Abstract
Heparin, a member of a family of molecules called glycosaminoglycans, is biosynthesized in mucosal mast cells. This important anticoagulant polysaccharide is primarily produced by extraction of the mast cell-rich intestinal mucosa of hogs. There is concern about our continued ability to supply sufficient heparin to support the worldwide growth of advanced medical procedures from the static population of adult hogs used as food animals. While the intestinal mucosa of adult pigs is rich in anticoagulant heparin (containing a few hundred milligrams per animal), little is known about how the content of heparin changes with animal age. Using sophisticated mass spectral analysis we discovered that heparin was largely absent from the intestinal mucosa of piglets. Moreover, while the related, nonanticoagulant heparan sulfate glycosaminoglycan was present in significant amounts we found little chondroitin sulfate E also associated with mast cells. Histological evaluation of piglet intestinal mucosa showed a very low mast cell content. Respiratory mast cells have been reported in baby pigs suggesting that there was something unique about the piglets used in the current study. These piglets were raised in the relatively clean environment of a university animal facility and treated with antibiotics over their lifetime resulting in a depleted microbiome that greatly reduced the number of mast cells and heparin content of the intestinal mucosal in these animals. Thus, from the current study it remains unclear whether the lack of intestinal mast cell-derived heparin results from the young age of these animals or their exposure to their depleted microbiome.
Keywords: antibiotics, heparin, intestine, microbiome, pig
Introduction
Heparin is a polyanionic linear polysaccharide that is commonly found together with other glycosaminoglycans (GAGs) in all animal tissues (Linhardt, 2003). Heparin has particular pharmacological importance as it is widely used as a clinical anticoagulant drug (Mulloy et al. 2016). Heparin was first prepared exactly 100 years ago by Jay McClean from canine liver (Linhardt, 1991). The commercialization of heparin was made possible when scientists at Connaught Laboratories first began producing heparin in large scale from food animal tissues such as bovine lung (Linhardt, 1991). In the 1990s, with the outbreak of bovine spongiform encephalopathy in the United Kingdom, most heparin production was moved to porcine intestine as the source material (Szajek et al. 2015). Today, porcine intestine is the world's major source for the 100 metric tons of pharmaceutical heparin produced annually (Bhaskar et al. 2012; Onishi et al. 2016). Today, pharmaceutical heparin is primarily prepared from the intestinal mucosa of adult hogs raised commercially on farms and are harvested at the slaughterhouse (Bhaskar et al. 2012).
Because of its clinical importance, heparin has been the most intensively studied GAG. These include studies of its biosynthesis (Lindahl, 1990; Esko and Selleck, 2002), structure (Li et al. 2012; Casu et al. 2015), biology (Kamhi et al. 2013; Linhardt and Toida, 2004; Stevens and Adachi, 2007; Rönnberg et al. 2012) and pharmacology (Bick et al. 2005; Mulloy et al. 2016), as well as clinical application (Bakchoul et al. 2016; Franchini et al. 2016). Heparin is biosynthesized as a proteoglycan by mast cells (Horner, 1986; Stevens and Adachi, 2007; Rönnberg et al. 2012). This biosynthesis begins in the endoplasmic reticulum where a serglycin core protein is made and a linkage region tetrasaccharide is attached to multiple serine residues of this core protein. As this nascent proteoglycan (PG) transits through the Golgi, a heparosan polysaccharide having repeating 1→4-linked β-d-glucuronic acid (GlcA) N-acetyl-α-d-glucosamine (GlcNAc) residues is extended from these linkage region tetrasaccharides and modified through the action of an N-deacetylase/N-sulfotransferase followed by C5-epimerase, 2- and 6-O-sulfotransferases and limited 3-O-sulfotransferase modification. The resulting GAG chains of the serglycin PG are rich (60–80%) in trisulfated repeating units of 1→4-linked 2-O-sulfo α-L-iduronic acid (IdoA2S) N-sulfo, 6-O-sulfo-α-D-glucosamine (GlcNS6S) residues. Heparin is closely related to heparan sulfate (Farrugia et al. 2015), while both GAGs are attached to different core proteins (i.e., heparin is attached only to serglycin and heparan sulfate is primarily attached to syndecans and glypicans), heparin and heparan sulfate are both biosynthesized in the same pathway. The heparan sulfate GAG is considerably less sulfated than heparin and is rich (>50%) in non-sulfated repeating units of 1→4-linked β-d-glucuronic acid (GlcA) α-d-N-acetylglucosamine (GlcNAc) residues. Heparan sulfate is isolated from porcine intestinal mucosa as a side-product in heparin production (Griffin et al. 1995). Mast cells sometimes contain chondroitin sulfate E attached to the core protein serglycin (Stevens et al. 1988). Chondroitin sulfate E is a more highly sulfated form of chondroitin sulfate, also biosynthesized in the Golgi by a different but similar process, containing a major repeating unit of alternating 1→4, 1→3 -linked β-d-glucuronic acid (GlcA) N-acetyl, 4-,6-di-O-sulfo-β-d-galactosamine (GalNAc4S6S).
Heparin PG is packed inside the granules of mucosal mast cells along with proteases and vasoactive amines, such as histamine (Horner, 1986; Stevens and Adachi, 2007; Rönnberg et al. 2012). When mast cells degranulate, as commonly occurs in an allergic response, the heparin is released as a GAG that has been proteolytically processed and also cleaved to smaller chain sizes through the action of lysosomal heparanase (Gong et al. 2003). The common features of tissues that are rich in heparin is that they are rich in mast cells. In mammals, such tissues include those of the lung, liver and intestine; these mast cells are believed to be important in responding to infections common in these tissues (Kamhi et al. 2013).
In the current study we examine the intestinal GAGs from baby pigs born at a breeder facility and raised in a clean university animal facility being fed formula and treated with antibiotics. This study may provide a better understanding of how an early, antibiotic-depleted microbiome impacts the amount structure of intestinal GAGs, such as heparin. Additional studies will be necessary to definitively establish whether a depleted microbiome and not the very young age of these animals is primarily responsible for the absence of intestinal mast cell-derived heparin.
Results and Discussion
Two baby pigs designated P and K were brought into the Northeastern Ohio Medical University animal facility at 3 days. From day 3 to 33 were fed milk formula and were administered a single dose of aminoglycoside antibiotic at day 4 (primarily effective against Gram negative bacteria) and a broad-spectrum beta-lactam antibiotic (effective against Gram negative and Gram positive bacteria) every three days through the duration of this study. At age 33 days, piglet K and P were killed at a weight of 3.8 and 5.2 kg, respectively. These animals were killed by an intracardiac injection of a pentobarbital sodium solution and the small intestine from each animal was recovered through dissection. Fecal matter was removed by flushing the intestines with saline solution and the mucosa lining the inside lumen of the intestine was recovered by inverting the intestine and scraping the lining with a spatula.
A simple three-step procedure was used to isolate GAGs from intestinal mucosa involving: (1) protease digestion; (2) strong anion exchange (SAX) chromatography on a spin column; and (3) methanol precipitation. This method results in the solubilization of tissue and the complete recovery of GAGs (Zhang et al. 2006). The total GAG isolated from each sample was next determined using carbazole assay for uronic acid (Bitter and Muir, 1962). The GAG content of the dried sample from piglet P (19.6 mg/g dry wt.) was very similar to that from piglet K (23.4 mg/g dry wt.)
The total GAG could be separated into the heparin/heparan sulfate family and chondroitin sulfate/hyaluronan family by selective treatment with polysaccharide lyases (Linhardt, 1994). Intact heparin/heparan sulfate GAGs could be prepared by treatment of a portion of the total GAG mixture with chondroitin ABC lyase, which converts the chondroitin sulfates/hyaluronan into disaccharide and tetrasaccharide products that can be removed from the heparin/heparan sulfate GAGs using a 3 kDa molecular weight cut-off (MWCO) spin column. Similarly, intact chondroitin sulfate/hyaluronan GAGs could be prepared by treatment of a portion of the total GAG mixture with a mixture of heparin lyases, which converts the heparin/heparan sulfate into disaccharide and tetrasaccharide products that can be removed from the chondroitin sulfate/hyaluronan GAGs using a 3 kDa MWCO spin column.
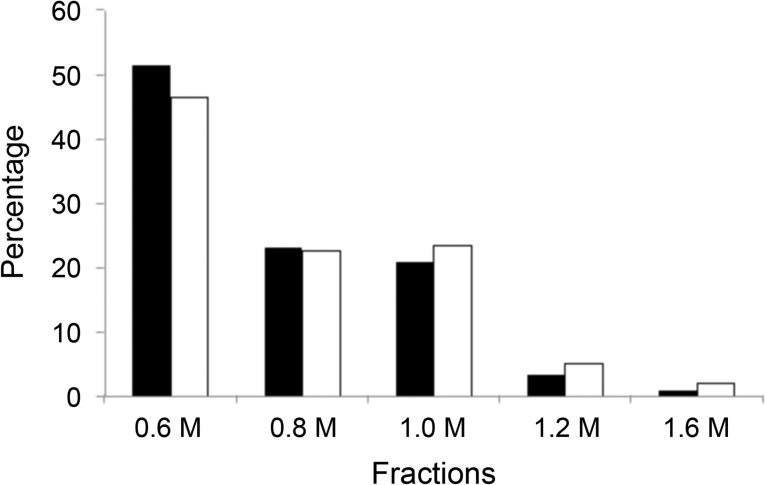
The same SAX chromatography step can be repeated to fractionate the intact heparin/heparan sulfate and chondroitin sulfate/hyaluronan GAGs into chains having low, intermediate and high levels of sulfation (Zhao et al. 2012). For example heparan sulfate with no sulfation can be eluted by washing the column with 0.6 M sodium chloride, heparan sulfate with low sulfation can be eluted by washing the column with 0.8–1.0 M sodium chloride and heparin/heparan sulfate with high sulfation could be completely eluted with 1.2–1.6 M sodium chloride, respectively. The results for P and K were very similar showing that almost 50% of total HS of both P and K were eluted in 0.6 M sodium chloride, only 4.5% of P and 7.3% of K were eluted in 1.2–1.6 M sodium chloride (Figure 1).
Fig. 1.
Percentage distribution (wt%) of HS eluting on SAX chromatography through stepwise washing at various sodium chloride concentrations ranging from 0.6 to 1.6 M. The dark bar corresponds to HS fractions from P and the open bar corresponds to HS fractions from B.
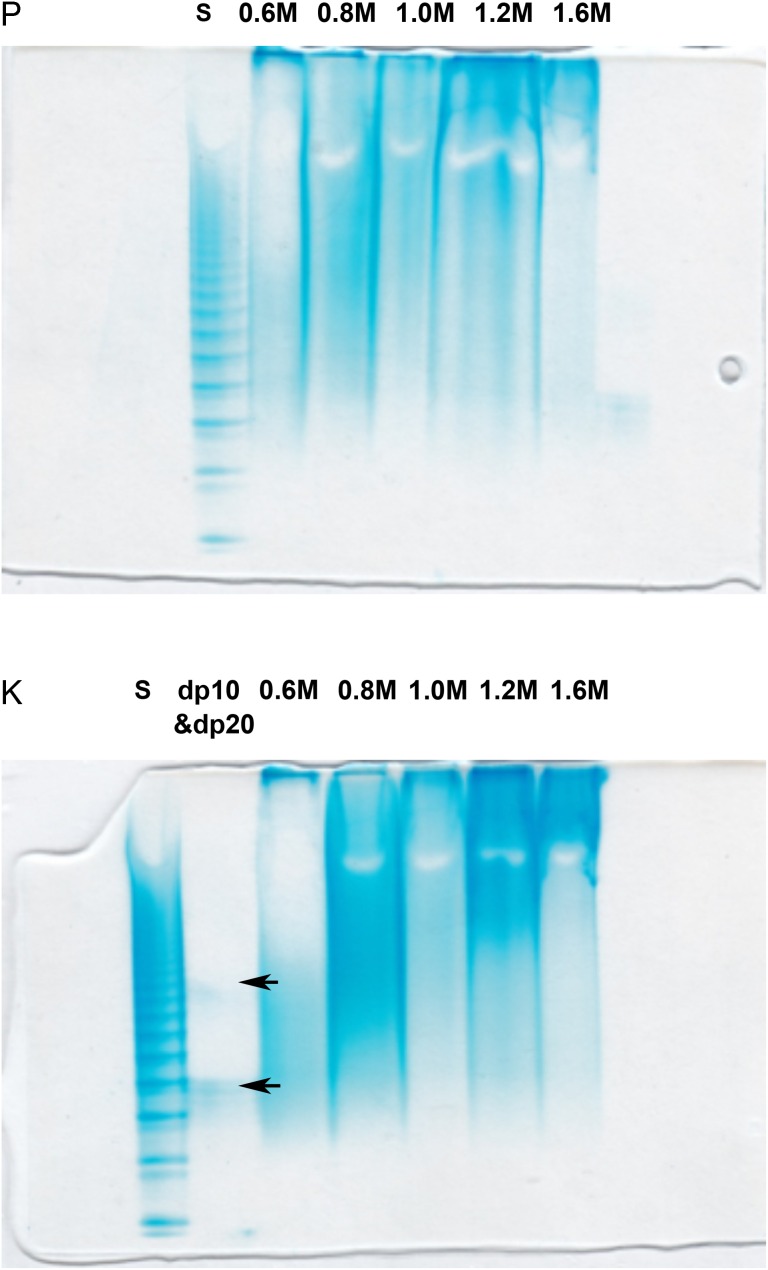
Heparin/heparan sulfate with different sulfation separated by stepwise method from P and K were analyzed by using polyacrylamide gel electrophoresis (PAGE) with Alcian blue staining. PAGE analysis established that heparin/heparan sulfate present in both fraction and showed a broad band of expected polydispersity and the average molecular weight (MWavg). Again, the results for P and K were very similar. The MWavg of 0.6 M sodium chloride eluted fraction of P was 6.6 kDa, 0.8 M was 11 kDa, 1.0 M was 15 kDa, 1.2 M was 17 kDa, 1.6 M was 18 kDa. Similar results were found in K samples as showed in Figure 2.
Fig. 2.
PAGE analysis using 15% resolving gel analysis of heparin/heparan sulfate fractions. The top gel shows fractions obtained from P and the bottom gel shows fractions obtained from K. The samples loaded in the lanes in both gels are labeled. S corresponds to a ladder of heparin oligosaccharide standards of known molecular weights, prepared enzymatically from bovine lung heparin (Edens et al. 1992). The sizes of the bands in this ladder were aligned using structurally defined oligosaccharides of degree of polymerization (dp)10 and dp20 indicated with black arrows shown in the lower gel (Pervin et al. 1995). The fractions were analyzed in the remaining lanes of both gels and are labeled as 0.6, 0.8, 1.0, 1.2 and 1.6 M corresponding to their elution described in Figure 1. This figure is available in black and white in print and in color at Glycobiology online.
An increased number of sulfate groups can impact molecular shape. A universal calibration method for gel permeation chromatography–high performance liquid chromatography (GPC–HPLC) measurement of the relative molecular mass properties of the most abundance three fractions of HS of each sample was further investigated using heparin as standard. The HPLC chromatography and calculation results are shown in Table I. The molecular weights determined by GPC–HPLC are similar to those estimated by PAGE analysis.
Table I.
Molecular Weight Analysis of heparin/heparan sulfate GAG component measured by HPLC-GPC.
| P | K | |||||
|---|---|---|---|---|---|---|
| 0.6 M | 0.8 M | 1.0 M | 0.6 M | 0.8 M | 1.0 M | |
| Mw (kDa) | 7.4 ± 0.1a | 12.0 ± 0.1 | 14.3 ± 0.3 | 7.4 ± 0.2 | 12.6 ± 0.2 | 13.7 ± 0.2 |
| Mn (kDa) | 6.9 ± 0.2 | 10.5 ± 0.1 | 12.5 ± 0.2 | 7.0 ± 0.1 | 10.9 ± 0.1 | 11.9 ± 0.3 |
| P | 1.07 | 1.13 | 1.14 | 1.06 | 1.15 | 1.16 |
aValues are mean from triplicate experiments and the standard deviations are shown.
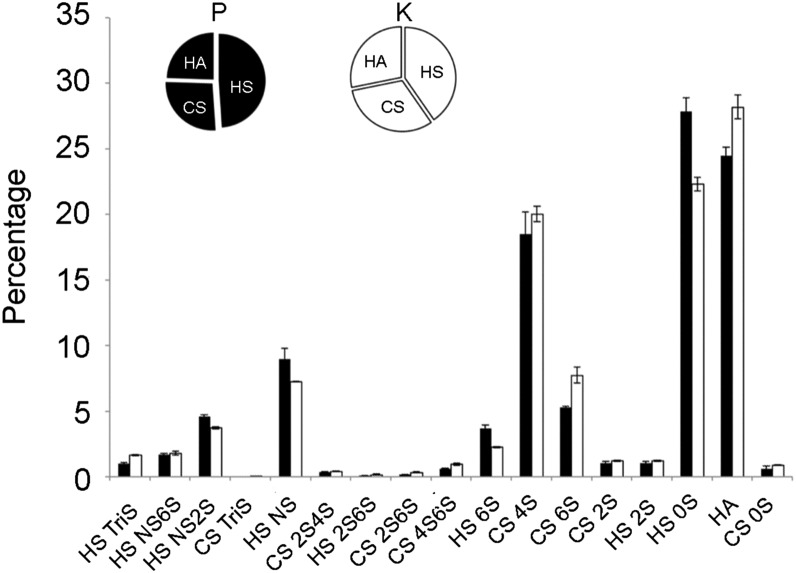
Compositional analysis of disaccharides gives important structural information and is an efficient method for measuring the variation of GAG structures. GAGs are enzymatically digested with heparin lyase I, II, III and chondroitin lyase ABC to obtain heparan sulfate, CS and disaccharides, these disaccharides were AMAC-labeled by reductive amination and analyzed by reversed-phase HPLC-MS/MS. The results for P and K were again very similar. Heparin/heparan sulfate was the most abundant GAG in both P and K samples; in P, 48.9% was heparin/heparan sulfate, 26.6% was chondroitin sulfates and hyaluronan was 24.5%, while 40.3% heparin/heparan sulfate, 31.5% chondroitin sulfates and 28.2% hyaluronan was found in K (Figure 3). Heparin/heparan sulfate, when treated with heparin lyases, affords disaccharides comprised primarily of ΔUA is 4-deoxy-β-l-threo-hex-4-enopyranosiduronic acid (ΔUA)-GlcNAc (Di-0S) (27.8%) and ΔUA-GlcNS (where S is sulfo) (Di-NS), (9.0%) in P and 22.3% Di-0S, 7.2% Di-NS in K, as shown in Figure 3.
Fig. 3.
Comparison of the GAG components and their disaccharide composition from piglets P and K as determined by HPLC-MS/MS. The pie charts show the proportions of HS, CS and HA from P (black) and K (white). The bar graph shows the mol% of the 17 disaccharides obtained following enzymatic treatment of P (black) and K (white).
After chondroitin lyase ABC digestion, heparin/heparan sulfate was acquired and separated by stepwise method, the disaccharides composition of fraction in each elution were next determined. The results showed that the 0 S disaccharide made up the vast majority of HS in each fraction (Table II). With an increasing of salt concentration, the content of ΔUA2S-GlcNS6S (Di-TriS), ΔUA-GlcNS6S (Di-NS6S) and ΔUA2S-GlcNAc6S (Di-UA2S6S) increased in the HS fractions, but Di-0S was still the most abundant disaccharide formed on lyase treatment of each HS fraction.
Table II.
Composition of HS and CS GAGs fractionated based on charge density.
| Sample | Ma | HSb | CSc | HS/CS | ||||||
| TrisHS | NS6SHS | 2S6SHS | 0SHS | 4S6SCS | 4SCS | 6SCS | 0SCS | mgd | ||
| P | 0.6 | 0.1 ± 0.02e | 0.5 ± 0.03 | 0 | 92.2 ± 0.91 | 0.3 ± 0.06 | 95.9 ± 1.05 | 3.9 ± 0.99 | 0 | 2.5/0.5 |
| 0.8 | 0.7 ± 0.03 | 1.6 ± 0.07 | 0.1 ± 0.01 | 76.1 ± 1.38 | 1.2 ± 0.22 | 73.4 ± 0.36 | 11.8 ± 0.59 | 12.8 ± 0.71 | 1.1/0.5 | |
| 1.0 | 1.9 ± 0.21 | 2.1 ± 0.31 | 0.1 ± 0.01 | 70.2 ± 1.43 | 3.3 ± 0.42 | 79.9 ± 1.06 | 10.1 ± 0.20 | 3.1 ± 0.34 | 1.0/0.1 | |
| 1.2 | 3.8 ± 0.06 | 4.8 ± 0.02 | 0.4 ± 0.06 | 55.2 ± 0.53 | 2.5 ± 0.65 | 82.8 ± 1.62 | 11.2 ± 1.36 | 0.8 ± 0.08 | 0.2/0.1 | |
| 1.6 | 1.5 ± 0.49 | 3.8 ± 0.02 | 4.1 ± 0.09 | 62.5 ± 2.14 | 6.6 ± 1.78 | 81.0 ± 1.74 | 12.0 ± 0.02 | 0.5 ± 0.06 | 0.1/0 | |
| K | 0.6 | 0 | 0.5 ± 0.15 | 0 | 90.7 ± 1.12 | 0.2 ± 0.09 | 93.4 ± 0.69 | 6.3 ± 0.57 | 0 | 2.0/0.6 |
| 0.8 | 1.4 ± 0.08 | 2.0 ± 0.14 | 0.1 ± 0.01 | 73.4 ± 1.65 | 1.8 ± 0.43 | 75.3 ± 1.32 | 9.5 ± 0.71 | 12.1 ± 1.35 | 1.0/0.5 | |
| 1.0 | 3.2 ± 0.19 | 3.3 ± 0.29 | 0.2 ± 0.06 | 61.1 ± 1.28 | 2.7 ± 1.34 | 71.9 ± 1.83 | 17.0 ± 1.33 | 6.0 ± 0.55 | 1.0/0.2 | |
| 1.2 | 7.3 ± 1.60 | 7.6 ± 0.46 | 1.4 ± 0.22 | 50.0 ± 1.28 | 4.0 ± 1.34 | 82.3 ± 2.73 | 8.2 ± 0.95 | 1.1 ± 0.25 | 0.2/0.1 | |
| 1.6 | 9.3 ± 0.70 | 8.3 ± 0.78 | 5.2 ± 1.01 | 50.6 ± 1.90 | 11.2 ± 1.58 | 74.6 ± 3.41 | 9.8 ± 1.50 | 0.6 ± .17 | 0.1/0 | |
aSodium chloride elution step strength.
bCalculated based on HS disaccharides corresponding 100%.
cCalculated based on CS disaccharides corresponding 100%.
dThe total amount of HS/CS isolated in each elution fraction from piglet P and K is shown.
eValues are mean ± SD from triplicate experiments.
Chondroitin sulfate E (GlcA-GalNAc4S6S) is a highly sulfated CS. It is known to serve a similar function as heparin within mast cell granules. Based on this understanding, we isolated the CS and examined its structure by disaccharide analysis. The results showed that ΔUA-GalNAc4S (Di-4S) was the most abundance disaccharide formed on lyase treatment of CS from both P and K (Table II). CS-E concentration increased in fractions eluted with high sodium chloride concentrations, and reached approximately 10% in the 0.6 M NaCl fraction.
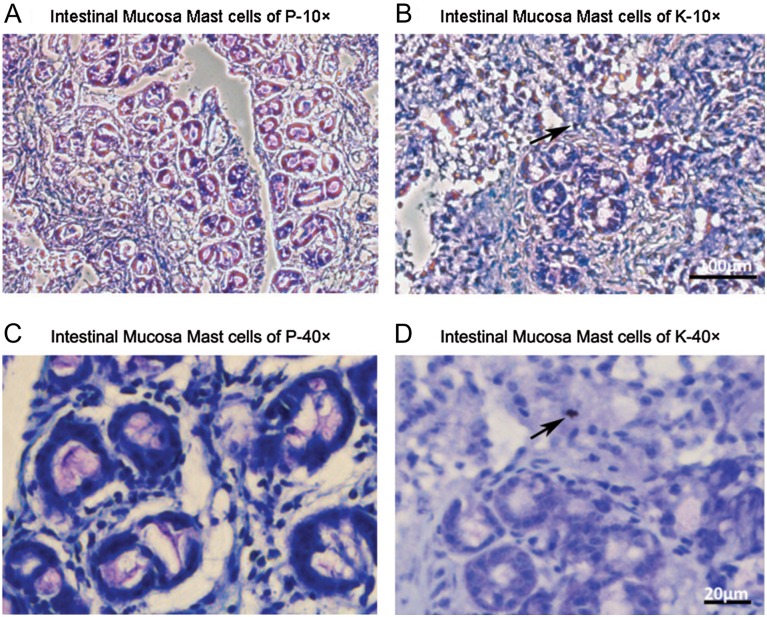
Mast cells are found in the connective tissue and their cytoplasm contains granules composed of heparin and histamine (Horner, 1986; Stevens and Adachi, 2007; Rönnberg et al. 2012). In human lung mast cells, in addition to heparin, also contain chondroitin sulfate E (Stevens et al. 1988). Approximately 25% of the chondroitin sulfate disaccharide units were chondroitin sulfate E type that on chondroitin lyase treatment results in ΔUA-GalNAc4S6S (Di-diSE). In toluidine blue staining, mast cells should stain red-purple and the background stain blue (Leclere et al. 2006; Rieger et al. 2013), the intestinal mucosa from P, we did not find any mast cells while in the intestinal mucosa from K, we found a single mast cell on the stained slide (Figure 4).
Fig. 4.
Micrographs of intestinal mucosa of piglets stained with toluidine blue. Mast cells are stained violet color with blue background (details in methods and material). (A and B) Intestinal sections of P and K, respectively, at 10× (Bar = 100 μm). (C and D) Intestinal sections of P and K, respectively, at 40× (Bar = 20 μm). Mast cells were not found in the P samples, while only one mast cell, indicated with black arrows, was present in the K samples. n = 4–5 sections per sample. This figure is available in black and white in print and in color at Glycobiology online.
The chemical, enzymatic and histological experiments described clearly demonstrate the absence of significant amounts of heparin in the intestinal mucosa of the piglets used this study. These results were surprising, as based on the heparin content of ~300 mg (Gunay and Linhardt 1999) from the intestinal mucosa of an adult pig weighing ~130 kg, we expected to find 9–12 mg of heparin in these 4–5 kg piglets. Instead, based on the content of heparin's characteristic trisulfated disaccharide, determined by disaccharide compositional analysis, we estimate that the intestinal mucosa of these piglets contain <53 μg of heparin. The presence of significant amounts of heparan sulfate (~2 mg/piglet), which shares a common biosynthetic pathway with heparin, clearly indicates that the heparin biosynthetic pathway is operating. We next looked at the total amount of chondroitin sulfate E, based on its characteristic 4S6SCS disaccharide, as mast cells have been found to contain this GAG chain in place of heparin on the serglycin core protein found in mast cell granules. Again we found very little chondroitin sulfate E, <100 μg in the intestinal mucosa of each piglet. Finally, we histologically examined retained frozen samples of intestinal mucosa for the presence of mast cells to confirm this surprising finding. We observed no mast cells in piglet P and only a single mast cell in piglet K. In contrast the intestinal mucosa of adult pigs is rich in mast cells with each stained field showing ten or more mast cells (Rieger et al. 2013). While it is possible that the absence of mast cells, heparin and chondroitin sulfate E in the piglets examined is the result of their young age, a previous study found mast cells in the respiratory track of new born and recently weaned pigs (Castillo Pena et al. 2009). While some developmental differences in the respiratory tract distributions of porcine mast cells were observed piglets clearly showed that mast cells were histologically present (Castillo Pena et al. 2009). One issue that we had not considered at the initiation of this study was that the early, antibiotic-depleted microbiome of the piglets might result in reduced mast cells and mast cell heparin (and/or chondroitin sulfate E). In mammalian lung, liver and intestinal tissues mast cells are believed to be important in responding to infections, ones caused by parasites and bacteria, common in these tissues (Kamhi et al. 2013). It is also well established that intestinal mast cells can proliferate in response to infection with parasites (Stevens et al. 1986). As far as we could tell, the piglets studied were parasite free and their routine treatment with antibiotics in the relatively clean environment of a university animal facility would certainly reduce the content and diversity of their microbiome. Further studies will be required to better understand the impact of microbiome content and diversity on intestinal mast cell content and its impact on the yield of heparin from hogs.
Materials and methods
Materials
Two baby pigs (Sus scrofa domesticus), age 3 days, were obtained from the Shupp Farm in (Wooster, Ohio). Actinase E was obtained from Kaken Biochemicals (Tokyo, Japan); Vivapure Q Maxi H spin columns were acquired from Sartoriou Stedim Biotech (Bohemia, NY). Recombinant Flavobacterial heparin lyases I, II and III, and chondroitin lyase ABC from Proteus vulgaris were expressed in our laboratory using Escherichia coli strains provided by Professor Jian Liu (College of Pharmacy, University of North Carolina). The 2-aminoacridone (AMAC), sodium cyanoborohydride (NaCNBH4), urea and 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate (CHAPS) were obtained from Sigma-Aldrich (St. Louis, MO).
Unsaturated disaccharide standards of HS/HP (Di-0S, ΔUA-GlcNAc; Di-NS, ΔUA-GlcNS; Di-6S, ΔUA-GlcNAc6S; Di-UA2S, ΔUA2S-GlcNAc; Di-UA2SNS, ΔUA2S-GlcNS; Di-NS6S, ΔUA-GlcNS6S; Di-UA2S6S, ΔUA2S-GlcNAc6S; Di-TriS, ΔUA2S-GlcNS6S) and unsaturated disaccharide standards of CS/DS (Di-0S, ΔUA-GalNAc; Di-4S, ΔUA-GalNAc4S; Di-6S, ΔUA-GalNAc6S; Di-UA2S, ΔUA2S-GalNAc; Di-diSB, ΔUA2S-GalNAc4S; Di-diSD, ΔUA2S-GalNAc6S; Di-diSE, ΔUA-GalNAc4S6S; Di-triS, ΔUA2S-GalNAc4S6S) were purchased from Seikagaku Corp (Japan).
Recovery of intestinal mucosa
The two baby pigs, designated K and P, were transported to Northeastern Ohio Medical University at three days of age. They were fed by their mother for the first three days of life and then bottle fed Solustart II, pig milk replacer formula, from Land O’ Lakes Animal Milk Products Company (St. Paul, MN) for the next 30 days. As far as we know, these animals were parasite-free. Each piglet received 5 mg of gentamicin at 4 days of age and received Excede® for veterinary use (Zoetis, Florham Park, NJ) 5 mg/kg every 3 days for the duration of the study. At age 33 days the piglets were killed and the mucosa was collected from their small intestines by first removing fecal matter, then inverting the intestine and scraping the intestine to collect the mucosa. The weight of piglet K and piglet P were 3.8 and 5.2 kg, respectively, at time of collection. Animals were housed in the NEOMED Comparative Medicine Unit. All protocols were approved by the NEOMED Institutional Animal Care and Use Committee (13–011). The two samples of intestinal mucosa were frozen at −80°C for processing.
Recovery of total GAGs from intestinal mucosa
Two samples of frozen intestinal mucosa from piglet K and P were processed separately to recover GAGs for analysis. A small amount (10 mg) of each sample frozen intestinal mucosa was retained for histology. The remaining mucosa samples from K and P were each weighed (4.3 and 7.0 g, respectively), freeze-dried and crushed into homogenized powders (500 and 887 mg, respectively). Dried samples were dissolved in 5 mL water and proteolyzed at 55°C with 10 mg/mL actinase E for 18 h, and freeze-dried. A 5 mL solution of 8 M urea containing 2 wt% CHAPS was added to each sample. The resulting cloudy solutions were clarified by being passed through a 0.2-µm MWCO membrane syringe filter. The filtered samples were loaded onto a Vivapure MAXI Q H spin column which pre-equilibrated with 3 mL of 8 M urea containing 2% CHAPS. The spin columns were then washed five-times with 5 mL 0.2 M NaCl. The GAG was then eluted from the spin column with washed three-1 mL volumes of 16% NaCl. These three-washes from each sample were combined and methanol was added to afford an 80 vol% aq. methanol solution that was stored overnight in an explosion-proof refrigerator at 4°C, resulting in precipitate that was recovered by centrifugation with 2500 × g for 30 min. The precipitate from K (11 mg) and P (17 mg) were dissolved in 0.5 mL of water for further analysis.
Isolation of heparin/heparan sulfate from the total GAG mixture
A portion of each GAG sample (~0.1 mg) was completely digested (repeated digestion failed to give additional product) with chondroitinase ABC (10 milliunits) at 37°C overnight to isolate heparin/heparan sulfate. The heparin/heparan sulfate components from K and P were purified with a Vivapure MINI Q H spin column and centrifugal filtration using a 3-kDa MWCO spin column. The total GAGs and heparin/heparan sulfate were quantified by a carbazole assay (Bitter and Muir, 1962).
Charge fractionation of heparin/heparan sulfate fractions
Heparin and heparan sulfate, having different levels of sulfation (Griffin et al. 1995), can be fractionated using SAX spin columns eluted in a stepwise fashion with various concentrations of sodium chloride (Zhao et al. 2012). The heparin/heparan sulfate samples were bound on the SAX columns, which had been pre-equilibrated with water, by centrifugation at 700 × g for 5 min. After the columns were washed twice with 0.2 M sodium chloride, heparin/heparan sulfate components with different levels of sulfation were eluted by washing with three-one mL volumes of 0.6, 0.8, 1.0, 1.2 and 1.6 M aqueous sodium chloride.
Charge fractionation of chondroitin sulfate fractions
Chondroitin sulfates, prepared by treating GAG mixture with heparin lyase I, II and III were also fractionated based on charge. SAX spin columns were loaded with the chondroitin sulfate mixture and fractionated in a stepwise fashion with the same salt concentrations used in heparin/heparan sulfate separation to obtain various chondroitin sulfate fractions for characterization.
Size analysis using polyacrylamide gel electrophoresis
PAGE was used to determine the average molecular weight (MWavg) and polydispersity of each fraction of the heparin/heparan sulfate samples. Samples were separated by a 15% total acrylamide (15% T) resolving gel containing 14.08% (w/v) acrylamide, 0.92% (w/v) N, N′-methylene-bis-acrylamide and 5% (w/v) sucrose. All monomer solutions were prepared in resolving buffer (0.1 M boric acid, 0.1 M Tris, 0.01 M disodium EDTA, pH 8.3). Stacking gel monomer solution was prepared in resolving buffer with the pH adjusted to 6.3 using HCl, and it contained 4.75% (w/v) acrylamide and 0.25% (w/v) N, N′-methylene-bis-acrylamide. A 10 cm × 7 mm diameter resolving gel column was cast from 4 mL of 15% T monomer solution containing 4 μL TEMED and 12 μL 10% APS. A stacking gel was cast from 1 mL stacking gel monomer solution containing 1 μL TEMED and 30 μL 10% APS. Phenol red dye was added to the sample for visualization of the ion front during electrophoresis. In each lane, ~5 µg of sample was subjected to electrophoresis. A standard composed of a mixture of heparin oligosaccharides with known molecular weights, prepared enzymatically from bovine lung heparin (Edens et al. 1992). The gel was visualized with alcian blue staining and then digitized with UN-Scan-it (Silk Scientific), and MWavg and polydispersity were calculated (Edens et al. 1992).
Molecular weight determination by GPC
HPLC-GPC was also used to determine the molecular weight and polydispersity of these samples. A guard column TSK SWXL 6 mm × 4 cm, 7 µm diameter was used to protect two analytical columns: TSK G4000 SWXL 7.8 mm × 30 cm, 8 µm in series with TSK G3000SWXL 7.8 mm × 30 cm, 5 µm (Tosoh Corporation, Tokyo, Japan). These columns were connected to an HPLC system consisting of Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector (Shimadzu, Kyoto, Japan). The mobile phase was 0.1 M ammonium acetate with 0.02% (w/v) sodium azide. Columns and refractive index detector were maintained at 30°C using an Eppendorf column heater (Eppendorf, Hamburg, Germany). The sample injection volume was 20 µL with concentration at 5 mg/mL. Flow rate was 0.6 mL/min. For molecular weight determination, USP Heparin Sodium Molecular Weight Calibrate RS was used to confirm system suitability (USP monograph, 2014).
Disaccharide analysis
The GAG substrate (1 µg/µL) was treated with a mixture of heparin lyase I, II, III (10 milliunits of each) and chondroitin lyase ABC (10 milliunit) and incubated at 37°C overnight. Heparin/heparan sulfate substrate was treated with heparin lyase I, II and III while chondroitin substrate was treated with chondroitin lyase ABC. The products were filtered using 3 K MWCO spin column and washed twice with distilled water. The freeze-dried samples were AMAC-labeled by adding 10 µL of 0.1 M AMAC in dimethylsulfoxide/acetic acid (17/3, v/v) incubating at room temperature for 10 min, followed by adding 10 µL of 1 M aqueous sodium cyanoborohydride and incubating for 1 h at 45°C. After centrifugation for 10 min at 10000 × g, each supernatant was recovered for HPLC-MS/MS. A mixture containing all 17 disaccharide standards was similarly AMAC-labeled and used as an external standard.
HPLC was performed on an Agilent 1200 LC system using an Agilent Poroshell 120 EC-C18 (2.7 µm, 3.0 × 50 mm) column. MPA was 50 mM ammonium acetate aqueous solution, and MPB was methanol. The flow rate was 300 µL/min. The concentration of MPB increased from 5% to 45% during 10 min, and then rose to 100% MPB in the following 0.2 min, and a 4 min flow of 100% MPB was applied to elute all compounds. A triple quadrupole mass spectrometry system equipped with an electrospray ionization source (Thermo Fisher Scientific, San Jose, CA) was used as detector. The online MS analysis was at the Multiple Reaction Monitoring mode.
Histological analysis of mucosa
This tissue on collection was immediately frozen at −80°C and was kept on dry ice for transportation. Frozen mucosa was defrosted immediately before being embedded in paraffin. Samples were deparaffinized and hydrated with distilled water, then stained with toluidine blue working solution for 2–3 min, washed with three changes of distilled water. Toluidine blue staining is useful for identifying mast cells (Leclere et al. 2006; Rieger et al. 2013). The stained cells were quickly dehydrated with 95% alcohol followed by two changes of 100% alcohol and cleaned by xylene solution. Mast cells were examined under light microscope (Olympus IX51) at 10× and 40×. Images were taken with an Olympus DP73 camera. Mast cells showed a violet color in a blue background.
Funding
This work was supported in part by the China scholarship council (Y.Y.). The work was also supported by Grants (R.J.L.) from the National Institutes of Health in the form of Grants HL125371 (R.J.L.), GM38060 (R.J.L.), GM090127 (R.J.L.), HL096972 (R.J.L.), HL10172 (R.J.L.) and NIH DC 009980 to R.Z.G.
Conflict of interest
None declared.
Abbreviations
GAGs, glycosaminoglycans; PG, proteoglycan; GlcA, β-D-glucuronic acid; GlcNAc, N-acetyl-α-d-glucosamine; S, sulfo; Ac, acetyl; GalNAc, β-d-galactosamine; SAX, strong anion exchange; MWCO, molecular weight cut-off; MWavg, average molecular weight; HPLC, high performance liquid chromatography; MS/MS, tandem mass spectrometry ΔUA, 4-deoxy-β-l-threo-hex-4-enopyranosiduronic acid; Di-0S, ΔUA-GlcNAc; Di-NS, ΔUA-GlcNS; Di-6S, ΔUA-GlcNAc6S; Di-UA2S, ΔUA2S-GlcNAc; Di-UA2SNS, ΔUA2S-GlcNS; Di-NS6S, ΔUA-GlcNS6S; Di-UA2S6S, ΔUA2S-GlcNAc6S; Di-TriS, ΔUA2S-GlcNS6S; Di-0S, ΔUA-GalNAc; Di-4S, ΔUA-GalNAc4S; Di-6S, ΔUA-GalNAc6S; Di-UA2S, ΔUA2S-GalNAc; Di-diSB, ΔUA2S-GalNAc4S; Di-diSD, ΔUA2S-GalNAc6S; Di-diSE, ΔUA-GalNAc4S6S; Di-triS, ΔUA2S-GalNAc4S6S; AMAC, 2-aminoacridone; CHAPS, 3-3-cholamidopropyl dimethylammonio-1-propanesulfonate; PAGE, polyacrylamide gel electrophoresis; GPC, gel permeation chromatography.
References
- Bakchoul T, Jouni R, Warkentin T E. 2016. Protamine (heparin)-induced thrombocytopenia: A review of the serological and clinical features associated with anti-protamine/heparin antibodies. J Thromb Haemost. 14:1685–1695. [DOI] [PubMed] [Google Scholar]
- Bhaskar U, Sterner E, Hickey A M, Onishi A, Zhang F, Dordick J S, Linhardt R J. 2012. Engineering of routes to heparin and related polysaccharides. Appl Microbiol Biot. 93:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick R L, Frenkel E P, Walenga J, Fareed J, Hoppensteadt D A. 2005. Unfractionated heparin, low molecular weight heparins, and pentasaccharide: Basic mechanism of actions, pharmacology, and clinical use. Hematol Oncol Clin North Am. 19:1–51. [DOI] [PubMed] [Google Scholar]
- Bitter T, Muir H M. 1962. A modified uronic acid carbazole reaction. Anal Biochem. 4:330–334. [DOI] [PubMed] [Google Scholar]
- Castillo Pena G A, Garrido Farina G I, Ochoa Uribe G, Garcia Tovar C G, Cruz Sanchez T A. 2009. Distribution of mast cells in the respiratory tract of the pig at three stages of development. J Anim Vet Adv. 8:2241–2246. [Google Scholar]
- Casu B, Naggi A, Torri G. 2015. Re-visiting the structure of heparin. Carbohydr Res. 11:60–8. [DOI] [PubMed] [Google Scholar]
- Edens R E, Al-Hakim A, Weiler J M, Rethwisch D G, Fareed J, Linhardt R J. 1992. Gradient polyacrylamide gel electrophoresis for determination of the molecular weight of heparin and low molecular weight heparin derivatives. J Pharm Sci. 81:823–827. [DOI] [PubMed] [Google Scholar]
- Esko J D, Selleck S B. 2002. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 71:435–471. [DOI] [PubMed] [Google Scholar]
- Farrugia B L, Lord M S, Melrose J, Whitelock J M. 2015. Can we produce heparin/heparan sulfate biomimetics using “mother-nature” as the gold standard. Molecules. 20:4254–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Liumbruno G M, Bonfanti C, Lippi G. 2016. The evolution of anticoagulant therapy. Blood Transfus. 14:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F, Jemth P, Escobar Galvis M L, Vlodavsky I, Horner A, Lindahl U, Li J P. 2003. Processing of macromolecular heparin by heparanase. J Biol Chem. 278:35152–35158. [DOI] [PubMed] [Google Scholar]
- Griffin C C, Linhardt R J, Van Gorp C L, Toida T, Hileman R E, Schubert R L, Brown S E. 1995. Isolation and characterization of heparan sulfate from crude porcine intestinal mucosa peptidoglycan heparin. Carbohydr Res. 276:183–197. [DOI] [PubMed] [Google Scholar]
- Gunay N S, Linhardt R J. 1999. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 25:5–16. [PubMed] [Google Scholar]
- Horner A A. 1986. Rat heparins. A study of the relative sizes and antithrombin-binding characteristics of heparin proteoglycans, chains and depolymerization products from rat adipose tissue, heart, lungs, peritoneal cavity and skin. Biochem J. 240:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi E E, Joo J, Dordick J S, Linhardt R J. 2013. Glycosaminoglycans in infectious disease. Biol Rev. 88:928–943. [DOI] [PubMed] [Google Scholar]
- Leclere M L, Desnoyers M, Beauchamp G, Lavoie J P. 2006. Comparison of four staining methods for detection of mast cells in equine bronchoalveolar lavage fluid. J Vet Intern Med. 20:377–381. [DOI] [PubMed] [Google Scholar]
- Li L, Ly M, Linhardt R J. 2012. Proteoglycan sequence. Mol Biosyst. 8:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U. 1990. Biosynthesis of heparin. Biochem Soc Trans. 18:803–805. [DOI] [PubMed] [Google Scholar]
- Linhardt R J. 2003. Heparin: Structure and activity. J Med Chem. 46:2551–2554. [DOI] [PubMed] [Google Scholar]
- Linhardt R J. 1991. Heparin: An important drug enters its seventh decade. Chem Ind. 2:45–50. [Google Scholar]
- Linhardt R J. 1994. Analysis of glycosaminoglycans with polysaccharide lyases. In: Varki A, editor. Current Protocols in Molecular Biology, Analysis of Glycoconjugates. Boston, MA: Wiley Interscience; V 2, 17.13.17-17.13.32. [DOI] [PubMed] [Google Scholar]
- Linhardt R J, Toida T. 2004. Role of glycosaminoglycans in cellular communication. Account Chem Res. 37:431–438. [DOI] [PubMed] [Google Scholar]
- Mulloy B, Hogwood J, Gray E, Lever R, Page C P. 2016. Pharmacology of heparin and related drugs. Pharmacol Rev. 68:76–141. [DOI] [PubMed] [Google Scholar]
- Onishi A, Ange K, Dordick J S, Linhardt R J. 2016. Heparin and anticoagulation: Glycosaminoglycans and related disorders. Front Biosci. 21:1372–1392. [DOI] [PubMed] [Google Scholar]
- Pervin A, Gallo C, Jandik K, Han X-J, Linhardt R J. 1995. Preparation and structural characterization of large heparin-derived oligosaccharides. Glycobiology. 5:83–95. [DOI] [PubMed] [Google Scholar]
- Rieger J, Twardziok S, Huenigen H, Hirschberg R M, Plendl J. 2013. Porcine intestinal mast cells. Evaluation of different fixatives for histochemical staining techniques considering tissue shrinkage. Eur J Histochem. 57:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg E, Melo F R, Pejler G. 2012. Mast cell proteoglycans. J Histochem Cytochem. 60:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R L, Adachi R. 2007. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 217:155–167. [DOI] [PubMed] [Google Scholar]
- Stevens R L, Lee T D, Seldin D C, Austen K F, Befus A D, Bienenstock J. 1986. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 137:291–295. [PubMed] [Google Scholar]
- Stevens R L, Fox C C, Lichtenstein L M, Austen K F. 1988. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc Natl Acad Sci USA. 85:2284–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajek A, Mulloy B, Keire D, Chase C, Al-Hakim A, Cairatti D, Gray E, Hogwood J, Morris T, Mourão P, et al. . 2015. Diversifying the global heparin supply chain: Reintroduction of bovine heparin in the United States. Pharm Tech. 39:11. [Google Scholar]
- United States Pharmacopeial Convention, USP37 2014. Official monograph, heparin sodium, D In: Molecular Weight Determinations Rockville, MD, United States Pharmacopeial Convention; p. 3224. [Google Scholar]
- Zhang F, Sun P, Munoz E, Chi L, Sakai S, Toida T, Zhang H, Mousa S, Linhardt R J. 2006. Microscale isolation and analysis of heparin from plasma using an anion exchange spin column. Anal Biochem. 353:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang B, Dutta P, Gasmili L, Zhang F, Linhardt R J. 2012. Cell-based microscale isolation of glycoaminoglycans for glycomics study. J Carbohyd Chem. 31:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]