Abstract
The heart-specific isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) is an important regulator of glycolytic flux in cardiac cells. Here, we present the crystal structures of two PFKFB2 orthologues, human and bovine, at resolutions of 2.0 and 1.8Å, respectively. Citrate, a TCA cycle intermediate and well-known inhibitor of PFKFB2, co-crystallized in the 2-kinase domains of both orthologues, occupying the fructose-6-phosphate binding-site and extending into the γ-phosphate binding pocket of ATP. This steric and electrostatic occlusion of the γ-phosphate site by citrate proved highly consequential to the binding of co-complexed ATP analogues. The bovine structure, which co-crystallized with ADP, closely resembled the overall structure of other PFKFB isoforms, with ADP mimicking the catalytic binding mode of ATP. The human structure, on the other hand, co-complexed with AMPPNP, which, unlike ADP, contains a γ-phosphate. The presence of this γ-phosphate made adoption of the catalytic ATP binding mode impossible for AMPPNP, forcing the analogue to bind atypically with concomitant conformational changes to the ATP binding-pocket. Inhibition kinetics were used to validate the structural observations, confirming citrate’s inhibition mechanism as competitive for F6P and noncompetitive for ATP. Together, these structural and kinetic data establish a molecular basis for citrate’s negative feed-back loop of the glycolytic pathway via PFKFB2.
Keywords: 6-Phosphofructo-2-kinase/Fructose-2,6-bisphosphatase; PFKFB2; crystal structure; glycolysis; citrate; inhibition; fructose-2,6-bisphosphate; heart; cardiac
Introduction
The bifunctional enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB), catalyzes both the synthesis and hydrolysis of fructose-2,6-bisphosphate (Fru-2,6-P2) as a functional homodimer (C. Wu et al. 2006). The dual enzymatic activities are provided by the two separate catalytic domains existing in a single protein subunit, to control the concentration of Fru-2,6-P2, the most potent allosteric activator of 6-phosphofructo-1-kinase (PFK), the rate-limiting enzyme of glycolysis (El-Maghrabi and Pilkis 1984; Pilkis and Granner 1992; Pilkis et al. 1995). An elevated cellular concentration of Fru-2,6-P2 increases glycolytic flux, whereas a lowered concentration of Fru-2,6-P2 decreases glycolysis. Reflecting the diversity of tissues and their physiological functions, different tissue-specific isoforms of PFKFB from four distinct genes (pfkfb1-4) are expressed, each with different kinetic properties, and a single isoform generally predominates in each tissue: PFKFB1, the liver form; PFKFB2, the heart form; PFKFB3, the inducible form; and PFKFB4, the testis form (Okar et al. 2001).
Because of the uniqueness in its structure/function relationships, the PFKFB enzyme system has long been the target of structure/function studies. As a result, a significant amount of functional data has been produced and the crystal structures of the rat liver Fru-2,6-P2ase domain, the rat testis form (PFKFB4), the human liver form (PFKFB1), and human cancer form (PFKFB3) have been determined (Hasemann et al. 1996; Kim et al. 2006; Y. H. Lee et al. 2003). These data altogether allowed us to understand the molecular mechanism of catalytic reactions and regulation of this enzyme system. Some are now serving as a molecular foundation for development of therapeutics for diabetes and cancer (Garber 2004; A. Minchenko et al. 2002).
However, one of the most fundamental questions yet to be answered about this enzyme system is how each PFKFB isoform performs its differential function in hosting tissues that have specific physiological roles and, accordingly, different optimum conditions for glucose metabolism. Reflecting such differences, the PFKFB isozymes have been shown to have different kinetic properties, as summarized in Figure 4.5. These kinetic differences suggest that glucose metabolism is uniquely related to the physiological roles of the given tissues and not just a simple housekeeping function for energy production (Y. H. Lee et al. 2003; Pilkis et al. 1995).
As an effort to address the tissue-type specifically differentiated structure/function relationships of PFKFB isoforms, we determined the crystal structures of heart isoforms of PFFKB, PFKFB2, from both H. sapiens and B. taurus. Analysis of their structures and kinetics in comparison with those already known from the previous PFKFB studies suggested a regulatory mechanism as yet unknown and we introduce the results here.
Materials and Methods
Crystallization of PFKFB2 Homologs
DNA sequences encoding full length constructs of the human and bovine PFKFB2 isoforms were cloned into a pET-3a vector (Novagen) allowing fusions to a 6xHis tag sequence at the N-termini. Both proteins were overexpressed in Escherichia coli BL21 C41(DE3) using 0.3mM IPTG at 18°C and purified using Ni-NTA affinity columns in conjunction with SP Sepharose cation exchange columns. Following purification by SP Sepharose, the proteins were dialyzed with pH 8.0 20 mM Tris•HCl, 10 mM NaPi, 5 mM β-Mercaptoethanol, and 5% glycerol and then concentrated to 8.0 mg/ml with Millipore centrifugal filter concentrators. Crystallization of the bovine PFKFB2 (bPFKFB2) were prepared via sitting drop vapor diffusion using a 1:1 mixture of protein sample to a mother liquor, pH 7.5 100mM HEPES, pH 7.0 0.5% tacsimate, 13–16% polyethylene glycol 3350, and 3% dioxane. The crystals were grown to a dimension of 0.2 × 0.2 × 0.05 mm within 2–3 weeks of incubation at 20°C. Crystals of the human PFKFB2 (hPFKFB2) were also prepared with the sitting drop vapor diffusion method using a mother liquor, pH 6.0 100mM MES, 0.5–3.0% polyethylene glycol 8000, 13–16% polyethylene glycol 3350, and 3% dioxane. Data quality crystals of hPFKFB2 were grown after 3–5 weeks of incubation at 12°C.
Data Collection and Processing
The crystals were soaked for cryo-protection and liganding in cryoprotectant solutions, containing the aimed ligands, for 0.5 to 2 hours prior to flash freezing at 77K using liquid N2. All cryoprotectant solutions were prepared by enriching the reservoir solution of each crystal with 35% ethylene glycol. The diffraction data was collected at both beamline 6C of the Pohang Accelerator Laboratory, Pohang, Korea, using the CCD detector Quantum 210 (ADSC) with a source wavelength of 1.23986 Å or at the Gulf Coast Consortium Protein Crystallography Beamline (PX1) in the Center for Advanced Microstructures and Devices (CAMD), Louisiana State University, Baton Rouge, LA., using a Mar 165 mm CCD detector with a source wavelength of 1.3808 Å. All diffraction data was processed and scaled using HKL2000.
Crystals of hPFKFB2 belong to the primitive orthorhombic space group P212121, having unit cell dimensions of a = 106.5 Å, b = 113.9 Å, c = 133.2 Å and an asymmetric unit consisting of two monomers. Crystals of bPFKFB2 belong to the C-centered orthorhombic space group, C2221, with unit cell dimensions being a = 82.1 Å, b = 169.5 Å, c = 85.3 Å, α =90, β =90, γ =90 and an asymmetric unit consisting of one monomer. Statistics of the reflection data are summarized in Table 1.
Table 1.
Statistics of reflection data and structure refinements. Liganding denotes the states in the 2-Kinase/2-Phosphatase. His-P is phosphorylated at His258. Rsym = Σh(Σj|Ih,j - <Ih>|/ΣIh,j), where h=set of Miller indices, j=set of observations of reflection h, and <Ih>=the mean intensity. RMSD values are deviations from ideal values. Rcrys = Σh || Fo,h | - |Fc,h || / Σh |Fo,h|. Rfree was calculated using 5% of the complete data set randomly excluded from refinement. The numbers in parentheses represent values from the highest resolution shell.
| Orthologue | Human | Bovine |
|---|---|---|
| Liganding | ATP•Citrate/His-P•F6P | ADP•Citrate/His-P•Citrate |
|
| ||
| Space Group | P212121 | C2221 |
|
| ||
| Unit Cell Dimensions (Å) | 106.5 × 113.9 × 133.2 | 82.1 × 169.5 × 85.3 |
|
| ||
| Resolution Range (Å) | 38.55-2.01 | 46.54 – 1.82 |
|
| ||
| Reflections [F ≥ σ(F)] | 106633 | 52149 |
|
| ||
| Completeness (%) | 0.98 (87.3) | 97.0 (81.5) |
|
| ||
| Redundancy | 4.2 (3.4) | 10.9 (10.9) |
|
| ||
| I/σ (I) | 16.2 (2.3) | 2.62 (1.82) |
|
| ||
| Rsym | 0.059 (0.420) | 0.050 (0.284) |
|
| ||
| Rcrys | 0.1490 | 0.1392 |
|
| ||
| Rfree | 0.1751 | 0.1623 |
|
| ||
| No. of Amino Acids | 840 | 423 |
|
| ||
| No. of Protein Atoms | 6925 | 3461 |
|
| ||
| No. of Hetero Atoms | 194 | 62 |
|
| ||
| RMSD | ||
| Bond Lengths (Å) | 0.016 | 0.009 |
| Angles (°) | 1.40 | 1.319 |
| Dihedral Angles (°) | 16.747 | 13.806 |
|
| ||
| Mean B factor | 39.70 | 25.82 |
| Protein Atoms (Å2) | 39.00 | 24.60 |
| Hetero Atoms (Å2) | 42.97 | 22.84 |
| Water Atoms (Å2) | 45.53 | 34.59 |
Structure determination and refinement
The structures of hPFKFB2 and bPFKFB2 were determined by molecular replacement using the Phaser software module implemented in the PHENIX program suite. Initial models for both orthologues were determined using the human liver PFKFB (1K6M) as a search model (Y. H. Lee et al. 2003). The final structures were achieved after iterated model rebuilding and refinement using PHENIX and Coot (P. D. Adams et al. 2010; Emsley and Cowtan 2004). The Rcrys/Rfree of the final models are 0.202/0.225 and 0.156/0.211 for hPFKFB2 and bPFKFB2, respectively.
The final models revealed the residues 31–450 out of 505 of hPFKFB2 and those of 28–450 of bPFKFB2. The missing residues are all from both the N- and C-terminal regulatory domains and are considered disordered, based on the results from mass spectroscopy of melt crystals (data not shown). For the human model, 93.2% of the 420 revealed residues lie within the ‘most favorable region’ of the main chain dihedral angle distribution, whereas none are found in the ‘disallowed region’. Similarly, for the 423 residues of the bovine orthologue, 90.9% are within the “most favorable region” and none are in the “disallowed region”.
Biochemical Assays
The 2-kinase inhibition assays were performed using a Fru-2,6-P2 assay modified for 96-well plates from the conventional method for both the wildtype hPFKFB2 and Y428A mutant (Van Schaftingen E, et al.). This assay consisted of two sequential steps: Fru-2,6-P2 production by PFKFB2 and allosteric activation of PFK-1 by the produced Fru-2,6-P2. For the first reaction, Fru-2,6-P2 synthesis by PFKFB2, was started by adding 130 nM PFKFB2 to mixtures containing 20 mM pH 8.0 TES, 1 mM DTT, 2 mM MgCl2, 0.5% tween, and varying concentrations of citrate, ATP and Fru-6-P, ranging from 0 to 200 mM for each. This reaction was allowed to run for 10 minutes at 25°C and then stopped by the addition of 0.1 M KOH. Aliquots of 1–4 μL of the first reaction were transferred, after pH neutralization, to the reactions of the second step, which consisted of 50 mM pH 8.0 Tris•HCl, 0.2 mM NADH, 5 mM DTT, 1 mM F-6-P, 2 mM MgCl2, 0.70 units/mL Aldolase, 0.45 units/mL glyceraldehyde dehydrogenase, 0.60 units/mL triose-phosphate isomerase, and 10 mU pyrophosphate-dependent phosphofructokinase. The reactions of second step were started by adding 0.5 mM sodium pyrophosphate and were then measured for changes in absorbance at 340 nm over a period of 30 minutes.
Accession numbers
Protein Data Bank: Coordinates and structure factors were deposited with the following codes: H5R5 (Bovine PFKFB2) and 5HTK (Human PFKFB2).
Results and Discussion
Overall Structures of PFKFB2
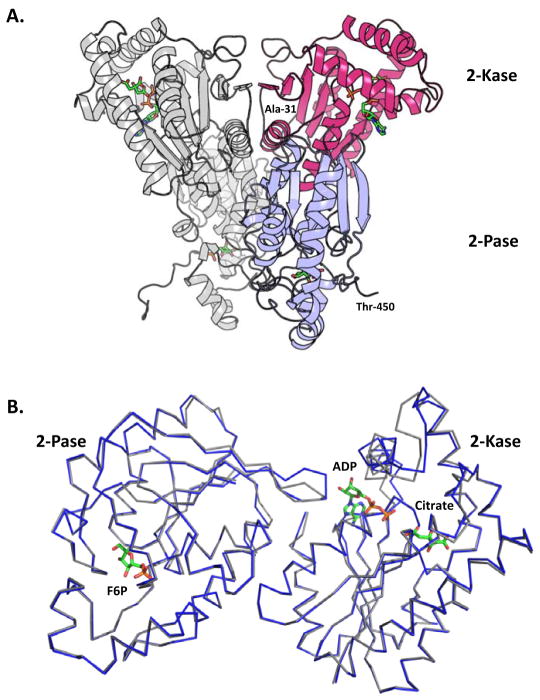
As summarized in the previous section and Table 1, crystal structures of the two PFKFB2 orthologs were determined – human (hPFKFB2) to 2.0Å and bovine (bPFKFB2) to 1.8Å – to elucidate any structural/functional differences in heart-type PFKFB compared to other isoforms. Similar to the other PFKFB isoforms, the crystal structure of the heart isozyme, for both human and bovine orthologues, shows a PFKFB-typical head-to-head homodimer arrangement (Figure 1a) with a dimeric interface constituted primarily through crystal contacts made between two kinase domains.
Figure 1.
Dimeric arrangement of heart form and a comparison of the structures of the human and bovine orthologues. (A) Head-to-head homodimer arrangement of the human PFKFB2. Shown in gray is a complete monomer, while the red and blue colors depict the 2-Kase and 2-Pase domains, respectively. Ligands are colored by element and are shown in stick form. (B) Superimposed C-alpha traces of bovine (blue) and human (light gray) kinase and phosphatase domains. The phosphatase domains contain Fru-6-P from the bovine orthologue in the F-2,6-P2 binding site. The kinase domains with ADP and Citrate from the bovine orthologue in the ATP and F6P binding sites, respectively.
Each PFKFB2 monomer consists of a single polypeptide chain that can be subdivided into four distinct regions: the 6-phosphofructo-2-kinase (2-Kase) domain, the fructose-2,6-bisphosphatase (2-Pase) domain, and two regulatory domains. The two catalytic domains are conserved among the different tissue isoforms with the sequence identity 76–83%, whereas the N-terminal and C-terminal domains are highly variable. Among 505 amino acid residues of PFKFB2, residues 1–37 and 451–505 constitute the N- and C-terminal regulatory domains, whereas residues 38–248 and 249–450 constitute the 2-Kase and 2-Pase domains, respectively.
The sequence identity shared between the human and bovine orthologs is larger than 95%, supporting the notion that the two PFKFB2 orthologs play a physiological role common to both human and swine. To analyze the structural differences between the two orthologs, Cα traces of hPFKFB2’s 2-Kase and 2-Pase domains were superimposed, separately, onto corresponding domains of bPFKFB2 (Figure 1b). It was not appropriate to compare the two domains together, because differences in liganding states of the orthologs cause differences in the domain-domain interface. In agreement with the high sequence identity, the two catalytic domains showed a high degree of overall similarity, with RMSD values of 1.46 Å and 0.68 Å, for the 2-Kase and 2-Pase domains, respectively. Although the discrepancy in the 2-Kase domains is higher than expected, no significant structural differences between the two orthologs, which would implicate coinciding differences in the structure/function relationship, are apparent.
The unexpectedly high RMSD for the 2-Kase domains is likely due to differences in liganding states of the two orthologs despite sharing similar liganding conditions during crystallization. For both the structures, Fru-6-P and citrate were in common, whereas AMPPNP – a non-hydrolyzable ATP analog – and ADP were exclusively included fill the ATP pockets of hPFKFB2 and bPFKFB2, respectively. Structural dissimilarities were concentrated to localized regions within-or-near the 2-Kase’s active site and correlate with citrate’s binding to the 2-Kase domain. Despite Fru-6-P being present in crystallization conditions for both orthologues, it didn’t complex with the 2-kinase domain of either, regardless of concentration. Instead, citrate, from an earlier purification step, occupies the Fru-6-P binding pocket. Interestingly, the binding of citrate appears to significantly influence ATP but not ADP binding to the 2-Kase domain. The details will be discussed in the following sections.
As indicated in Table 1, His258 were found as phosphorylated histidine in both the structures despite the fact that no Fru-2,6-P2 was involved in crystallization attempts. No other isoforms have shown phosphorylated histidine unless the crystals are soaked with high concentrations of Fru-2,6-P2 to induce the phosphorylated histidine as a catalytic intermediate state (Kim et al. 2007). The only possibility is that the phosphorylated histidine was formed by Fru-2,6-P2 synthesized during the PFKFB2 expression in E. coli. In addition, it has to be presumed that PFKFB2 has the phosphor-histidine state, which is more stable than that of any other isoforms.
Citrate binding to the 2-Kase domain
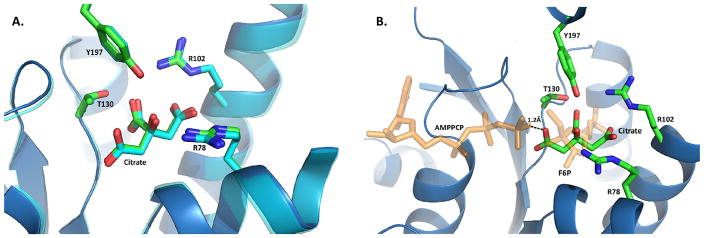
There was no Fru-6-P molecule bound to the 2-Kinase domain despite being present in the crystallization mixture, because the 2-Kase, which follows an ordered reaction, requires ATP binding prior to Fru-6-P binding. Instead, citrate was found to be bound to the Fru-6-P pocket of the 2-Kase domains of both hPFKFB2 and bPFKFB2 with strong hydrogen bonds to Thr130 and Tyr197 and salt bridges with Arg78 and Arg102 (Figure 2a). Except for Arg102, which is conserved though, all of these residues are known to be involved in the catalytic binding of Fru-6-P to the 2-Kase domain. This binding mode of citrate is conserved between the bovine and human orthologs. It is known that the 2-Kase of PFKFB2, the heart isoform, is very sensitive to inhibition from products of TCA cycle such as succinate and citrate. A previous structural study has suggested that succinate behaves like a competitive inhibitor against Fru-6-P (Galluzzi et al. 2013).
Figure 2.
Citrate binding in the 2-Kase domain. (A) Superimposed structures of the citrate binding pocket for human (blue) and bovine (light gray) orthologues. Residues forming interactions with citrate are represented as sticks, with a ribbon diagram representing the mainchain. Human PFKFB2 is depicted as a blue ribbon with sticks containing green carbon atoms. In contrast, bovine PFKFB2 is represented as a light gray ribbon with sticks containing white carbon atoms. (B) AMPPCP and F6P from PFKFB3 (2DWP) overlaid onto the citrate binding site of human PFKFB2. The distance between the carboxy arm from citrate and nearest atom from the overlaid AMPPCP is shown. Both AMPPCP and F6P are semi-transparent colored orange.
However, citrate bound to PFKFB2 suggests an inhibition pattern, which is different from that of succinate, which was previously studied (Yuen et al. 1999). While primarily located in the Fru-6-P binding site, citrate, unlike succinate, impedes the γ-phosphate of AMPPNP from occupying its normal location for the Fru-2,6-P2 synthesis. When citrate from hPFKFB2 was superimposed onto a pseudo-Michaelis complex of PFKFB3 (2DWP), one of the three carboxy group arms of citrate is only 1.2Å away from the γ-phosphate of AMPPCP (Figure 2b) (Kim et al. 2007). Such close proximity of two negatively charged acidic groups is both sterically and electrostatically unfavorable for ATP binding but ADP binding is not significantly influenced. Thus, it has been suggested that ATP binding to the 2-Kase domain in the known catalytic mode is not favored in the presence of citrate. And, considering perfect conservation of all the residues for citrate, binding of citrate to the four PFKFB isoforms would be very similar.
Conserved catalytic binding of ATP and ADP to the 2-Kase domain
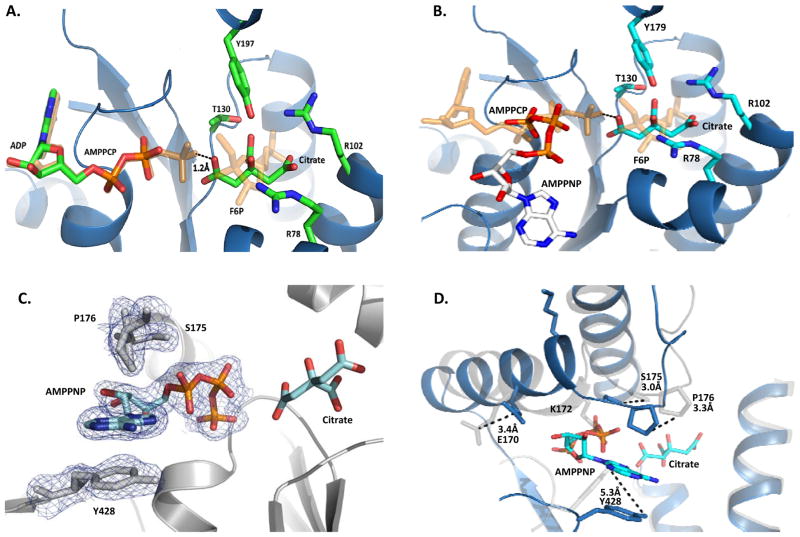
Both hPFKFB2 and bPFKFB2 were co-crystallized in the presence of ADP and AMPPNP, respectively. As expected, the binding of ADP in the bPFKFB2 structure is analogous to the catalytic mode of ADP and ATP, which were well characterized and elucidated in previous studies of the PFKFB protein system (Figure 3a) (Kim et al. 2007; Y. H. Lee et al. 2003). The adenine moiety resides in a partly solvent-exposed hydrophobic pocket stabilized primarily through CH-π contributions made by Gly51, Tyr54, and Val221. A single hydrogen bond is formed between the adenine N6 and the Oδ of Asn168. The nucleotide diphosphates, on the other hand, occupy a highly anionic phosphate binding loop created by a Walker A motif – conserved as -49GLPARGKT56- in all PFKFB isoforms (Walker et al. 1982). Additional hydrogen bonds to the phosphate moieties are contributed from the side chains of Lys172 and Tyr428. This binding is well conserved among all PFKFB isoforms. However, Tyr428 plays a different role upon binding of AMPPNP in the presence of citrate bound to 2-Kase.
Figure 3.
Ligand Binding within the 2-Kase Domain. (A) Bovine PFKFB2 complexed with ADP and Citrate. Superimposed are AMPPCP and F6P from the pseudo-substrate complex with PFKFB3 (2DWP), showing the catalytic ATP and F6P binding modes, respectively. (B) Human PFKFB2 complexed with AMPPNP and Citrate. Superimposed are AMPPCP and F6P from the pseudo-substrate complex with PFKFB3 (2DWP), showing the catalytic ATP and F6P binding modes, respectively. (C) Unbiased Fo-Fc omit map of AMPPNP and neighboring residues in human PFKFB2. Contoured at 3.0σ (D) Ribbon diagram view of human PFKFB2 (blue) super-imposed onto the bovine orthologue (light gray). The sidechains and their positional differences are shown for residues involved in AMPPNP binding.
An alternative ATP binding mode to the 2-Kase domain caused by citrate binding
Surprisingly, the binding mode of AMPPNP in the presence of citrate is markedly different from the conventional mode of catalytic ATP binding for the Fru-2,6-P2 synthesis, when it was revealed by the |Fo| - |Fc| omit map (Figure 3c). When AMPPNP from the hPFKFB2•AMPPNP complex was superimposed onto ADP of bPFKFB2•ADP complex, as can be seen in Figure 3b, the ribose and adenine rings of AMPPNP are rotated 115° and 180°, respectively, relative to those of ADP. Furthermore, the centroid of AMPPNP’s adenine ring is 14Å shifted outward from that of ADP. Consequently, few molecular interactions are shared among the bindings of adenosine moieties of AMPPNP and ADP to PFKFB2 and, thus, the nucleoside binding sites for AMPPNP are remarkably different from that of ADP (Figure 3c). From the AMPPNP-complexed structure, it can be seen that the α-carbon of Pro176 shifts ~3.3Å, placing the residue’s R-group perpendicular to AMPPNP’s planar ring, allowing for CH-π contributions. More significant contributions come by way of π-π stacking interactions with the phenol side chain of Tyr428, which is otherwise involved in interaction with the phosphate moieties. For the π-π stacking between the adenine ring and Tyr428, the β-hairpin containing Tyr428 shifted 4Å and the phenol sidechain rotated away from the position for its conventional interaction with the α-phosphate moiety of ADP (Figure 3d).
As a consequence, the γ-phosphate of AMPNP occupies the site for the β-phosphate of ADP or catalytic ATP, sharing many contact residues with the α- and β-phosphates of ADP. Of the five residues forming the Walker A motif, Tyr54 is the only notable absence. As for sidechain interactions, the phosphate stabilizing hydrogen bond of Tyr428 is replaced by Ser174. Interactions with Lys172, on the other hand, are completely lost, with the residue flipping outward towards the solvent. This atypical binding of AMPPNP is inappropriate for the 2-Kase catalytic reaction and appeared to be caused by the citrate binding to 2-Kase as described in the following section.
Citrate inhibition at the active site of the 2-Kinase domain
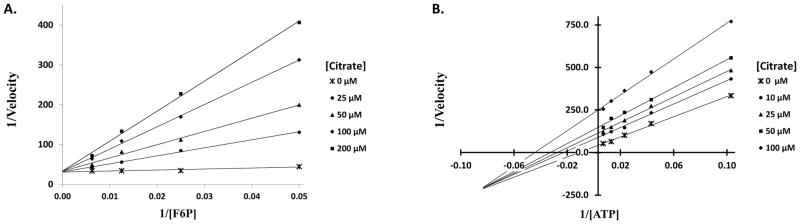
Although previous studies have reported citrate to inhibit PFKFB enzymes, particularly the heart isotype, the inhibition mechanism has remained unknown (Ros and Schulze 2013). From the human structure of PFKFB2 presented here, it can now be seen that citrate competitively blocks F6P binding while simultaneously prohibiting ATP, or any γ-phosphate containing analogues, from binding in a catalytically active position. Inhibition studies were conducted to validate these structural observations. Non-linear regression analyses of the data supported the structural findings, showing citrate to act as a competitive inhibitor for F6P with a Ki of 80.3±6.5μM and non-competitive inhibitor for ATP with a Kis of 50.23±8.0μM and Kii of 58.75±9.9μM, as shown in the double-reciprocal plot (Figure 4) and summarized in Table 2. It was well accepted that PFKFB3 catalyzes the reaction of Fru-2,6-P2 synthesis with ordered bindings of ATP first and then Fru-6-P. Because citrate can bind to both the free enzyme (E) and the ATP bound enzyme (E•ATP), citrate is a non-competitive inhibitor. The noncompetitive inhibition agrees with the observation that citrate influences but does not completely block ATP from binding.
Figure 4.
2-Kase inhibition by Citrate. (A) Double reciprocal plot with F6P as the variable substrate showing competitive inhibition, where v=Vmax[S]/{Km(1+[I]/Kis)+[S]} (B) Double reciprocal plot with ATP as the variable substrate showing non-competitive inhibition, where v=Vmax[S]/{Km(1+[I]/Kis)+[S](1+[I]/Kii)}.
Table 2.
Citrate inhibition properties on wild-type and mutant PFKFB2.
| Human PFKFB2 | KmATP (μM) | KmF6P (μM) | kcat (s−1) | Variable Substrate | Fixed Substrate | Citrate Kis (μM) | Citrate Kii (μM) | Inhibition Type |
|---|---|---|---|---|---|---|---|---|
| Wild-Type | 38.8±5.3 | 80.3±7.6 | 0.17±0.053 | ATP | F6P | 50.23±8.0 | 58.75±9.9 | NC |
| F6P | ATP | 80.03±6.5 | C | |||||
| Y428A | 40.7±4.2 | 13.8±4.3 | 1.5±0.039 | ATP | F6P | 2.43±1.0 | 3.05±1.4 | NC |
| F6P | ATP | 67.30±4.2 | C |
Fixed Substrates: Concentrations of ATP and Fru-6-P were kept as near saturating 250μM.
Inhibition: NC, noncompetitive; C, competitive
To validate biological significance of the citrate binding and the consequentially altered binding of ATP, a site-directed mutant, Y428A, the π-π stack provider for the altered ATP binding mode was mutated to alanine, was prepared and its kinetic properties were analyzed in comparison to the wild-type, as shown in Table 2. The Y428A mutant PFKFB2 exhibited kinetic properties, which are uniquely different from those of the wild type. Although the change in Km for ATP is modest, a 6-fold increase in its affinity for Fru-6-P and a near 10-fold increase in its kcat were observed. The activity increase was probably driven by an increased affinity for Fru6-P, although a notable 20-fold increase in affinity for the inhibitor, citrate, from 50.2 μM to 2.4 μM was also observed as counteract. This difference is likely attributable to a reduction in the non-catalytic, inhibitory binding of ATP in response to the lost π-π stacking interactions between phenyl ring and the adenine base of the inhibitory ATP binding.
The apparent differences in kinetic properties between wild-type and the mutant strongly suggest that Tyr428 makes significant contributions to citrate inhibition of 2-Kase by allowing ATP to bind in a non-catalytic, inhibitory fashion. As a consequence, citrate is not only an apparent competitive inhibitor against Fru-6-P but also makes ATP act as an apparent inhibitor. The present study suggests that the inhibitory ATP binding significantly depends on Tyr428 mediated π-π stack interactions between the adenine base moiety of ATP and the tyrosine side chain. When these π-π stacking interactions were prevented, as with the Y428A mutant, the inhibitory binding of ATP was decreased, making the overall inhibition potency of citrate weaker. In addition, it is likely that the mutant have a difference in the conformational change required for ordered bindings of ATP and Fru-6-P, because Tyr428 is an important residue for ATP binding. As consequence, the Fru-6-P pocket is more stable such that bindings of both Fru-6-P and citrate are increased as manifested in their Km and Ki, respectively.
The suggested model is in agreement with the previously accepted model of energy metabolism within cardiac cells. It has been well established that the heart cells depend mainly on oxidation of fatty acids and ketone bodies as energy source and that glycolysis is mostly down regulated in the heart through the so called “glucose-sparing effect”. This suggests a possible model of the glucose-sparing effect. An unnecessary increase in the myocardial levels of Fru-2,6-P2, the most potent allosteric activator of the glycolysis rate-regulator protein, phosphofructokinase, is avoided by the citrate-sensitive 2-Kase domain in PFKFB2 isoform.
Highlights.
Solved the crystal structure of PFKFB2.
Structurally determined the molecular basis for citrate’s inhibition of PFKFB2.
Validated citrate’s inhibition model using enzyme kinetics.
Demonstrated that citrate binding induces ATP to bind in a non-catalytic, inhibitory fashion.
Acknowledgments
The authors would like to thank Derek L. Classert for his helpful advice regarding manuscript revisions. Additionally, the authors thank Dr. Grover Wardrop for his suggestions and advice regarding inhibition kinetics. Lastly we thank Dr. Henry Bellamy at the protein crystallography beamline at The Center for Advanced Microstructures and Devices, LSU.
Abbreviations
- PFKFB2
Heart-type 6-Phosphofructo-2-kinase/Fructose-2,6-bisphosphatase PFKFB3
- F-6-P
fructose-6-phosphate
- F-2,6-P2
fructose-2,6-bisphosphate
- AMPPNP
Adenylyl-imidodiphosphate
Footnotes
Additional Footnote:
The atomic coordinates and structure factors (code H5R5 and 5HTK for bovine and human PFKFB2, respectively) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
References
- 1.Wu C, et al. Roles for fructose-2,6-bisphosphate in the control of fuel metabolism: beyond its allosteric effects on glycolytic and gluconeogenic enzymes. Adv Enzyme Regul. 2006;46:72–88. doi: 10.1016/j.advenzreg.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.El-Maghrabi MR, Pilkis SJ. Rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase: a review of relationships between the two activities of the enzyme. J Cell Biochem. 1984;26(1):1–17. doi: 10.1002/jcb.240260102. [DOI] [PubMed] [Google Scholar]
- 3.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 4.Pilkis SJ, et al. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu Rev Biochem. 1995;64:799–835. doi: 10.1146/annurev.bi.64.070195.004055. [DOI] [PubMed] [Google Scholar]
- 5.Okar DA, et al. PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem Sci. 2001;26(1):30–5. doi: 10.1016/s0968-0004(00)01699-6. [DOI] [PubMed] [Google Scholar]
- 6.Hasemann CA, et al. The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies. Structure. 1996;4(9):1017–29. doi: 10.1016/s0969-2126(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 7.Kim SG, et al. Crystal structure of the hypoxia-inducible form of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3): a possible new target for cancer therapy. J Biol Chem. 2006;281(5):2939–44. doi: 10.1074/jbc.M511019200. [DOI] [PubMed] [Google Scholar]
- 8.Lee YH, et al. Tissue-specific structure/function differentiation of the liver isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 2003;278(1):523–30. doi: 10.1074/jbc.M209105200. [DOI] [PubMed] [Google Scholar]
- 9.Garber K. Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96(24):1805–6. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 10.Minchenko A, et al. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem. 2002;277(8):6183–7. doi: 10.1074/jbc.M110978200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SG, et al. A direct substrate-substrate interaction found in the kinase domain of the bifunctional enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Mol Biol. 2007;370(1):14–26. doi: 10.1016/j.jmb.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Walker JE, et al. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–51. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 15.Van Schaftingen E, et al. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129(1):191–5. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, et al. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–46. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 17.Yuen MH, et al. A switch in the kinase domain of rat testis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochemistry. 1999;38(38):12333–42. doi: 10.1021/bi991268+. [DOI] [PubMed] [Google Scholar]