Abstract
Spinal metabotropic serotonin receptors encode transient experiences into long-lasting changes in motor behavior (i.e. motor plasticity). While interactions between serotonin receptor subtypes are known to regulate plasticity, the significance of molecular divergence in downstream G protein coupled receptor signaling is not well understood. Here we tested the hypothesis that distinct cAMP dependent signaling pathways differentially regulate serotonin-induced phrenic motor facilitation (pMF); a well-studied model of spinal motor plasticity. Specifically, we studied the capacity of cAMP-dependent protein kinase A (PKA) and exchange protein activated by cAMP (EPAC) to regulate 5-HT2A receptor-induced pMF within adult male rats. Although spinal PKA, EPAC and 5-HT2A each elicit pMF when activated alone, concurrent PKA and 5-HT2A activation interact via mutual inhibition thereby blocking pMF expression. Conversely, concurrent EPAC and 5-HT2A activation enhance pMF expression reflecting additive contributions from both mechanisms. Thus, we demonstrate that distinct downstream cAMP signaling pathways enable differential regulation of 5-HT2A-induced pMF. Conditional activation of independent signaling mechanisms may explain experience amendable changes in plasticity expression (i.e. metaplasticity), an emerging concept thought to enable flexible motor control within the adult central nervous system.
Keywords: motor neuron, phrenic, spinal cord, respiratory plasticity, 5-HT7 receptor, 5-HT2 receptor, exchange protein activated by cAMP, protein kinase A, EPAC, PKA
Introduction
Serotonin elicits long-lasting motor plasticity via G protein-coupled receptors (GPCRs; Brunelli et al., 1976; Randić et al., 1993; Clark and Kandel, 1993), with different receptor subtypes giving rise to plasticity via independent signaling pathways (reviewed in Barbas et al., 2003). Concurrent activation of multiple serotonin receptor subtypes reveals inhibitory inter-receptor cross-talk interactions thereby regulating serotonin-induced plasticity (Seol et al., 2007; Treviño et al., 2012; Hoffman et al., 2013; MacFarlane et al., 2014). Although signaling pathways downstream from individual GPCRs are known to diverge, it is not known how signaling divergence differentially impacts serotonin-induced plasticity.
Serotonin-induced motor plasticity is a major feature of the neural system controlling breathing (Mitchell and Johnson, 2003; Feldman et al., 2003). For example, serotonin elicits plasticity in respiratory defense reflexes of gastropod mollusks (Glanzman et al., 1989; Macket et al., 1989; Levy and Susswein, 1993), and enhances spinal respiratory motor control in mammals (Bach and Mitchell, 1996; Baker-Herman and Mitchell, 2002). In rats, selective activation of spinal Gq-coupled serotonin 2A receptors (5-HT2A; MacFarlane et al., 2011) or Gs-coupled serotonin 7 receptors (5-HT7; Hoffman and Mitchell, 2011) elicits long-lasting phrenic motor facilitation (pMF). When multiple spinal serotonin receptors are stimulated with non-specific serotonin, pMF expression exhibits a bell-shaped dose response curve; low serotonin doses elicit pMF through Gq associated 5-HT2 receptors, but high serotonin doses elicit pMF only when spinal Gs associated 5-HT7 receptors are blocked (Macfarlane and Mitchell, 2009). Thus, there is a poorly understood interplay between Gq and Gs-coupled serotonin receptors within the spinal motor network regulating the expression of serotonin-induced pMF (MacFarlane and Mitchell, 2009; Hoffman et al., 2013).
Due to differences in cAMP binding affinity (Dostmann and Taylor, 1991; Ponsioen et al., 2004; Zhou et al., 2016), cell type and sub-cellular distribution (Seino and Shibasaki, 2005), cAMP can independently activate cAMP-dependent protein kinase A (PKA) versus exchange protein activated by cAMP (EPAC), thus enabling distinct functional outcomes from Gs-coupled receptor signaling. For example, netrin-1 receptors differentially activate PKA and EPAC to dynamically regulate spinal axonal growth (Murray et al., 2009). While netrin-1 induced, cAMP-dependent, EPAC signaling promotes growth cone extension early in development, PKA signaling predominates later in development switching netrin-1/cAMP effects to growth cone repulsion. Thus, EPAC and PKA underlie contrasting time-specific and context-specific functions within the developing nervous system.
Here, we tested the hypothesis that EPAC and PKA differentially regulate serotonin-induced pMF. Using recently available, highly selective, drugs to manipulate spinal cAMP signaling (Table 1), we investigated the functional significance of distinct downstream cAMP signaling mechanisms on 5-HT2A induced pMF. We demonstrate that whereas PKA constrains 5-HT2A induced pMF, EPAC and 5-HT2A co-activation exert additive effects, enhancing pMF expression. Thus, cAMP signaling differentially regulates serotonin-induced pMF. This is the first demonstration that downstream signaling from a single intracellular molecule enables differential regulation of plasticity within the adult nervous system. While the present studies do not conclusively confirm that downstream cAMP signaling divergence occurs within a single cell, or cell type (i.e. neuron vs astrocyte vs glia), these observations provide evidence that flexible signaling through distinct PKA vs EPAC mechanisms may explain a number of emergent properties of serotonin-induced neuroplasticity of spinal motor networks, including metaplasticity (Huang et al., 1992; Kirkwood et al., 1995; Abraham and Bear, 1996; Fischer et al., 1997; Mitchell and Johnson, 2003).
Table 1. Published selectivity of PKA/EPAC activators and inhibitors.
from cell culture assays. The volume:conc (concentration) values listed in column 1 were concentration and volume used for intrathecal injections in the present in-vivo study.
| Drug (volume:conc) | EPAC Ka | PKA Ka | PKA Ki | EPAC Ki |
|---|---|---|---|---|
| 6-Bnz-cAMPa (10μL:100μM) | NSa | 2.7μM | - | - |
| 8-pCPT-2′-O-Me-cAMPa (10μL:100μM) | 1.8μM | 190μM | - | - |
| Rp-8-Br-cAMPa (10μL:1mM) | - | - | 8.5μM | NSi |
| ESI-05b (10μL:2M) | - | - | NSi | 0.43μM |
concentration for half of maximum cAMP induced response
concentration for inhibition of half maximum cAMP induced response
non-significant activating effect; ≥100 fold Ka difference
non-significant inhibitory effect; ≥100 fold Ki difference
Materials/Methods
Animals
Adult male Sprague-Dawley rats (2–5 months old; colony 218A, Harlan; Indianapolis, IN) were doubly housed, with food and water ad libitum, a 12h light/dark cycle, and controlled humidity/temperature. The University of Wisconsin Institutional Animal Care and Use Committee approved all animal procedures.
Neurophysiology experiments
Anesthesia was induced with isoflurane in a closed chamber and then maintained via nose cone (3.5% isoflurane in 50% O2, balance N2). Rats were tracheotomized and pump ventilated (2.5ml per breath; frequency adjusted to regulate end-tidal PCO2 between 40–50mmHg; Rodent Ventilator, model 683; Harvard Apparatus; South Natick, MA, USA) with an inspiratory mixture of 50% O2; 2% CO2; balanced N2 Followed by bilateral vagotomy in the mid-cervical region to eliminate ventilator entrainment of breathing efforts. An arterial catheter was placed into the right femoral artery to enable blood sampling for blood-gas analysis during protocols. To enable intrathecal drug delivery, a dorsal laminectomy and durotomy (C1/C2) was performed, a silicone catheter (OD 0.6mm; Access Technologies, IL, USA; primed with drug/vehicle) was inserted through a small hole in the dura and advanced caudally (~3mm) until resting at the C3-C4 spinal region. To minimize unintended drug diffusion from the catheter it was not placed until the stabilization period at the end of surgical preparations. The left phrenic and left hypoglossal (XII) nerves were isolated via a dorsal approach, cut distally, de-sheathed, submerged in mineral oil and then placed on bipolar silver wire electrode. After nerve dissection, rats were slowly converted to urethane anesthesia (1.8 g/kg, i.v. via tail vein catheter). Rectal body temperature (Traceable™, Fisher Scientific; Pittsburgh, PA, USA) was maintained within ± 1.0 of 37.5 °C using a custom temperature-controlled surgical table. A flow-through capnoguard with sufficient response time to measure exhaled CO2 in rats (Capnoguard, Novametrix; Wallingford, CT; USA) was used to monitor and control end-tidal CO2 (via adjustments to ventilator frequency). A heparinized plastic capillary tube (250×125 μl cut in half) was used to sample arterial blood to measure gas tensions (PaO2, PaCO2), pH and base excess (ABL 800Flex, Radiometer; Copenhagen, Denmark). Intravenous fluid infusions at a rate of 1mL/Hr (1:10:5 by volume of NaHCO3/Lactated Ringer’s/Hetastarch) were used to maintain blood pressure, acid/base and fluid balance from induction with isoflurane to euthanasia (overdose with urethane) following neurophysiological recordings.
Phrenic nerve activity was amplified (x10,000: A-M Systems, Everett, WA), band-pass filtered (100Hz to 10kHz), full-wave rectified, processed with a moving averager (CWE 821 filter; Paynter, Ardmore, PA: time constant, 50ms) and analyzed using a WINDAQ data-acquisition system (DATAQ Instruments, Akron, OH). Peak integrated phrenic burst frequency, amplitude, and mean arterial blood pressure (MAP) were analyzed in 60sec bins prior to obtaining blood samples. Data were included only if PaCO2 was maintained within ± 1.5mmHg of baseline (set by recruitment threshold; approx. 45mmHg), base excess was within ± 3mEq/L of 0mEq/L, MAP had decreased less than 30mmHg of baseline values (approx. 120mmHg), and PaO2 decreased less than 50mmHg from baseline (approx. 300mmHg) while remaining above 150mmHg for the entire protocol. There was no significant drift tendency in any of the physiological variables as assessed via 2-way ANOVA (Table 2).
Table 2. Physiology variables.
There were no consistent differences of PaCO2, PaO2, mean arterial pressure (MAP) or phrenic burst frequency within any individual group or amongst different groups. Differences between groups at a given time point (*) and differences from baseline within individual groups (**) are denoted within the table; p < 0.05. Values expressed as means ± SEM.
| Time (min) | Vehicle Control (N = 13) | Veh + 5-HT2A (N = 6) | EPACi + 5-HT2A (N = 5) | EPACa + 5-HT2A (N = 6) | PKAi + 5-HT2A (N = 5) | PKAa + 5-HT2A (N = 7) | |
|---|---|---|---|---|---|---|---|
| PaCO2 (mmHg) | Baseline | 48.6 ± 0.9 | 48.5 ± 1.1 | 48.3 ± 0.8 | 48.0 ± 1.6 | 48.0 ± 0.6 | 48.4 ± 0.6 |
| 30 | 48.7 ± 0.9 | 49.3 ± 0.9 | 47.5 ± 0.8 | 48.0 ± 1.9 | 48.1 ± 0.6 | 48.0 ± 0.9 | |
| 60 | 48.3 ± 0.9 | 49.3 ± 1.0 | 48.7 ± 0.7 | 48.2 ± 1.8 | 48.8 ± 0.6 | 48.5 ± 0.6 | |
| 90 | 48.4 ± 1.0 | 48.2 ± 1.5 | 48.6 ± 0.8 | 48.3 ± 1.6 | 47.8 ± 0.6 | 48.7 ± 0.6 | |
|
| |||||||
| PaO2 (mmHg) | Baseline | 316.2 ± 10.4 | 296.3 ± 16.4 | 340.6 ± 12.1 | 349.7 ± 8.5 | 333.0 ± 13.1 | 324.1 ± 16.8 |
| 30 | 321 ± 7.6 | 302.7 ± 15.0 | 339.6 ± 8.0 | 343.3 ± 5.4 | 343.4 ± 6.2 | 344.0 ± 14.2 | |
| 60 | 323.9 ± 8.0 | 314.7 ± 7.3 | 329.2 ± 8.4 | 342.7 ± 7.0 | 332.8 ± 6.8 | 348.7 ± 14.5 | |
| 90 | 325.5 ± 7.3 | 312.8 ± 10.9 | 328.0 ± 5.3 | 339.7 ± 7.2 | 321.6 ± 5.4 | 347.6 ± 13.6 | |
|
| |||||||
| MAP (mmHg) | Baseline | 120.6 ± 6.0 | 106.1 ± 10.1 | 124.2 ± 5.1 | 106.7 ± 11.8 | 107.1 ± 6.3 | 111.9 ± 11.9 |
| 30 | 110.3 ± 6.5 | 104.9 ± 10.8 | 114.1 ± 10.5 | 97.8 ± 11.6 | 102.4 ± 6.7 | 94.1 ± 9.6 | |
| 60 | 102.9 ± 6.2 | 94.9 ± 8.8 | 108.3 ± 4.0 | 96.5 ± 10.6 | 97.9 ± 6.5 | 89.6 ± 8.3 | |
| 90 | 103.2 ± 6.2 | 95.6 ± 9.8 | 100.1 ± 10.1 | 84.8 ± 7.6 | 83.0 ± 7.2 | 77.4 ± 9.2 | |
|
| |||||||
| Phrenic Burst Frequency (Burst/min) | Baseline | 49.0 ± 1.2 | 49.1 ± 2.9 | 49.3 ± 2.2 | 49.3 ± 2.1 | 47.8 ± 2.2 | 45.7 ± 2.0 |
| 30 | 47.9 ± 1.7 | 48.9 ± 2.5 | 48.4 ± 2.3 | 50.3 ± 1.2 | 48.9 ± 1.9 | 46.5 ± 2.3 | |
| 60 | 46.6 ± 2.0 | 48.8 ± 1.7 | 49.6 ± 2.8 | 50.2 ± 0.6 | 48.7 ± 1.9 | 45.5 ± 2.5 | |
| 90 | 47.7 ± 1.8 | 48.3 ± 2.2 | 49.8 ± 1.8 | 51.0 ± 0.9 | 49.6 ± 2.2 | 45.8 ± 2.3 | |
|
| |||||||
| Time (min) | Veh + EPACa (N = 6) | EPACi + EPACa (N = 4) | PKAi + EPACa (N = 4) | Veh + PKAa (N = 7) | PKAi + PKAa (N = 6) | EPACi + PKAa (N = 4) | |
|
| |||||||
| PaCO2 (mmHg) | Baseline | 50.4 ± 1.7 | 48.7 ± 1.4 | 48.0 ± 1.8 | 49.4 ± 1.2 | 48.4 ± 0.8 | 50.8 ± 1.0 |
| 30 | 50.1 ± 1.7 | 49.5 ± 1.3 | 48.0 ± 1.9 | 50.2 ± 1.0 | 48.2 ± 1.0 | 51.3 ± 1.2 | |
| 60 | 50.9 ± 1.6 | 48.2 ± 1.1 | 48.5 ± 1.0 | 49.8 ± 1.4 | 48.9 ± 0.8 | 51.1 ± 1.6 | |
| 90 | 50.2 ± 1.7 | 48.3 ± 1.4 | 48.6 ± 1.3 | 49.5 ± 1.2 | 48.8 ± 0.9 | 50.3 ± 1.2 | |
|
| |||||||
| PaO2 (mmHg) | Baseline | 311.5 ± 10.9 | 329.0 ± 15.1 | 274.0 ± 39.6 | 316.7 ± 17.5 | 315.7 ± 14.0 | 291.2 ± 25.1 |
| 30 | 320.0 ± 6.9 | 322.3 ± 16.2 | 330.3 ± 12.9 | 311.9 ± 12.7 | 307.2 ± 16.3 | 306.7 ± 10.8 | |
| 60 | 315.8 ± 8.5 | 310.0 ± 14.6 | 333.7 ± 14.3 | 267.5 ± 44.2 | 309.8 ± 14.6 | 305.2 ± 13.3 | |
| 90 | 315.8 ± 9.3 | 310.5 ± 13.6 | 336.7 ± 10.9 | 273.0 ± 40.0** | 306.4 ± 10.9 | 311.2 ± 3.8 | |
|
| |||||||
| MAP (mmHg) | Baseline | 121.4 ± 5.8 | 118.9 ± 10.1 | 130.9 ± 10.6 | 113.3 ± 5.3 | 103.6 ± 6.9 | 116.3 ± 7.4 |
| 30 | 109.9 ± 2.9 | 99.5 ± 11.1 | 110.5 ± 13.3 | 102.5 ± 5.5 | 91.7 ± 1.7 | 92.8 ± 7.9 | |
| 60 | 108.2 ± 2.8 | 100.5 ± 7.7 | 106.4 ± 8.6 | 100.4 ± 7.4 | 91.2 ± 5.9 | 89.9 ± 10.0 | |
| 90 | 98.8 ± 2.7 | 86.7 ± 12.7 | 104.3 ± 9.7 | 89.7 ± 6.5 | 84.6 ± 3.0 | 89.8 ± 10.1 | |
|
| |||||||
| Phrenic Burst Frequency (Burst/min) | Baseline | 50.2 ± 2.8 | 54.5 ± 2.5 | 51.7 ± 1.1 | 45.8 ± 1.5 | 41.7 ± 3.3 | 56.0 ± 1.3* |
| 30 | 50.8 ± 2.2 | 52.4 ± 1.5 | 50.6 ± 0.5 | 47.7 ± 1.7 | 48.4 ± 4.9 | 58.7 ± 1.1* | |
| 60 | 51.8 ± 2.1 | 53.6 ± 2.2 | 51.1 ± 0.5 | 47.2 ± 1.9 | 48.2 ± 3.3 | 57.2 ± 1.1* | |
| 90 | 51.3 ± 2.0 | 54.4 ± 1.3 | 50.3 ± 1.2 | 46.8 ± 1.9 | 44.3 ± 3.7 | 58.3 ± 1.3* | |
One hour after conversion to urethane adequate levels of anesthesia were confirmed by an absence of response (movement, arterial blood pressure, phrenic nerve activity) to toe pinch. Rats were then paralyzed with pancuronium bromide (2.5mg/kg, i.v.) and baseline end-tidal CO2 levels were set 2–3mmHg above the recruitment threshold for each individual rat (described in Bach and Mitchell, 1996). After 20min of stable nerve recordings a blood sample was drawn to establish baseline blood gas values. Rats then received the first of two series of intrathecal injections. Fifteen minutes after completion of the first injection series, rats received the second series as outlined below.
Drugs
The following drugs were obtained from Santa Cruz (Dallas, TX, USA): 6-Bnz-cAMP (PKA selective activator; PKAa), 8-pCPT-2′-Me-O-cAMP (EPAC selective activator; EPACa) and Rp-8-Br-cAMP (PKA selective inhibitor; PKAi). 2,5-Dimethoxy-4-iodoamphetamine (DOI; 5-HT2A receptor agonist; 5HT2a) was ordered from Sigma-Aldrich (St. Louis, MO) while ESI-05 (EPAC selective inhibitor; EPACi) was obtained from BioLog Life Science Institute (Germany). All drugs were initially dissolved in dimethylsulfoxide (DMSO) and then diluted with saline (maximum DMSO concentration of 20%) before use. Aliquots of stock solutions remained viable for up to one week if stored frozen (−20° C) in 100% DMSO; after this time unused drug solutions were discarded. Prior studies using a similar protocol confirmed that EPACa is a selective EPAC activator (Fields et al. 2015) and DOI is a selective 5-HT2A receptor agonist (MacFarlane et al. 2011). Separate studies using cell culture assays have shown that ESI-05 and Rp-8-Br-cAMP are selective inhibitors of EPAC (Tsalkova et al. 2012; Rehmann 2013) and PKA (Poppe et al. 2008; Harmati et al. 2011) respectively (Table 1). In addition, crossover control studies in which an EPAC selective inhibitor were shown to have no effect on PKA induced pMF (and vice versa; PKA selective inhibitor was shown to have no effect on EPAC induced pMF) were done to confirm selectivity of the cAMP analogue drugs within our in-vivo model.
Experimental groups
All drugs were delivered as a single injection bolus over a 2min period with the exception of the 5-HT2A agonist, which was delivered as 3 smaller injections of 5μL over a 1min period, each separated by 5min intervals to establish intermittent receptor activation. Previous studies have shown that intermittent 5-HT2A agonist injections are required to elicit this form of pMF (MacFarlane et al 2011), whereas single injections (not intermittent) are necessary for PKA and EPAC induced pMF (Fields et al. 2015). To confirm individual molecules are sufficient to elicit pMF, intrathecal 5-HT2A receptor agonist (3×6μL, 100μM), PKAa (10μL, 100μM) or EPACa (10μL, 100μM) injections were given intrathecally. To maintain volume consistency vehicle injections were given in each of these groups via a second intrathecal catheter. Dosing for 5-HT2A (MacFarlane et al., 2011) and EPACa (Fields et al., 2015) were determined from previous studies; a limited dose response curve was completed for PKAa (data not shown). Intrathecal injections of the 5-HT2A agonist, PKAa, or EPACa, gave rise to pMF without affecting hypoglossal (XII) nerve activity. XII nerve activity serves as an internal control to confirm pMF is due to spinal mechanisms and not drug diffusion to brainstem respiratory centers which would elicit motor facilitation in both phrenic and XII nerves (Baker-Herman and Mitchell, 2001). For cAMP crosstalk groups either PKAa or EPACa were given via a second catheter 15min prior to 5-HT2A receptor agonist injections at the same dose sufficient to elicit pMF when given alone.
To better understand signaling pathways necessary for pMF, additional rat groups were pretreated with PKAi (10μL, 1mM) or EPACi (10μL, 2mM) 15min prior to 5-HT2A agonist (3×6μL, 100μM), PKAa (10μL, 100μM), or EPACa (10μL, 100μM) injections. All inhibitors were given intrathecally via a second catheter over 2min. “Time post injection” was started after the final injection of the second series.
Statistical analyses
Peak amplitude and frequency of integrated phrenic bursts were averaged in 60sec bins at baseline (pre-injection), and at 30, 60 and 90min after the final intrathecal injection. Amplitude is expressed as a percent change from baseline in each rat; frequency is expressed as change from baseline in bursts/min. Phrenic nerve burst frequency did not change significantly in any group (Table 2). Statistical comparisons were made for experimental, vehicle and drug control groups using two-way repeated measures ANOVA with Tukey post hoc test to identify statistically significant pair-wise differences. All values are expressed as means ± SEM. Significance was accepted as p ≤ 0.05. p values are relative to baseline phrenic nerve amplitude for the respective group unless otherwise noted. Since none of the control groups exhibited significant pMF, and since there were no significant differences between any of the control groups (vehicle + vehicle, n = 5; EPACi + vehicle, n = 4; PKAi + vehicle, n = 4), they were combined into a single, master control group (n = 13). Individual group data from 5-HT2A agonist and control groups are repeated in figures 1 and 2. Group numbers are defined in the figure legends and in Table 2.
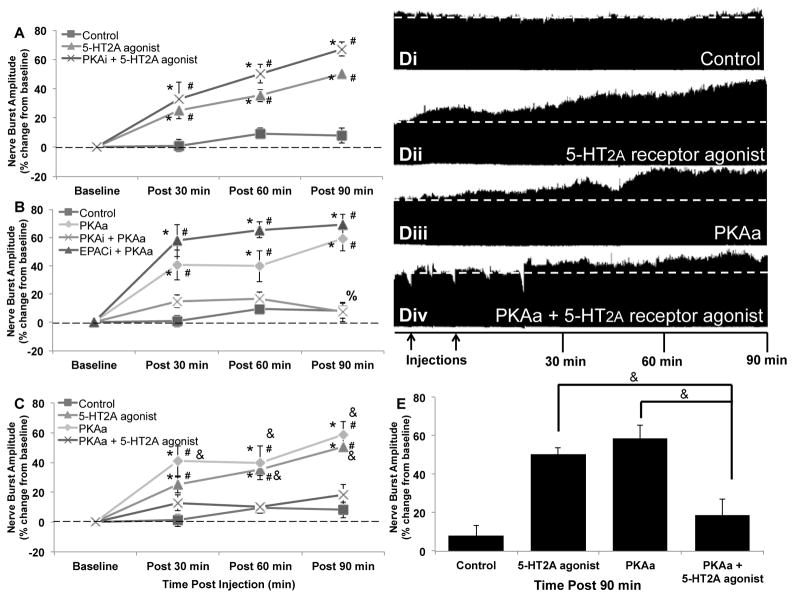
Figure 1. PKA constrains 5-HT2A receptor-induced phrenic motor facilitation.
A) intermittent intrathecal injections of 5-HT2A receptor agonist (3×6μL, 100μM) elicited pMF (90min: 50.6 ± 3.1%; n = 6; p < 0.001) were not affected by PKAi (10μL, 1mM; 90min: 67.2 ± 4.9%; n = 5; p = 0.098 relative to 5-HT2A agonist induced pMF). B) Intrathecal injections of PKAa (10μL, 100μM) elicited pMF (90min: 58.9 ± 8.6%; n = 7; p < 0.001), an effect that was undermined by PKAi (90min: 7.3 ± 6.7%; n = 6; p < 0.001), but not EPACi pretreatment (90min: 69.1 ± 7.3%; n = 4; p = 0.819). C) Concurrent application of PKAa and 5-HT2A receptor agonist limited the capacity for either to elicit pMF (90min: 18.6 ± 6.5%; n = 7; p < 0.001 relative to PKAa or 5-HT2A receptor agonist-induced pMF). D) Representative phrenic neurograms; i) vehicle control, ii) vehicle + 5-HT2A receptor agonist, iii) vehicle + PKAa and iv) PKAa + 5-HT2A receptor agonist. First arrow represents pretreatment injection; second arrow represents either start of intermittent 5-HT2A agonist injections or start of single PKAa injection. E) Summary of data from A–C at 90min post-final injection. Data represent mean values ± 1 SEM. Significant differences from baseline (#), control (*), PKAa (%), or PKAa + 5-HT2A receptor agonist (&).
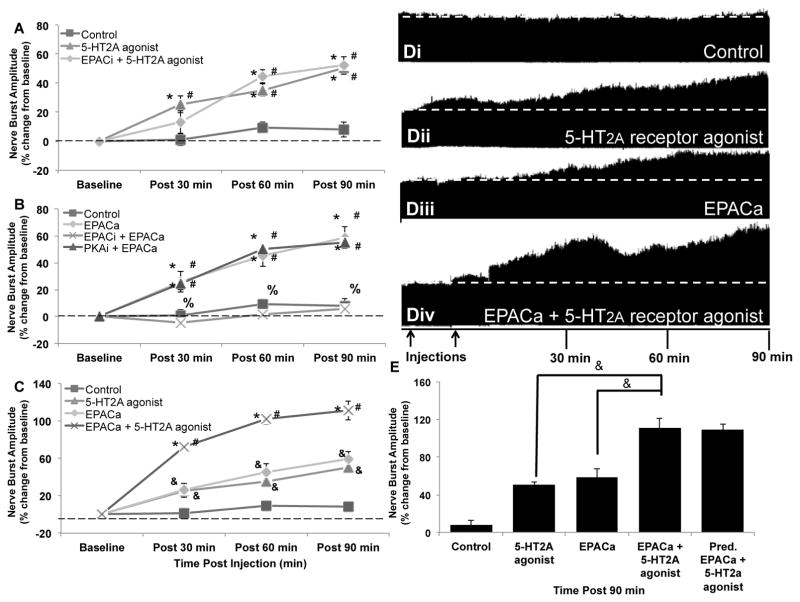
Figure 2. EPAC additively enhances 5-HT2A receptor-induced phrenic motor facilitation.
A) Intermittent intrathecal injections of 5-HT2A receptor agonist (3×6μL, 100μM) elicited pMF (90min: 50.6 ± 3.1%; n = 6; p < 0.001); this pMF was not affected by EPACi pretreatment (10μL, 2mM; 90min: 52.4 ± 6.1%; n = 5; p < 0.001 versus 5-HT2A-induced pMF). B) Intrathecal EPACa injections (10μL, 100μM) elicited pMF (90min: 58.9 ± 8.2%; n = 6; p < 0.001), an effect constrained by EPACi (90min: 6.2 ± 16.7%; n = 4; p < 0.001 versus EPACa induced pMF), but not PKAi (10μL, 1mM; 90min: 55.2 ± 2.5%; n = 4; p = 0.979 versus EPACa induced pMF). C) Concurrent EPACa and 5-HT2A agonist injections elicited an enhanced pMF significantly greater than EPACa or 5-HT2A-induced pMF alone (90min: 110.9 ± 10.0%; n = 6; p < 0.001 versus EPACa or 5-HT2A agonist-induced pMF). D) Representative phrenic neurograms; i) vehicle control, ii) vehicle + 5HT2A receptor agonist, iii) vehicle + EPACa and iv) EPACa + 5-HT2A receptor agonist. First arrow represents pretreatment injection; second arrow represents either start of intermittent 5-HT2A agonist injections or start of single EPACa injection. E) Summary of data from A–C; the actual pMF from combined EPACa and 5-HT2A activation was not different from predicted pMF resulting from additive contributions from the pMF elicited when each molecule is activated alone. Data represent mean values ± 1 SEM. Significant differences from baseline (#), control (*), EPACa (%), or EPACa + 5-HT2A agonist (&).
Results
PKA activation elicits pMF, but constrains 5-HT2A induced pMF
Intermittent, intrathecal 5-HT2A agonist injections (2,5-Dimethoxy-4-iodoamphetamine; 3 × 6μL, 100μM) elicited a 50% increase in phrenic nerve inspiratory amplitude; pMF (Fig. 1, A; 50.6 ± 3.1% 90 min post-injection; n = 6; p < 0.001). 5-HT2A induced pMF was not affected by pretreatment with the selective PKA inhibitor, Rp-8-Br-cAMP (PKAi; 10μL, 1mM; 67.2 ± 4.9% 90 min post-injection; n = 5; p = 0.1 versus 5-HT2A agonist alone), confirming that PKA activity is not necessary for 5-HT2A induced pMF.
6-Bnz-cAMP is a cell permeable cAMP analogue that preferentially activates PKA (PKAa) versus EPAC (Table 1). Intrathecal 6-Bnz-cAMP injections (10μL, 100μM) elicited progressive increases in phrenic nerve burst amplitude (Fig. 1, B; 58.9 ± 8.6% at 90 min post-injection; n = 7; p < 0.001), demonstrating PKA activity is sufficient to elicit pMF. Although PKAa-induced pMF was attenuated by PKAi (Fig. 1, B; 7.3 ± 6.7%; n = 6; p < 0.001 vs. PKAa alone), it was unaffected by EPAC inhibition (EPACi) with the selective inhibitor, ESI-05 (10μL, 2mM; Fig. 1, B; 69.1 ± 7.3%; n = 4; p = 0.819 vs PKAa alone). Thus, we confirm PKAa-induced pMF requires PKA, but not EPAC activation.
Although 5-HT2A and PKA activation each elicit pMF alone, concurrent PKAa and 5-HT2A receptor activation prevented pMF expression (Fig. 1, C; 30min post-injection: 12.9 ± 5.4%; 60min: 9.9 ± 3.4%; 90min: 18.6 ± 6.5%; n = 7; p < 0.001 vs. 5-HT2A or PKAa-induced pMF). Thus, concurrent PKA and 5-HT2A activation are mutually inhibitory, disenabling pMF.
EPAC activation elicits pMF, and enhances 5-HT2A induced pMF
Pre-treatment with an EPAC inhibitor (ESI-05; 10μL, 2mM) had no effect on 5-HT2A induced pMF (Fig. 2, A; 52.4 ± 6.1% 90 min post-injection; n = 5; p < 0.001 vs. 5-HT2A agonist induced pMF), demonstrating EPAC activity plays no role in 5-HT2A-induced pMF.
8-pCPT-2′-Me-O-cAMP is a cAMP analogue with high relative selectivity for EPAC (EPACa) versus PKA activation (Table 1). Intrathecal EPACa (10μL, 100μM) elicited pMF (Fig. 2, B; 58.9 ± 8.2% 90 min post-injection; n = 6; p < 0.001) similar to our previous report (Fields et al., 2015). EPACa induced pMF was attenuated by EPACi (Fig. 2, B; 6.2 ± 16.7%; n = 4; p < 0.001 vs. EPACa alone), but not PKAi (Fig. 2, B; 55.2 ± 2.5%; n = 4; p = 0.979 vs. EPACa alone). Thus, EPACa induced pMF requires EPAC, not PKA activity.
Concurrent spinal EPAC and 5-HT2A activation gave rise to pMF greater than that elicited by either drug alone (Fig. 2, C; 110.9 ± 10.0% 90 min post-injection; n = 6; p < 0.001 vs. EPACa or 5-HT2A agonist alone). Combined EPACa + 5-HT2A agonist-induced pMF was additive (i.e. equal to the sum of pMF induced by each drug alone (Fig. 2, E; p = 0.999 vs. EPACa plus 5-HT2A agonist induced pMF). Thus, EPAC and 5-HT2A make independent pMF contributions, much in contrast to the mutual inhibition observed with concurrent PKA and 5-HT2A activation.
Discussion
Serotonin elicits multiple forms of sensory-motor plasticity through its actions onto diverse GPCR subtypes (Brunelli et al., 1976; Randić et al., 1993; Clark and Kandel, 1993). However, the functional implications of serotonin receptor co-activation have seldom been explored. In spinal pMF, Gq-coupled 5-HT2A and Gs-coupled (cAMP-linked) 5-HT7 receptors give rise to pMF through mechanistically distinct signaling cascades (MacFarlane et al., 2011; Hoffman and Mitchell, 2011; Fields et al., 2015). Although each receptor is sufficient to elicit pMF when stimulated alone, inter-receptor, cross-talk inhibition within the spinal respiratory control network limits pMF when they are co-activated (MacFarlane et al., 2009). Here we provide the first evidence that divergent cAMP signaling enables differential regulation of serotonin Gq receptor-induced spinal motor plasticity.
Whereas PKA activity attenuates 5-HT2A receptor-induced pMF, EPAC enhances pMF by combining (additively) with 5-HT2A receptor-induced pMF. We propose that a shift in cAMP signaling from PKA to EPAC predominance may relieve cross-talk constraints, potentially enabling independent contributions of 5-HT2A and 5-HT7 receptors for enhanced spinal motor plasticity. Whereas PKA is activated at with transient nanomolar cAMP levels (Dostmann and Taylor, 1991), EPAC activation requires relatively prolonged cAMP levels in the micromolar range (Ponsioen et al., 2002; Zhou et al., 2016). Different cAMP affinities and activation paradigms suggest that stimulation of Gs protein coupled receptors will initially activate PKA with EPAC signaling following only with greater/stronger receptor activation. However, certain growth/trophic factors can change the relative cAMP sensitivity of PKA and EPAC, shifting activation thresholds in favor of EPAC signaling (Vasko et al., 2014). For example, although nerve growth factor-1 (NGF-1) does not affect plasticity expression in a well-studied model of sensory hypersensitivity, it does convert the plasticity from PKA- to EPAC-dependence (Vasko et al., 2014). Similar effects could shift the PKA/EPAC balance downstream from 5-HT7 receptors, enable 5-HT7 receptors to contribute rather than constrain serotonin-induced plasticity, potentially explaining enhanced serotonin-dependent plasticity observed with preconditioning experiences known to increase growth/trophic factor expression (Kinkead et al., 1998; Johnson et al., 2000; Ling et al., 2001; Wilkerson and Mitchell, 2009).
Direct manipulation of cAMP signaling with selective drugs may enable modulation of cross-talk interactions to enhance serotonin-induced pMF for experimental or therapeutic advantage. For example, serotonin-induced, 5-HT2A-dependent, spinal motor plasticity may be enhanced via PKA inhibition to relieve inhibitory cross-talk constraints; conversely, selective EPAC activation will contribute to serotonin induced, 5-HT2A-dependent, pMF via additive contributions from the mechanistically distinct Gs associated pathway (Fields et al., 2015). By enhancing respiratory control through spinal motor plasticity we may restore lost breathing capacity in severe clinical disorders such as cervical spinal injury (Lovett-Barr et al., 2012) or motor neuron disease (Nichols et al., 2013).
Here we utilized recently available, highly selective, drugs to independently manipulate PKA and EPAC activity. While rodent knockout models are often used to assure target selectivity (vs drugs), a recent study demonstrated that plasticity investigations are sometimes hampered in EPAC knockout mice due to compensatory signaling responses to gene deletions. For example, although PKA does not contribute to forskolin-induced, cAMP dependent, mossy fiber plasticity in wild-type mice, PKA inhibition suppresses mossy fiber plasticity in EPAC knockout mice, suggesting a compensatory/supportive role for PKA revealed only when EPAC signaling is impaired during development (Fernandes et al., 2015). Furthermore, despite the potential for off-target effects when using pharmacological approaches, literature supports the selectivity of the drugs used here (Table 1), and we have confirmed selectivity within our conditions with carefully designed control experiments cross-comparing EPAC and PKA activators/inhibitors.
Although contrasting roles for EPAC and PKA have been emphasized in embryonic model systems (Murray et al., 2009), the present data are the first to confirm differential actions in the fully mature adult nervous system. This is an important advancement as it supports the idea that spinal motor networks retain their capacity to adapt important motor behaviors long after maturation. Due to the limited capacity of spinal motor neurons to replicate, functional flexibility through plasticity represents an important target for therapeutic intervention in neural disorders that compromise essential motor behaviors; such as breathing. These possibilities await further exploration.
Highlights.
cAMP initiates distinct PKA and EPAC signaling cascades in phrenic motor neurons
PKA and EPAC differentially regulate serotonin-induced spinal, phrenic motor plasticity
Acknowledgments
We thank Drs. BJ Dougherty and AG Huxtable for helpful critiques of this manuscript. Funded by NIH grants RO1 HL80209 and RO1 HL69064. DPF supported by Advanced Opportunity Fellowship at the University of Wisconsin, a NIH NRSA (F30 HL126351) and the University of Wisconsin Medical Scientist Training Program (NIH T32 GM008692).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Clark GA, Kandel ER. Induction of long-term facilitation in aplysia sensory neurons by local application of serotonin to remote synapses. PNAS. 1993;90:11411–11415. doi: 10.1073/pnas.90.23.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostmann WR, Taylor SS. Identifying the molecular switches that determine whether (Rp)-cAMPS functions as an antagonist or an agonist in the activation of cAMP-dependent protein kinase I. Biochemistry. 1991;30:8710–8716. doi: 10.1021/bi00099a032. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Riordan S, Nomura T, Remmers CL, Kraniotis S, Marshall JJ, Kukreja L, Vassar R, Contractor A. Epac2 mediates cAMP-Dependent Potentiation of Neurotransmission in the Hippocampus. J Neurosci. 2015;35:6544–6553. doi: 10.1523/JNEUROSCI.0314-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophys. 2015;114:2015–2022. doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TM, Blazis DEJ, Priver NA, Carew TJ. Metaplasticity at identified inhibitory synapses in Aplysia. Nature. 1997;389:860–865. doi: 10.1038/39892. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Llyod PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmati G, Bányász T, Bárándi L, Szentandrássy N, Horváth B, Szabó G, Szentmiklósi JA, Szénási G, Nánási PP, Magyar J. Effects of β-adrenoceptor stimulation on delayed rectifier K(+) currents in canine ventricular cardiomyocytes. Br J Pharmacol. 2011;162:890–896. doi: 10.1111/j.1476-5381.2010.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation elicits long-lasting phrenic motor facilitation. J Physiol. 2011;589:1397–1407. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neurosci. 2013;250:632–643. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:750–753. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci. 2000;20:RC77. doi: 10.1523/JNEUROSCI.20-10-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear M. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Levy M, Susswein AJ. Separate neural pathways respond to different noxious stimuli affecting respiratory pump frequency in Aplysia fasciata. Brain Res. 1993;616:218–229. doi: 10.1016/0006-8993(93)90212-6. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience. 2011;178:45–55. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989;9:4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–15444. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Randić M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neursci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmann H. EPAC-inhibitors: facts and artefacts. Sci Rep. 2013;3:3032. doi: 10.1038/srep03032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir R, Lee HK, Kirkwood A. Neuromodulators Control the Polarity of Spike-Timing-Dependent Synaptic Plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño M, Huang S, He K, Ardiles A, Pasquale R, Guo Y, Palacios A, Huganir R, Kirkwood A. Push-pull neuromoulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL, Jr, Cheng X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. PNAS. 2012;109:18613–18618. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko MR, Habashy-Malty R, Guo C, Duarte DB, Zhang Y, Nicol GD. Nerve growth factor mediates a switch in intracellular signaling for PGE2-induced sensitization of sensory neurons from protein kinase A to Epac. PloS One. 2014;9:e104529. doi: 10.1371/journal.pone.0104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Tanaka KF, Matsunaga S, Iseki M, Watanabe M, Matsuki N, Ikegaya Y, Koyama R. Photoactivated adenylyl cyclase (PAC) reveals novel mechanisms underlying cAMP-dependent axonal morphogenesis. Sci Rep. 2016;5:19679. doi: 10.1038/srep19679. [DOI] [PMC free article] [PubMed] [Google Scholar]