Abstract
Background
Systemic inflammatory response and nutritional status are important to the prognosis of patients with colorectal cancer (CRC). This study aimed to investigate the prognostic value of the combination of preoperative hemoglobin, lymphocyte, albumin, and neutrophil (HLAN) in patients with locally advanced CRC (LACRC).
Material/Methods
We performed a retrospective analysis in 536 LACRC patients undergoing radical surgery. The value of HLAN was defined as follow: HLAN=Hemoglobin (g/L)×Lymphocyte (/L)×Albumin (g/L)/Neutrophil (/L)/100. The X-tile program was used to determine the optimal cut-point of HLAN, and the prognostic value of HLAN for overall survival (OS) was evaluated with the Cox proportional hazard model.
Results
The cut-point of HLAN was set at 19.5. Compared with the high-HLAN group, the low-HLAN group had a 1.50-fold (95% confidence interval 1.09–2.05) increased risk of death and a significantly lower OS rate (P<0.001). Furthermore, the risk stratification model based on HLAN (AUC=0.72) displayed better accuracy in OS prediction than the TNM system (AUC=0.61).
Conclusions
HLAN is a valuable prognostic marker for patients with LACRC.
MeSH Keywords: Albumins, Colorectal Neoplasms, Hemoglobins, Lymphocytes, Neutrophils, Prognosis
Background
Colorectal cancer (CRC) is a major public health issue, with an estimated 1.4 million new cases and 693,900 deaths occurring in 2012 worldwide [1]. Surgery is the mainstay of curative treatment for patients with locally advanced CRC (LACRC). Unfortunately, the prognosis is unsatisfactory, and about 50% of patients will develop recurrence or metastasis after radical resection [2]. Tumor-node metastasis (TNM) stage has been widely recognized as the primary prognostic factor, but it is not entirely reliable, especially in patients with localized disease [3]. Therefore, it is very important and urgent to find more effective biomarkers to identify patients with poor prognosis, and to guide treatment according to the predictive risk.
Accumulating evidence supports the involvement of systemic inflammatory response and nutritional status in the cancer development and progression [4–6]. The immune or nutritional status of the host can be assessed by hematological examination [5], and many hematological indexes have been reported to have prognostic value in various cancers, including CRC, such as C-reactive protein level [7], hemoglobin level [8], albumin level [9], and lymphocyte [10] and neutrophil counts [11]. Further studies indicated that the integration of these hematological indexes can improve the predictive accuracy, like the Glasgow prognostic score (GPS) [12], prognostic nutritional index (PNI) [13], and neutrophil-to-lymphocyte ratio (NLR) [14]. However, the prognostic significance of the combination of hemoglobin, lymphocyte, albumin, and neutrophil (HLAN) in cancer has not been well investigated to date. Accordingly, we performed a retrospective analysis in 536 LACRC patients undergoing radical surgery and systematically evaluated the prognostic value of HLAN for survival.
Material and Methods
Study population
We included 536 CRC patients recruited from Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine between January 2009 and December 2010 in this retrospective study, with follow-up to January 2016. All patients were diagnosed with stage II or III disease according to the seventh edition of American Joint Committee on Cancer (AJCC) TNM staging system, and they all underwent radical surgery. None of the patients had another previous malignancy, end-stage liver disease, or chronic inflammatory disease, including autoimmune disorders and infection.
Data collection
Baseline demographic and clinical data were abstracted from patients’ medical records, including age, sex, smoking and drinking history, family cancer history, date of diagnosis, and some tumor characteristics, such as tumor location, differentiation grade, vessels/nerves invasion, and tumor stage. Information on vital status was obtained from the medical records or telephone follow-up. Moreover, 4 preoperative hematologic indexes, including hemoglobin level, albumin level, and lymphocyte and neutrophil counts, were also collected to establish a new index HLAN. The value of HLAN was defined as follow: HLAN=Hemoglobin (g/L)×Lymphocyte (/L)×Albumin (g/L)/Neutrophil (/L)/100. This study was approved by the institutional review board of our hospital and all participants provided written informed consent.
Statistical analysis
The endpoint of this study was overall survival (OS), which was calculated from the date of diagnosis to death from any cause or the last follow-up. Statistical analyses to identify prognostic factors were performed using SPSS software (SPSS 19.0, IBM, Chicago, IL, USA). The optimal cut-point of HLAN was determined through use of the X-tile program (Version 3.6.1, Yale University, USA) [15]. Survival curves were made by the Kaplan-Meier method and compared by the log-rank test. To identify the significant prognostic factors for OS, the Cox proportional hazard model was used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs). A risk stratification model was established according to the number of risk factors. The predictive accuracy of this risk model and the TNM system was compared by receiver operating characteristic (ROC) curve analysis [16]. The area under the curve (AUC) was calculated with the use of R software (Version 3.2.0, R Foundation for Statistical Computing). Two-sided P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 536 patients with LACRC were enrolled in this study, and the demographic and clinical characteristics are summarized in Table 1. There were 216 females (40.3%) and 320 males (59.7%), with a median age of 61 years (range, 21–92). Of these, 264 (49.3%) had rectal tumors and 272 (50.7%) had colonic tumors; 259 patients (48.3%) were stage II and 277 (51.7%) were stage III. There were 177 deaths (33.0%), with a median follow-up time of 66 months, and the 5-year OS rate for all patients was 69.0%.
Table 1.
Selected characteristics of 536 patients with LACRC.
| Characteristic* | No. (%) of patients |
|---|---|
| Age (years), median (range) | 61 (21–92) |
| Gender | |
| Female | 216 (40.3%) |
| Male | 320 (59.7%) |
| Smoking history | |
| No | 427 (79.7%) |
| Yes | 109 (20.3%) |
| Alcohol-drinking history | |
| No | 421 (78.5%) |
| Yes | 115 (21.5%) |
| First-degree relative cancer history | |
| Yes | 82 (15.3%) |
| No | 454 (84.7%) |
| Tumor location | |
| Rectum | 264 (49.3%) |
| Colon | 272 (50.7%) |
| Differentiation grade | |
| Well/moderate | 360 (67.2%) |
| Poor/mucinous | 176 (32.8%) |
| Vessels/nerves invasion | |
| Negative | 462 (86.2%) |
| Positive | 74 (13.8%) |
| TNM stage | |
| II | 259 (48.3%) |
| III | 277 (51.7%) |
| HLAN, median (range) | 18.7 (0.6–70.1) |
LACRC – locally advanced colorectal cancer; TNM – tumor-node-metastasis; HLAN=Hemoglobin (g/L)×Lymphocyte (/L)× Albumin (g/L)/Neutrophil (/L)/100.
Prognostic value of HLAN
The median value of HLAN was 18.7 (range, 0.6–70.1). With the use of the X-tile program, the optimal cut-point of HLAN was set at 19.5, and patients were divided into either high-HLAN (n=260, 48.5%) or low-HLAN (n=276, 51.5%) groups (Figure 1). Univariate analysis showed that age, first-degree relative cancer history, differentiation grade, vessels/nerves invasion, TNM stage, and HLAN were associated with OS (all P<0.05) (Table 2). Multivariate analysis with backward stepwise regression identified that age, first-degree relative cancer history, differentiation grade, vessels/nerves invasion, TNM stage, and HLAN were significant independent prognostic factors of LACRC patients (Table 2). Compared with the high-HLAN group, the low-HLAN group had a 1.50-fold (95% CI 1.09–2.05; P=0.012) increased risk of death and a significantly lower 5-year OS rate (76.5 vs. 62.0%; log-rank P<0.001) (Figure 2).
Figure 1.
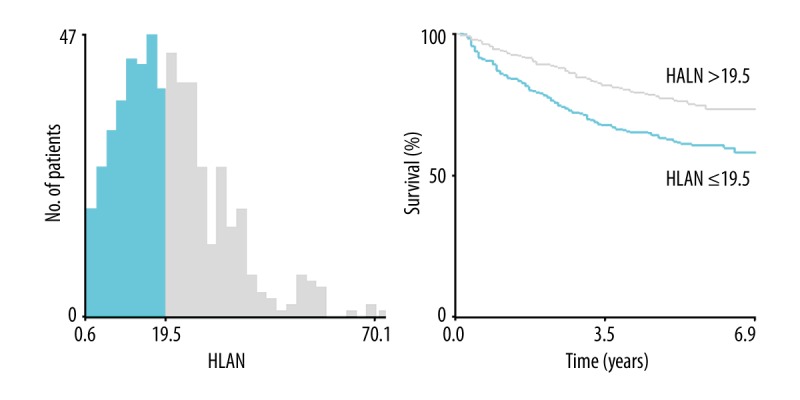
Optimal cut-off point selection of HLAN by X-tile program. The optimal cut-off point of HLAN was set at 19.5. HLAN=Hemoglobin (g/L)×Lymphocyte (/L)×Albumin (g/L)/Neutrophil (/L)/100.
Table 2.
Univariate and multivariate analyses for overall survival.
| Variable* | Univariate | Multivariate | |
|---|---|---|---|
| P value | HR (95% CI) ** | P value | |
| Age (years) | |||
| ≤65 | 1.00 | ||
| >65 | <0.001 | 1.87 (1.38–2.53) | <0.001 |
| Gender | |||
| Female | |||
| Male | 0.164 | ||
| Smoking history | |||
| No | |||
| Yes | 0.187 | ||
| Alcohol-drinking history | |||
| No | |||
| Yes | 0.169 | ||
| First-degree relative cancer history | |||
| Yes | 1.00 | ||
| No | 0.029 | 1.83 (1.12–2.98) | 0.016 |
| Tumor location | |||
| Rectum | |||
| Colon | 0.882 | ||
| Differentiation grade | |||
| Well/moderate | 1.00 | ||
| Poor/mucinous | <0.001 | 1.73 (1.28–2.34) | <0.001 |
| Vessels/nerves invasion | |||
| Negative | 1.00 | ||
| Positive | <0.001 | 1.65 (1.14–2.40) | 0.008 |
| TNM stage | |||
| II | 1.00 | ||
| III | <0.001 | 2.08 (1.51–2.86) | <0.001 |
| HLAN | |||
| >19.5 | 1.00 | ||
| ≤19.5 | <0.001 | 1.50 (1.09–2.05) | 0.012 |
TNM – tumor-node-metastasis; HLAN=Hemoglobin (g/L)×Lymphocyte (/L)×Albumin (g/L)/Neutrophil (/L)/100;
HR – hazard ratio; CI – confidence interval.
Figure 2.
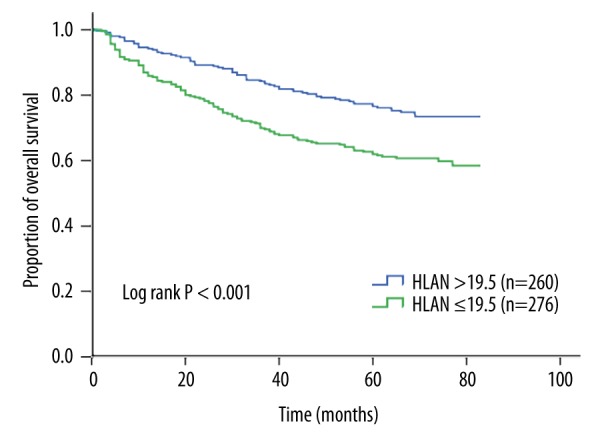
Kaplan-Meier curves for overall survival according to HLAN. The 5-year overall survival rate of patients with HLAN ≤19.5 was significantly lower than that of patients with HLAN >19.5 (62.0 vs. 76.5%, log-rank P<0.001). HLAN=Hemoglobin (g/L)×Lymphocyte (/L) ×Albumin (g/L)/Neutrophil (/L)/100.
Risk stratification model
The 6 independent risk factors, including lower HLAN, identified by multivariate analysis were used to establish a risk stratification model. Patients were categorized into 3 distinct risk groups according to the number of risk factors and similar HRs. Compared with the low-risk group (0–2 risk factors; n=230), the medium-risk group (3 risk factors; n=163) and the high-risk group (4–6 risk factors; n=143) had a 2.74-fold (95% CI 1.81–4.14; P<0.001) and 4.89-fold (95% CI 3.29–7.26; P<0.001) higher risk of death, respectively. The 5-year OS rates of the 3 groups were 86.1%, 65.0%, and 46.2%, respectively, with significant difference (log-rank P<0.001) (Figure 3). We subsequently compared the predictive power of the risk stratification model with the 7th edition of AJCC TNM system. The AUC for our model was 0.72 (95% CI 0.68–0.77), which was higher than that for the TNM system (AUC=0.61). Furthermore, the analysis showed that multiple risk factors had a significant dose-dependent effect on patient survival (P for trend <0.001).
Figure 3.
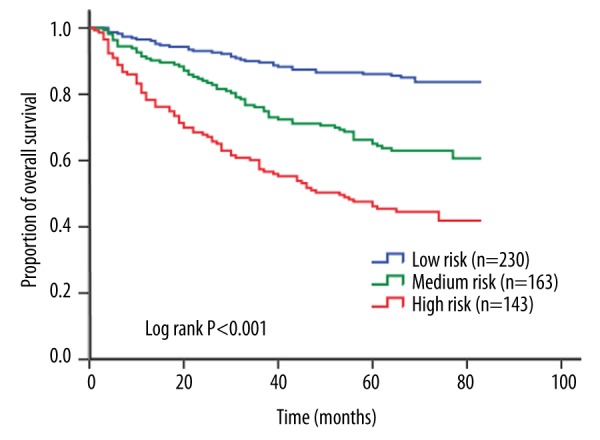
Kaplan-Meier curves for overall survival according to risk stratification model. The 5-year overall survival rates were 86.1%, 65.0%, and 46.2% for patients with low risk, medium risk, and high risk, respectively, with significant difference (log-rank P<0.001).
Discussion
In this study, we developed a novel index – HLAN and found that it was significantly associated with the prognosis of patients with LACRC. Moreover, the risk stratification model based on HLAN displayed better accuracy in survival prediction than did the TNM system. To the best of our knowledge, this is the first study to investigate the prognostic value of HLAN in CRC.
It is well known that systemic inflammation and nutritional status play important roles in the prognosis of cancer patients [5,17,18]. Both hemoglobin and albumin are commonly used markers for assessing patient nutritional status. With the progression of cancer, the levels of hemoglobin and albumin fall sharply because malnutrition and systemic inflammatory response to tumor suppress their synthesis [8,19]. Large studies have indicated that anemia and malnutrition can cause many adverse clinical consequences, including decreased quality of life, reduced response to treatment, increased risk of chemotherapeutic toxicity, and a reduction in cancer survival [9,20]. Neutrophil and lymphocyte counts can be used to reflect the balance between tumor-promoting inflammatory response and antitumor immune function [21]. Neutrophils plays a key role in tumor proliferation and metastasis by releasing reactive oxygen species (ROS), vascular endothelial growth factor (VEGF), and other factors [22,23]. On the other hand, the function of lymphocytes is to induce cytotoxic cell death and cytokine production that inhibit cancer development [24,25]. Therefore, an increased number of neutrophils or a decreased number of lymphocytes confers a poor prognosis [21]. We integrated these 4 factors to form a superior index (HLAN), and for clinical convenience we divided this index by 100. Our results indicated the prognostic value of HLAN in LACRC patients, suggesting that lower HLAN was strongly correlated with a worse clinical outcome. In addition, the X-tile program, which is a new tool for biomarker assessment [15], was used in this study for the optimal cut-point selection of HLAN. We found the cut-off value of 19.5 had a relatively high sensitivity and specificity for distinction.
Hematological indexes are promising prognostic factors of cancer patients. However, a single index may not have sufficient predictive power for clinical application. The joint analysis of multiple markers can enhance the predictive power. A large European study on the prognosis of renal cell carcinoma patients revealed that the concordance index (C-index, equivalent to AUC) of the original Leibovich model was 0.79, compared with 0.81 when NLR was supplemented [26]. Huang et al. [27] performed an analysis in 349 patients with hepatocellular carcinoma and showed that the combined GPS and Cancer of the Liver Italian Program (CLIP) score (C-index=0.705) were superior to CLIP alone (C-index=0.686) in prognostic ability. Liu et al. [28] also found that when serum albumin, lactate dehydrogenase, and total bilirubin were added into the traditional prognostic model (including age and nuclear grade) for breast cancer, the AUC increased from 0.748 to 0.766. When we incorporated HLAN and other significant predictors revealed in the present study, the AUC for the risk stratification model reached 0.72. Compared with the TNM system, our model showed better predictive accuracy, highlighting the cumulative effect of multiple markers in prognostic prediction. This risk model may allow clinicians to identify LACRC patients at high risk of poor survival before the treatment and to make better clinical decisions and follow-up surveillance for patients.
Some limitations of this study should be acknowledged. First, these analyses are based on data obtained from a single institution in China. Second, this was a retrospective study, and the potential for selection bias is unavoidable. In addition, other CRC risk factors not included in this study cannot be examined for confounding effects. As a result, large prospective studies are required to further confirm our findings.
Conclusions
Our study demonstrates that HLAN is an independent prognostic factor for survival of patients with LACRC, and the risk stratification model based on HLAN can serve as a useful guide in clinical practice.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81272480) and the Natural Science Foundation of Shanghai (No. 15411969900, ZK2015A32, and 201540132)
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2012;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol. 2009;3:131–44. doi: 10.1586/egh.09.8. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Bekaii-Saab T, Chan E, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:519–28. doi: 10.6004/jnccn.2013.0069. [DOI] [PubMed] [Google Scholar]
- 4.Shalapour S, Karin M. Immunity, inflammation, and cancer: An eternal fight between good and evil. Clin Invest. 2015;125:3347–55. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–26. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 6.Ramos Chaves M, Boleo-Tome C, Monteiro-Grillo I, et al. The diversity of nutritional status in cancer: New insights. Oncologist. 2010;15:523–30. doi: 10.1634/theoncologist.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–55. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–21. [PubMed] [Google Scholar]
- 9.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB. 2007;9:456–60. doi: 10.1080/13651820701774891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–58. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 12.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–40. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–49. doi: 10.1007/s00432-014-1714-3. [DOI] [PubMed] [Google Scholar]
- 14.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–59. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 16.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 17.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 18.Brookes GB. Nutritional status – a prognostic indicator in head and neck cancer. Otolaryngol Head Neck Surg. 1985;93:69–74. doi: 10.1177/019459988509300114. [DOI] [PubMed] [Google Scholar]
- 19.Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32:S118–25. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 20.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med. 2004;116:11–26. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–13. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 22.Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–64. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman PM. Pathobiology of the neutrophil-intestinal epithelial cell interaction: role in carcinogenesis. World J Gastroenterol. 2010;16:5790–800. doi: 10.3748/wjg.v16.i46.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90:2053–58. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901–7. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Xu L, Luo Y, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol. 2014;31:883. doi: 10.1007/s12032-014-0883-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Meng QH, Ye Y, et al. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36:243–48. doi: 10.1093/carcin/bgu247. [DOI] [PubMed] [Google Scholar]


