± 10 |
| Diabetes mellitus, n(%) | 575 (29) | 695 (30) | 76 (26) |
| Current drinkers, n(%) | 804 (41) | 1341 (58)* | 177 (62)* |
| Low-density-lipoprotein cholesterol, mg/dL | 110 ± 35 | 106 ± 33* | 104 ± 28* |
| High-density-lipoprotein cholesterol, mg/dL | 54 ± 14 | 52 ± 14* | 52 ± 13* |
| Glomerular filtration rate, mL/min/1.73m2 | 70 ± 16 | 71 ± 17 | 74 ± 17*† |
| Carotid-femoral pulse wave velocity, m/s | 11.7 ± 3.4 | 11.7 ± 4.0 | 11.4 ± 3.3 |
Legend. Normal distributed and non-normal distributed continuous variables are presented as mean ± standard deviation and median [25th-75th percentile], respectively.
p<0.05 vs. never smokers and
p<0.05 vs. former smokers
Tables 2 and 3 present the associations between smoking status and measures of cardiac structure and function. When compared to never and former smokers, current smokers had greater LV mass index, LV mass/volume ratio, and higher prevalence of LV hypertrophy and concentric LV hypertrophy after adjusting for age, sex, race, body mass index, diabetes, anti-hypertensive medications, systolic blood pressure, current alcohol consumption, heart rate and carotid-femoral pulse wave velocity (Table 2). Current smokers also had worse LV diastolic function, as evidenced by higher E/E′ ratio (Table 3). We observed a marginally higher LV ejection fraction in current smokers, but the difference in this variable between current and never smokers became non-significant (p=0.07) after additional adjustment for LV mass/volume ratio (Table 3). Furthermore, global longitudinal LV strain, which has been reported as a more sensitive measure of systolic function (24), did not differ among the smoking groups. Lastly, no significant association between right ventricular structural and functional features and smoking status was detected.
Table 2. Echocardiographic morphological characteristics of the participants according to smoking status.
| Smoking status | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Never | Former | Current | Never | Former | Current | |
| LV end-diastolic diameter, cm | 4.36 ± 0.01 | 4.37 ± 0.01 | 4.35 ± 0.03 | 4.35 ± 0.01 | 4.35 ± 0.01 | 4.38 ± 0.03 |
| LV end-systolic diameter, cm | 2.57 ± 0.01 | 2.59 ± 0.01 | 2.57 ± 0.03 | 2.57 ± 0.01 | 2.59 ± 0.01 | 2.59 ± 0.03 |
| LV Posterior wall thickness, mm | 9.2 ± 0.03 | 9.3 ± 0.03 | 9.3 ± 0.08 | 9.2 ± 0.03 | 9.2 ± 0.03 | 9.3 ± 0.08 |
| Septal wall thickness, mm | 10.3 ± 0.03 | 10.3 ± 0.03 | 10.4± 0.09 | 10.2 ± 0.03 | 10.3 ± 0.03 | 10.5± 0.09†§ |
| LV EDV, mL | 80.8 ± 0.4 | 80.3 ± 0.4 | 77.9 ± 1.1* | 80.2 ± 0.4 | 80.0 ± 0.4 | 79.0 ± 1.1 |
| LV mass, g | 144 ± 1 | 145 ± 1 | 145 ± 2 | 142 ± 1 | 143 ± 1 | 149 ± 2†‡ |
| LV mass index, g/m2 | 77.5 ± 0.4 | 77.2 ± 0.4 | 80.0 ± 1.1*‡ | 76.7 ± 0.4 | 76.7 ± 0.4 | 80.4 ± 1.1†§ |
| LV mass/EDV ratio, g/mL | 1.84 ± 0.01 | 1.86 ± 0.01 | 1.91 ± 0.03* | 1.83 ± 0.01 | 1.84 ± 0.01 | 1.93 ± 0.03†§ |
| Relative wall thickness | 0.428 ± 0.002 | 0.427 ± 0.002 | 0.429 ± 0.004 | 0.426 ± 0.002 | 0.427 ± 0.002 | 0.428 ± 0.005 |
| LV hypertrophy, % | 10 | 10 | 13 | 9 | 10 | 15†‡ |
| LV concentric hypertrophy, % | 7 | 6 | 9‡ | 6 | 6 | 10*‡ |
| LV eccentric hypertrophy, % | 3 | 4 | 4 | 3 | 4 | 5 |
| LV concentric remodeling, % | 38 | 40 | 37 | 38 | 41* | 36 |
| LV normal geometry, % | 52 | 50 | 50 | 53 | 49 | 49 |
| Left atrial volume index, mL/m2 | 25.4 ± 0.2 | 25.5 ± 0.2 | 24.7 ± 0.5 | 25.3 ± 0.2 | 25.4 ± 0.2 | 25.0 ± 0.5 |
| RV end-diastolic area, cm2 | 19.5 ± 0.1 | 19.5 ± 0.1 | 18.8 ± 0.3*‡ | 19.3 ± 0.1 | 19.3 ± 0.1 | 19.1 ± 0.3 |
| RV end-systolic area, cm2 | 9.2 ± 0.1 | 9.2 ± 0.1 | 8.9 ± 0.2*‡ | 9.2 ± 0.1 | 9.1 ± 0.1 | 9.0 ± 0.2 |
Legend. Continuous variables are presented as mean ± standard error. LV – left ventricular; EDV – end-diastolic volume; RV – right ventricular.
p<0.05 and
p<0.01 vs. never smokers and
p<0.05 and
p<0.01 vs. former smokers.
Model 1: Adjusted for age, sex and race
Model 2: Further adjusted for body mass index, diabetes, anti-hypertensive medications, systolic blood pressure, current alcohol consumption, heart rate and carotid-femoral pulse wave velocity.
Table 3. Echocardiographic functional characteristics of the participants according to smoking status.
| Smoking status | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Never | Former | Current | Never | Former | Current | |
| E/A ratio | 0.84 ± 0.01 | 0.85 ± 0.01 | 0.88 ± 0.02 | 0.84 ± 0.01 | 0.84 ± 0.01 | 0.88 ± 0.02‡ |
| E/E′ ratio | 11.0 ± 0.1 | 11.1 ± 0.1 | 11.6 ± 0.2†‡ | 10.9 ± 0.1 | 11.0 ± 0.1 | 11.7 ± 0.2†§ |
| Global longitudinal LV strain, % | -18.2 ± 0.1 | -18.0 ± 0.1* | -18.0 ± 0.2 | -18.2 ± 0.1 | -18.1 ± 0.1 | -18.0 ± 0.1 |
| LV ejection fraction, % | 65.8± 0.1 | 65.6 ± 0.1 | 66.5 ± 0.4‡ | 65.8± 0.1 | 65.7 ± 0.1 | 66.6 ± 0.4*‡‖ |
| RV fractional area change (%) | 52.6 ± 0.2 | 52.5 ± 0.2 | 52.7 ± 0.5 | 52.5 ± 0.2 | 52.7 ± 0.2 | 52.8 ± 0.5 |
| Tricuspid annulus PSV (cm/s) | 12.0 ± 0.1 | 11.9 ± 0.1 | 11.8 ± 0.2 | 12.0 ± 0.1 | 11.9 ± 0.1 | 11.8 ± 0.2 |
Legend. Continuous variables are presented as mean ± standard error. LV – left ventricular; E/A – early to late transmitral inflow velocity ratio; E/E′ - early transmitral inflow to early mitral relaxation velocity ratio; RV – right ventricular; PSV – peak-systolic velocity.
p<0.05 and
p<0.01 vs. never smokers and
p<0.05 and
p<0.01 vs. former smokers;
p>0.05 compared to never smokers after further adjustment for LV mass/end-diastolic volume ratio.
Model 1: Adjusted for age, sex and race
Model 2: Further adjusted for body mass index, diabetes, anti-hypertensive medications, systolic blood pressure, current alcohol consumption, heart rate and carotid-femoral pulse wave velocity.
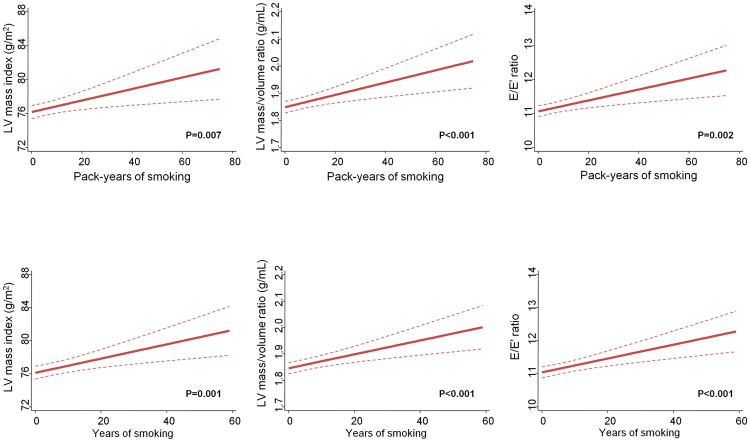
Estimated pack-years and years of smoking, which are measures of cumulative cigarette exposure, were associated with greater LV mass index, LV mass/volume ratio and worse diastolic function (higher E/E′ ratio) in multivariable analysis (Figure).
Figure.
Linear regression analysis of echocardiographic features, as a function of pack-years and years of smoking among never (0 pack-years) and current smokers. The 95% confidence intervals are indicated by the dash lines. Models are adjusted for age, sex, race, body mass index, diabetes, anti-hypertensive medications, systolic blood pressure, current alcohol consumption, heart rate and carotid-femoral pulse wave velocity. LV – left ventricular; E/E′ - early transmitral inflow to early mitral relaxation velocity ratio.
To account for possible bias due to selective attrition before visit 5 due to non-attendance among living cohort participants, we included estimated inverse probability of attendance as weights in the multivariable models (Supplemental Tables 1 and 2). This approach did not change the associations between smoking variables and echocardiographic parameters, except for the differences in the prevalence of LV concentric hypertrophy between current and never smokers, which became non significant after applying calculated inverse probability weights. We did not find any significant interaction by sex and race for the relationship between smoking and cardiac structural and functional features. Lastly, further adjustment for spirometric markers of lung function obtained at visit 5 (forced vital capacity or forced expiratory volume in 1 second) and markers of socio-economic status (income and education level) did not exert substantial changes in the association between smoking and echocardiographic parameters.
Discussion
In a large sample of elderly individuals free of overt coronary artery disease and heart failure, current smokers had higher LV mass and LV mass/volume ratio, higher prevalence of LV hypertrophy, and worse diastolic function, as reflected by higher E/E′ ratio, compared to never smokers and former smokers. Furthermore, pack-years and years of smoking were associated with greater LV mass index, LV mass/volume ratio, and worse diastolic function among current and never smokers in multivariable analysis. These findings suggest that active smoking and cumulative cigarette exposure were associated with alterations in LV structure and function.
Elevated LV mass and LV hypertrophy are acknowledged risk factors for heart failure (25). In the present study, LV mass and prevalence of LV hypertrophy were higher in current smokers compared to never and former smokers. Although previous studies have reported conflicting results (8, 9), our findings are in agreement with data from other large general populations (5, 6, 7), which showed greater LV mass in active smokers. The average difference in LV mass between current smokers and non-smokers in our adjusted analysis was approximately seven grams, similar in magnitude to data from the Cardiovascular Health Study (5), Multi-Ethnic Study of Atherosclerosis (6) and The Study of Health in Pomerania (7). Furthermore, we found that current smokers had a higher LV mass/volume ratio and a trend toward higher prevalence of LV concentric hypertrophy in comparison with the other smoking groups, suggesting that active smoking may also favor the development of LV concentricity, a feature that has been related to worse cardiovascular prognosis independent of LV mass (26). Whether these relatively modest differences in LV geometry and mass can be related to future outcomes remains to be established.
Previous studies showed contradictory results regarding the relationship between smoking and LV diastolic function in general populations (12, 13, 14). In addition, significant associations between smoking and impaired LV diastolic function have been reported only in women (13, 14). In the present report, we found that E/E′ ratio, a marker of LV filling pressure, was elevated in current smokers and this association was not influenced by sex. Our larger sample size probably increased the ability to detect significant associations in the whole sample, which could account for the differences between our findings and those of previous reports (12, 13, 14). Overall, these results suggest that active smoking was associated with impaired LV diastolic function, which might help explain the higher risk of incident heart failure reported for smokers in epidemiological studies (2, 3).
Smoking is known to increase the risk of cardiovascular atherosclerotic diseases in a dose-dependent fashion (27). However, the impact of intensity and duration of cigarette smoking on cardiac structure and function has been uncertain. We found that cumulative cigarette exposure was associated with higher LV mass and worse diastolic function in active smokers. In addition, we observed similar echocardiographic features between former and never smokers, suggesting that the potential effects of tobacco on the myocardium might be reversible after smoking cessation. Nevertheless, it is possible that residual myocardial alterations may persist after smoking cessation, because subtle reductions in LV function (11) and a modestly higher risk of heart failure (3) among former smokers have been reported by others.
In our population, smoking was not associated with global longitudinal LV strain, which has been reported as a more sensitive measure of systolic function (24), thus suggesting that smoking was not related to significant variations in LV systolic function. We also observed that LV ejection fraction, a less informative measure of systolic function when compared to global longitudinal strain (24, 28), was marginally higher in current smokers than in the other smoking groups. However, LV ejection fraction may be influenced by cardiac geometry (29) and is known to increase in parallel with LV mass/volume ratio (30). In accordance with this notion, we found that the difference in LV ejection fraction between current and never smokers became no longer apparent after adjusting for LV mass/volume ratio in multivariable analysis, suggesting that the higher LV ejection fraction seen in current smokers could be more a consequence of variation in LV geometry rather than resulting from enhanced LV systolic function.
A variety of mechanisms might explain the association between smoking and LV remodeling and dysfunction. Smoking-associated cardiac alterations could be a result of tobacco-induced increases in blood pressure levels (15) or a consequence of coexisting risk factors, such as alcohol consumption (16). However, we found no differences in brachial blood pressure levels among the smoking groups and the association between smoking and echocardiographic parameters was independent of blood pressure levels and active alcohol consumption. Previous studies have also hypothesized that the adverse effects of tobacco on the myocardium could be driven by smoking-induced increases in arterial stiffness (7). In contrast to this hypothesis, we provided novel evidence that the association between smoking and higher LV mass and worse LV function was independent of carotid-femoral pulse-wave-velocity, an acknowledged marker of arterial stiffness. Lastly, it is possible that the changes in cardiac parameters were a result of direct effects of tobacco on the myocardium. This hypothesis is supported by experimental evidence showing that cigarette smoke may stimulate myocardial hypertrophy and dysfunction, by inducing neurohumoral changes, oxidative stress and activation of matrix-metalloproteinases and mitogen-activated protein-kinases (4). Nevertheless, additional studies are necessary to discern the exact mechanisms by which smoking influences cardiac structure and function.
Some limitations of this study should be highlighted. Cigarette smoking information was obtained through interviewer-administered questionnaires and no validation with biochemical analyses was attempted. Thus, participants may have underreported their smoking habits, although this would likely have biased our results toward the null. In addition, we estimated the number of pack-years smoked between visits 4 and 5 by assuming that smoking intensity obtained at visit 4 persisted over the interval from visit 4 to visit 5, which might limit the accuracy of total pack-years used in our analyses. However, years of smoking, an alternative measure of smoking burden (31), was calculated up to visit 5 and showed quite similar associations with echocardiographic parameters when compared to total pack-years, suggesting that total pack-years used in our analysis may have provided a good estimation of cumulative cigarette exposure. As in any observational cross-sectional study, residual confounding cannot be excluded, and the associations observed between smoking and variation in cardiac structure and function cannot be assumed to be causal. In our analysis we did not account for multiple testing, as such typical corrections for multiplicity (i.e. Bonferroni) can be overly conservative in the presence of correlated outcomes, and it is known that in general, echocardiography parameters are not independent of each other. However, all associations between smoking status and echocardiographic parameters (except for the adjusted differences on LV hypertrophy prevalence between current and former smokers) would remain significant considering a Bonferroni level of significance of P<0.05/6 (=0.0083), accounting for six echocardiography measures. It is possible that survival bias and selection bias may have influenced our estimates. Given that current smokers have a higher rate of death, and incident heart failure and cardiovascular events compared to never and former smokers (32), our analysis possibly included only the smokers who were relatively healthier and therefore did not include a substantial number of smokers who had more expressive cardiac remodeling and dysfunction but died prior to the analysis. This raises the assumption that similar or even greater associations between smoking and adverse cardiac characteristics would have been observed among subjects who did not survive. Conversely, it should be acknowledged that our sample of current smokers, which only included elderly individuals without coronary disease or heart failure may represent a biologically unique group of individuals, not necessarily reflecting the full spectrum of the smoking population. Furthermore, selective attrition before visit 5 may have introduced biased estimates in our analysis. However, our sensitivity analysis using inverse probability attrition weighting to account for non-attendance among living cohort participants, demonstrated consistent results when compared to the primary analysis.
In summary, we found that active smoking and cumulative cigarette exposure were associated with higher LV mass, LV mass/volume ratio and worse diastolic function in an elderly community-based population free of overt coronary artery disease and heart failure. These findings suggest that active smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure reported for smokers independent of coronary artery disease.
Supplementary Material
Clinical Perspective.
Cigarette smoking has been associated with higher risk of incident heart failure independent of coronary artery disease, but the impact of tobacco use on cardiac structure and function in the general population is uncertain. We studied 4,580 elderly subjects free of overt coronary artery disease and heart failure and found that current smokers had greater left ventricular (LV) mass index, LV mass/volume ratio, higher prevalence of LV hypertrophy, and worse diastolic function as compared to never and former smokers. Furthermore, estimated pack-years and years of smoking, measures of cumulative cigarette exposure, were associated with greater LV mass and worse diastolic function in current smokers. These findings suggest that active smoking is associated with alterations in LV structure and function, which might help explain the higher risk of heart failure reported for smokers independent of coronary artery disease.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. This work was also supported NHLBI cooperative agreement NHLBI-HC-11-08 (S.D.S.), grants R00-HL-107642 (S.C.) and K08-HL-116792 (A.M.S.), and a grant from the Ellison Foundation (S.C.). A.G. was supported by the Portuguese Foundation for Science and Technology Grant HMSP-ICS/007/2012. W.N.J. was supported by the Brazilian National Council for Scientific and Technological Development Grant 249481/2013-8.
Footnotes
Disclosures: None.
References
- 1.Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J. 2013;34:3259–3267. doi: 10.1093/eurheartj/eht352. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–342. doi: 10.1016/j.ahj.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Cardiac remodeling induced by smoking: concepts, relevance, and potential mechanisms. Inflamm Allergy Drug Targets. 2012;11:442–447. doi: 10.2174/187152812803589958. [DOI] [PubMed] [Google Scholar]
- 5.Gardin JM, Arnold A, Gottdiener JS, Wong ND, Fried LP, Klopfenstein HS, O'Leary DH, Tracy R, Kronmal R. Left ventricular mass in the elderly. The Cardiovascular Health Study. Hypertension. 1997;29:1095–1103. doi: 10.1161/01.hyp.29.5.1095. [DOI] [PubMed] [Google Scholar]
- 6.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markus MR, Stritzke J, Baumeister SE, Siewert U, Baulmann J, Hannemann A, Schipf S, Meisinger C, Dörr M, Felix SB, Keil U, Völzke H, Hense HW, Schunkert H MONICA/KORA Augsburg Cohort Study. Effects of smoking on arterial distensibility, central aortic pressures and left ventricular mass. Int J Cardiol. 2013;168:2593–2601. doi: 10.1016/j.ijcard.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa T, Boden-Albala B, Eguchi K, Jin Z, Sacco RL, Homma S, Di Tullio MR. Impaired flow-mediated vasodilatation is associated with increased left ventricular mass in a multiethnic population. The Northern Manhattan Study. Am J Hypertens. 2010;23:413–419. doi: 10.1038/ajh.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne JR, James LE, Eleftheriou KI, Hawe E, Mann J, Stronge A, Banham K, World M, Humphries SE, Pennell DJ, Montgomery HE. The association of left ventricular mass with blood pressure, cigarette smoking and alcohol consumption; data from the LARGE Heart study. Int J Cardiol. 2007;120:52–58. doi: 10.1016/j.ijcard.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Roman MJ, Paranicas M, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Rodeheffer RJ, Cowan LD, Howard BV. A population-based assessment of left ventricular systolic dysfunction in middle-aged and older adults: the Strong Heart Study. Am Heart J. 2001;141:439–446. doi: 10.1067/mhj.2001.113223. [DOI] [PubMed] [Google Scholar]
- 11.Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, Jerosch-Herold M, Arnett DK, Lai S, Bluemke DA, Lima JA. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 13.Bennet L, Larsson C, Söderström M, Råstam L, Lindblad U. Diastolic dysfunction is associated with sedentary leisure time physical activity and smoking in females only. Scand J Prim Health Care. 2010;28:172–178. doi: 10.3109/02813432.2010.506803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011;24:322–332.e6. doi: 10.1016/j.echo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Feng D, Liu T, Su DF, Wang H, Ding P, He YH, Deng XQ, Hou MJ, Ling WH, Chen WQ. The association between smoking quantity and hypertension mediated by inflammation in Chinese current smokers. J Hypertens. 2013;31:1798–1805. doi: 10.1097/HJH.0b013e328362c21a. [DOI] [PubMed] [Google Scholar]
- 16.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–462. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study I. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, Heiss G. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2016;29:114–122. doi: 10.1093/ajh/hpv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Selnes OA, Schneider AL, Wagenknecht LE, Windham BG, Wruck LM, Mosley TH. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal Cardiovascular Health During Adult Life and Cardiovascular Structure and Function Among the Elderly. Circulation. 2015;132:1979–89. doi: 10.1161/CIRCULATIONAHA.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 25.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail. 2014;2:512–522. doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Jain A, McClelland RL, Polak JF, Shea S, Burke GL, Bild DE, Watson KE, Budoff MJ, Liu K, Post WS, Folsom AR, Lima JA, Bluemke DA. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA) Circ Cardiovasc Imaging. 2011;4:8–15. doi: 10.1161/CIRCIMAGING.110.959403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–90. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 28.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manisty CH, Francis DP. Ejection fraction: a measure of desperation? Heart. 2008;94:400–401. doi: 10.1136/hrt.2007.118976. [DOI] [PubMed] [Google Scholar]
- 30.Yeon SB, Salton CJ, Gona P, Chuang ML, Blease SJ, Han Y, Tsao CW, Danias PG, Levy D, O'Donnell CJ, Manning WJ. Impact of age, sex, and indexation method on MR left ventricular reference values in the Framingham Heart Study offspring cohort. J Magn Reson Imaging. 2015;41:1038–1045. doi: 10.1002/jmri.24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thun MJ, Myers DG, Day-Lally C, Namboodiri MM, Calle EE, Flanders WD, Adams SL, Heath CW., Jr . Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control Smoking and Tobacco Control Monograph No 8. Bethesda (MD): U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1997. Age and the exposure-response relationships between cigarette smoking and premature death in Cancer Prevention Study II; pp. 383–475. [Google Scholar]
- 32.Nadruz W, Jr, Gonçalves A, Claggett B, Querejeta Roca G, Shah AM, Cheng S, Heiss G, Ballantyne CM, Solomon SD. Influence of cigarette smoking on cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Heart Fail. 2016;18:629–37. doi: 10.1002/ejhf.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.