Abstract
Aim
Pulmonary hypertension (PH) develops in 25–40% of bronchopulmonary dysplasia (BPD) patients, substantially increasing mortality. We have previously found that asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide (NO) production, is elevated in patients with BPD-associated PH. ADMA is metabolized by NG,NG- dimethylarginine dimethylaminohydrolase (DDAH). Presently, we test the hypothesis that there are single nucleotide polymorphisms (SNPs) in DDAH1 and/or DDAH2 associated with the development of PH in BPD patients.
Methods
BPD patients were enrolled (n=98) at Nationwide Children’s Hospital. Clinical characteristics and 36 SNPs in DDAH1 and DDAH2 were compared between BPD-associated PH patients (cases) and BPD-alone patients (controls).
Results
In BPD patients, 25 (26%) had echocardiographic evidence of PH (cases). In this cohort, DDAH1 wildtype rs480414 was 92% sensitive and 53% specific for PH in BPD, and the DDAH1 SNP rs480414 decreased the risk of PH in an additive model of inheritance (OR=0.39; 95% CI [0.18–0.88], p=0.01).
Conclusion
The rs480414 SNP in DDAH1 may be protective against the development of PH in patients with BPD. Furthermore, the DDAH1 rs480414 may be a useful biomarker in developing predictive models for PH in patients with BPD.
Keywords: neonate, prematurity, urea, nitric oxide
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common complication following preterm birth (1). The lungs in patients with BPD are characterized by areas of emphysema surrounded by atelectasis with widespread bronchial and bronchiolar mucosal hyperplasia and metaplasia (2, 3). Pulmonary hypertension (PH) is a relatively common complication of BPD, estimated to develop in 25–40% of patients with BPD (3,5–7) and the development of PH is associated with a marked increase in morbidity and mortality in BPD patients (1, 4, 5). The PH in BPD patients is characterized by decreased vascular surface area and vasoconstriction, which contribute to increased vascular resistance leading to the higher pulmonary arterial pressures found in BPD patients with PH (6, 7).
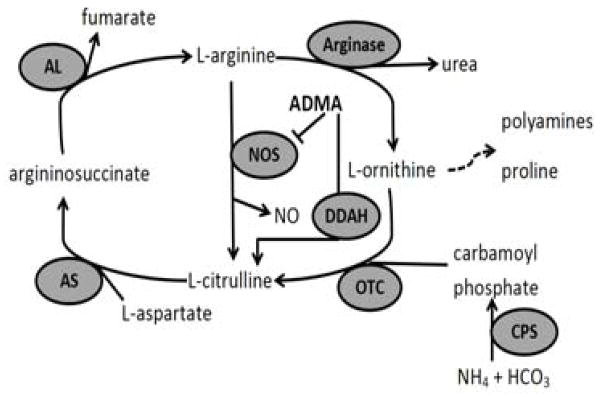
Nitric oxide (NO) is an endogenous pulmonary vasodilator produced by endothelial NO synthase (eNOS) (8), and decreased eNOS-derived NO underlies many types of PH. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of eNOS, is a biomarker for some cardiovascular diseases and/or mortality in adults (9, 10). Recently, we found that BPD patients with PH had significantly higher plasma levels of ADMA than did patients with BPD alone, and this increase in plasma ADMA levels was seen prior to the onset of BPD (11). ADMA is hydrolyzed to citrulline by NG,NG-dimethylarginine dimethylaminohydrolase (DDAH) (Figure 1), and there are 2 isoforms encoded by 2 different genes DDAH1 and DDAH2 (12–16). In adults with type II diabetes, single nucleotide polymorphisms (SNP) in DDAH1 and DDAH2 have been correlated with serum ADMA levels (17). Therefore, we hypothesized that SNPs in DDAH would be associated with the development of PH in BPD patients, and that these SNPs may be biomarkers for the development of PH in BPD patients.
Figure 1. Arginine metabolism in the pulmonary vascular wall.
Arginine metabolism occurs in endothelial cells with effects on vascular smooth muscle cells. L-arginine/NO pathway enzymes; carbamoyl phosphate synthetase (CPS), ornithine transcarbamylase (OTC), argininosuccinate synthetase (AS), argininosuccinate lyase (AL), arginase, nitric oxide synthase (NOS). Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of NOS. Dimethylarginine dimethylaminohydrolase (DDAH) degrades ADMA to L-citrulline.
METHODS
The Institutional Review Board at Nationwide Children’s Hospital (NCH) approved this study. All patients admitted to the Nationwide Children’s Hospital NICU between September 1, 2009 and December 31, 2013 with the diagnosis of BPD, were eligible for this study. BPD was defined according to the NICHD consensus statement as a supplemental oxygen requirement at 28 days of life (2). Some of the patients enrolled in the current study have been enrolled in previous research studies (11, 18). Enrollment, clinical data abstraction, and specimen collection were completed through the Ohio Perinatal Research Network (OPRN) and Perinatal Research Repository (PRR) at The Research Institute at NCH, Columbus, Ohio, USA.
Pulmonary Hypertension
PH was identified in BPD patients as evidence of abnormally elevated pulmonary arterial pressure on echocardiography in a structurally normal heart after 28 days of age (cases). Elevated pulmonary arterial pressure on echocardiography was defined by the presence of any of the following four criteria: 1. right ventricular hypertrophy 2. flattening of the intraventricular septum 3. tricuspid regurgitation (TR) and/or 4. pulmonary regurgitation (4, 19–22). Infants with BPD who did not have PH according to these criteria were considered controls. BPD patients with congenital heart disease were excluded from the study. Patients with anatomical causes of PH, including congenital diaphragmatic hernia and lung hypoplasia, were excluded from the study.
Single Nucleotide Polymorphisms
Patient blood samples were collected at the earliest convenient date after enrollment. Since patient DNA sequence was expected to remain stable throughout the patient’s lifetime, variation in collection date was allowed and occurred at less than one year of age. Blood samples were collected, maintained on ice for no more than 6 hours, and centrifuged. DNA was isolated from blood leukocytes and assayed by Sequenom MassArray (Sequenom, San Diego, CA). There are 2 isoforms of DDAH, DDAH1 and DDAH2. Since there are thousands of DDAH SNPs, and we were limited to 30–50 SNPs on our available technology, we selected DDAH SNPs from the NCBI/SNP database for their putative impact on protein function. Furthermore, a literature search was performed on all DDAH SNPs previously associated with disease. This inquiry resulted in a list of SNPs that had been identified as important in other cardiovascular diseases and/or were located in a region of the gene that might affect transcription. Consequently, we studied 36 SNPs: 32 in DDAH1 and 4 in DDAH2.
Statistics
Demographic and clinical characteristics were compared between cases and controls. Continuous, normally distributed variables were compared using the Student’s t-test. Categorical variables were compared using the χ2 test. Calculated minor allele frequencies (MAF) of cases and controls were compared using the χ2 test. Analysis of the distributions of genotypes was performed by χ2 analysis with 1 degree of freedom (23). A p-value < 0.05 was considered to be significant. Logistic regression was used to analyze seven SNPs in DDAH1, whose association with PH had a p<0.10. Genetic models assuming recessive, dominant, and additive modes of inheritance of the resulting seven DDAH1 SNPs were analyzed to estimate the odds ratio of the risk of individual genotypes in developing PH in BPD. There were insufficient patient samples to correlate DDAH1 SNPs with previously studied ADMA levels (11). STATA/IC 12.0 (STATA Corp., College Station, TX) statistical software was used to complete all of the analyses in this study.
Results
In this cohort of 98 patients with BPD, 25 (26%) had echocardiographic evidence of PH (cases). The remaining 73 patients had BPD alone (controls). Demographic and clinical characteristics are shown in Table 1. Overall, there were no significant differences between cases and controls, except for gender. More controls were male than cases (78% versus 44%, p<0.01). Interestingly, we found that cases of PH were more likely to require and/or receive >30% O2 at 36 weeks CGA (52% versus 29%, p=0.04) and conventional mechanical ventilation (40% versus 18%, p=0.02), as compared to BPD controls. We found that both inhaled NO and sildenafil treatments were used infrequently in this preterm cohort of BPD patients, and that sildenafil use was higher in BPD and PH patients than in BPD alone (12% versus 1%, p=0.02). Lastly, one patient died, and this patient had BPD-associated PH. The minor allele frequencies (MAF) for cases and controls for the 36 SNPs analyzed in DDAH1 and DDAH2 are shown in Table 2. We found that the SNP (A-allele) rs480414 in DDAH1 was less common in cases (MAF= 0.20) than in controls (MAF=0.34, p=0.03).
Table 1.
Demographic and clinical characteristics.
| BPD alone (n=73) | BPD+PH (n=25) | p-value | |
|---|---|---|---|
| Gestational age, weeks | 27 ± 3 | 27 ± 3 | 0.60 |
| Birth weight, grams | 978 ± 518 | 982 ± 479 | 0.98 |
| Gender (male), n (%) | 57 (78) | 11 (44) | <0.01 |
| Race (caucasian), n (%) | 47 (64) | 19 (76) | 0.29 |
| Age at admission, days | 45 ± 93 | 29 ± 45 | 0.35 |
| APGAR score at 5 minutes | 6 ± 3 | 6 ± 2 | 0.37 |
| Ventilation, days | 29 ± 33 | 34 ± 39 | 0.60 |
| >30% O2 at 36wks CGA, n (%) | 21 (29) | 13 (52) | 0.04 |
| CPAP at 36wks CGA, n (%) | 12 (16) | 5 (20) | 0.69 |
| CMV at 36wks CGA, n (%) | 13 (18) | 10 (40) | 0.02 |
| Inhaled Nitric oxide, n (%) | 6 (8) | 4 (16) | 0.27 |
| Sildenafil, n (%) | 1 (1) | 3 (12) | 0.02 |
| Intestinal perforation, n (%) | 2 (3) | 3 (12) | 0.07 |
| Patent ductus arteriosus, n (%) | 38 (52) | 17 (68) | 0.17 |
| Discharge weight, grams | 3889 ± 1647 | 3867 ± 1079 | 0.95 |
| Oxygen at discharge, n (%) | 52 (71) | 19 (76) | 0.65 |
| death | 0 (0) | 1 (4) | 0.09 |
Continuous variables were compared using the Student’s t-test and presented as mean ± standard deviation. Categorical variables were compared using the χ2 test and presented as number and percentage. CGA, corrected gestational age; CPAP, continuous positive airway pressure; CMV, conventional mechanical ventilation.
Table 2.
Description and calculated minor allele frequencies (MAF) for 36 SNPs in DDAH1 and DDAH2.
| Gene | SNP-rs | Nucleotide change | Functional consequence | Calculated MAF: BPD alone (n=73) | Calculated MAF: BPD+PH (n=25) | p-value |
|---|---|---|---|---|---|---|
| DDAH1 | 2935 | G>A | intron variant | 0.130 | 0.100 | 0.29 |
| DDAH1 | 233080 | G>A | intron variant | 0.322 | 0.280 | 0.29 |
| DDAH1 | 233112 | G>C | utr variant 3 prime | 0.329 | 0.420 | 0.12 |
| DDAH1 | 233115 | C>A | utr variant 3 prime | 0.315 | 0.420 | 0.09 |
| DDAH1 | 233130 | G>C | intron variant | 0.315 | 0.420 | 0.09 |
| DDAH1 | 480414 | G>A | intron variant | 0.342 | 0.200 | 0.03 |
| DDAH1 | 539714 | T>C | intron variant | 0.171 | 0.220 | 0.22 |
| DDAH1 | 553257 | T>G | intron variant | 0.192 | 0.180 | 0.43 |
| DDAH1 | 669173 | T>C | intron variant | 0.411 | 0.400 | 0.45 |
| DDAH1 | 877041 | G>A | intron variant | 0.356 | 0.300 | 0.24 |
| DDAH1 | 954353 | T>G | upstream variant 2KB | 0.390 | 0.340 | 0.26 |
| DDAH1 | 974874 | A>G | intron variant | 0.240 | 0.320 | 0.13 |
| DDAH1 | 986639 | G>C | intron variant | 0.164 | 0.220 | 0.19 |
| DDAH1 | 1241321 | T>G | intron variant | 0.301 | 0.340 | 0.31 |
| DDAH1 | 1403951 | G>A | intron variant | 0.459 | 0.480 | 0.40 |
| DDAH1 | 1403955 | A>G | intron variant | 0.390 | 0.500 | 0.09 |
| DDAH1 | 1498373 | C>A | intron variant | 0.260 | 0.360 | 0.09 |
| DDAH1 | 1498374 | G>T | intron variant | 0.158 | 0.200 | 0.24 |
| DDAH1 | 2177461 | G>C | intron variant | 0.336 | 0.440 | 0.09 |
| DDAH1 | 2474123 | G>A | intron variant; upstream variant 2KB | 0.370 | 0.440 | 0.19 |
| DDAH1 | 3738111 | T>G | intron variant | 0.130 | 0.100 | 0.29 |
| DDAH1 | 3753793 | G>C | upstream variant 2KB | 0.322 | 0.240 | 0.13 |
| DDAH1 | 7521189 | G>A | intron variant | 0.479 | 0.440 | 0.31 |
| DDAH1 | 10782551 | G>A | intron variant | 0.075 | 0.100 | 0.29 |
| DDAH1 | 11161614 | T>G | intron variant | 0.178 | 0.200 | 0.36 |
| DDAH1 | 11161618 | C>T | intron variant | 0.322 | 0.400 | 0.16 |
| DDAH1 | 11161637 | A>G | intron variant | 0.308 | 0.260 | 0.26 |
| DDAH1 | 12132677 | A>C | intron variant | 0.151 | 0.200 | 0.07 |
| DDAH1 | 12568675 | T>C | intron variant | 0.082 | 0.080 | 0.48 |
| DDAH1 | 13373844 | A>C | intron variant | 0.233 | 0.220 | 0.43 |
| DDAH1 | 17384213 | G>A | intron variant | 0.103 | 0.160 | 0.14 |
| DDAH1 | 17590006 | A>G | intron variant | 0.158 | 0.220 | 0.16 |
| DDAH2 | 707916 | T>G | intron variant; upstream variant 2KB; utr variant 5 prime | 0.425 | 0.440 | 0.43 |
| DDAH2 | 805304 | C>T | downstream variant 500B; upstream variant 2KB | 0.425 | 0.440 | 0.43 |
| DDAH2 | 805305 | G>C | intron variant; upstream variant 2KB; utr variant 5 prime | 0.370 | 0.360 | 0.45 |
| DDAH2 | 2272592 | G>T | downstream variant 500B; upstream variant 2KB | 0.158 | 0.120 | 0.26 |
SNP, single nucleotide polymorphism; MAF, minor allele frequency; BPD, bronchopulmonary dysplasia; PH, pulmonary hypertension; rs, NCBI dbSNP number; DDAH, dimethylarginine dimethylaminohydrolase.
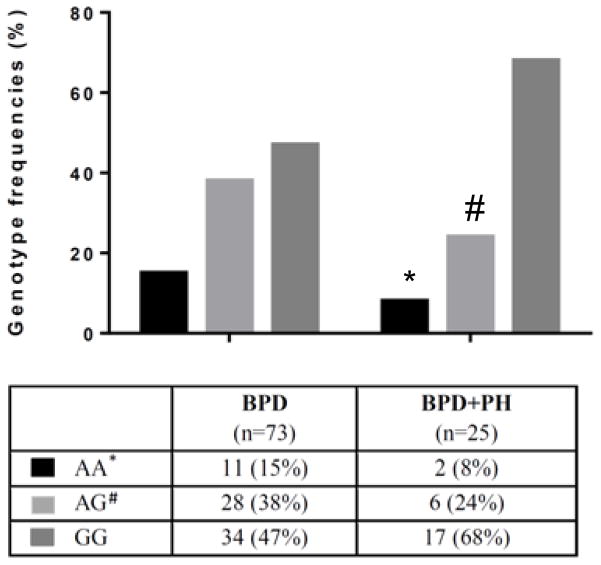
The genotype frequencies for the DDAH1 SNP rs480414 are shown in Figure 2. The percentage of patients with at least one copy of the A-allele (AA+AG) was lower in the BPD+PH group than in the controls [8/25 (32%) vs. 39/73 (53%), p<0.01]. Calculations for Hardy-Weinberg equilibrium were not significant for cases, controls, or cases + controls. Sensitivity and specificity were calculated to determine the predictive value of the DDAH1 wildtype (G-allele) rs480414 for BPD-PH disease. BPD-PH patients were found to have DDAH1 wildtype (G-allele) rs480414 in 23/25 BPD-PH cases, for a calculated sensitivity of 92%. BPD-PH patients were found to have DDAH1 SNP (A-allele) rs480414 in 39/73 controls, for a calculated specificity of 53%.
Figure 2. Genotype frequencies for the rs480414 SNP in the DDAH1 gene in BPD+PH patients.
A is the minor allele (SNP), and G is the major allele (wildtype). The percentage of patients with BPD+PH for each genotype: AA [2/25 (8%)]; AG [6/25 (24%)]; GG [17/25 (68%)]. * BPD+PH different from BPD alone, p<0.01; # BPD+PH different from BPD alone, p<0.05.
In order to assess the predictive value of DDAH1 SNPs for BPD-associated PH, we constructed genetic models based on three modes of inheritance (recessive, dominant, and additive) for seven DDAH1 SNPs for which the MAF associations had a p-value of <0.1 (see Table 3). In the additive model of inheritance, the odds of PH were decreased by 61% for each copy of the minor allele (A) of the rs480414 SNP in patients with BPD (OR per additional A allele 0.39 [0.18–0.88], p=0.01).
Table 3.
Genetic modeling of BPD-associated pulmonary hypertension: rs480414 SNP in DDAH1.
| Genetic model | BPD alone, n (%) | BPD+PH, n (%) | p-value1 | OR (95% CI)2 | p-value2 |
|---|---|---|---|---|---|
| rs233115 | |||||
| Recessive | 34 (46.6) | 8 (32.0) | 0.20 | 0.54 (0.21–1.41) | 0.20 |
| Dominant | 39 (53.4) | 17 (68.0) | 0.20 | 1.85 (0.71–4.83) | 0.20 |
| Additive | 8 (11.0) | 4 (16.0) | 0.32 | 1.60 (0.83–3.09) | 0.16 |
| rs233130 | |||||
| Recessive | 34 (46.6) | 8 (32.0) | 0.20 | 0.54 (0.21–1.41) | 0.20 |
| Dominant | 39 (53.4) | 17 (68.0) | 0.20 | 1.85 (0.71–4.83) | 0.20 |
| Additive | 8 (11.0) | 4 (16.0) | 0.32 | 1.60 (0.83–3.09) | 0.16 |
| rs480414 | |||||
| Recessive | 34 (46.6) | 17 (68.0) | 0.06 | 2.44 (0.94–6.35) | 0.06 |
| Dominant | 39 (53.4) | 8 (32.0) | 0.06 | 0.41 (0.16–1.07) | 0.06 |
| Additive | 11 (15.1) | 1 (4.0) | 0.06 | 0.39 (0.18–0.88) | 0.01 |
| rs1403955 | |||||
| Recessive | 29 (39.7) | 5 (20.0) | 0.07 | 0.38 (0.13–1.12) | 0.06 |
| Dominant | 44 (60.3) | 20 (80.0) | 0.07 | 2.64 (0.89–7.81) | 0.06 |
| Additive | 12 (16.4) | 5 (20.0) | 0.37 | 1.47 (0.77–2.80) | 0.24 |
| rs1498373 | |||||
| Recessive | 42 (57.5) | 11 (44.0) | 0.24 | 0.58 (0.23–1.45) | 0.24 |
| Dominant | 31 (42.5) | 14 (56.0) | 0.24 | 1.72 (0.69–4.31) | 0.24 |
| Additive | 6 (8.2) | 4 (16.0) | 0.49 | 1.45 (0.75–2.79) | 0.27 |
| rs2177461 | |||||
| Recessive | 32 (43.8) | 7 (28.0) | 0.16 | 0.50 (0.19–1.34) | 0.16 |
| Dominant | 41 (56.2) | 18 (72.0) | 0.16 | 2.01 (0.75–5.39) | 0.16 |
| Additive | 7 (9.6) | 4 (16.0) | 0.25 | 1.76 (0.88–3.50) | 0.11 |
| rs12132677 | |||||
| Recessive | 35 (48.0) | 9 (36.0) | 0.30 | 0.61 (0.24–1.56) | 0.30 |
| Dominant | 38 (52.1) | 16 (64.0) | 0.30 | 0.54 (0.21–1.41) | 0.20 |
| Additive | 9 (12.3) | 4 (16.0) | 0.51 | 1.41 (0.74–2.68) | 0.30 |
Genotype frequencies analyzed with chi-square test.
Univariate logistic regression.
SNP, single nucleotide polymorphism; BPD, bronchopulmonary dysplasia; rs, NCBI dbSNP number; DDAH, dimethylarginine dimethylaminohydrolase.
Discussion
The major objective of the present study was to investigate the association of SNPs in DDAH1 and DDAH2 with the development of PH in patients with BPD. The major findings were that 1) BPD-PH patients had more severe lung disease than patients with BPD alone, 2) the DDAH1 rs480414 SNP (A-allele) is associated with a lower incidence of PH in patients with BPD, 3) the DDAH1 rs480414 wildtype (G-allele) was 92% sensitive and 53% specific for PH in BPD patients, and 4) the risk of PH was decreased by 61% for each copy of the minor allele (A) of the rs480414 SNP in patients with BPD. These findings are consistent with our hypothesis that SNPs in DDAH are associated with the development of PH in BPD patients. In this cohort of BPD patients, DDAH1 rs480414 SNP appears to play a protective role against the development of PH.
DDAH activity is important in regulating intracellular ADMA concentrations and thus, modulating NO production in vivo (12–14, 24). NO works to maintain vascular homeostasis by promoting vasodilation, suppressing inflammation, smooth muscle cell proliferation, and platelet adhesion and aggregation (17, 25, 26). In adults with type 2 diabetes, 5 SNPs in DDAH1 have been associated with plasma levels of ADMA, 3 of the SNPs (rs13373844, rs7521189, and rs669173) resulted in lower levels of plasma ADMA (19). Since we found that the rs480414 SNP in DDAH1 was associated with a lower incidence of PH in BPD, and since we previously reported that BPD patients with PH had higher plasma levels of ADMA (12), it is reasonable to postulate that BPD patients with the rs480414 SNP might have lower plasma levels of ADMA. This postulate would be consistent with the notion that the rs480414 SNP resulted in greater DDAH1 activity, however, further studies are needed to determine the actual effect of the rs480414 SNP on DDAH1 protein function (17).
The rs480414 G/A SNP is located in the intron of the DDAH1 gene on chromosome 1p22. Although the intron is a non-coding region, recent studies have shown that mutations in non-coding parts of the genome can be associated with disease progression (27). Introns can work to positively regulate gene expression by stimulating mRNA accumulation through a process known as intron-mediated enhancement (28). Alternatively, alterations in intron DNA may affect pre-mRNA splicing, a process that determines the pattern of exons and/or introns that are selectively included or excluded when generating mature mRNA. These alternative spliced mRNAs may have subsequent differential effects on protein function, such as changes in protein-protein interaction, subcellular localization, and flux through metabolic pathways (29). Further studies are necessary to elucidate the effects of this SNP on DDAH protein levels/activity.
There are several limitations to this study. First, BPD is a rare disease as defined by the Office of Rare Diseases at the NIH, so the sample size in this single center study over a reasonable time period is small. However, our findings suggest that a SNP in DDAH1 may be a potential novel biomarker for BPD-associated PH. Furthermore, several other SNPs had a p-value in this relatively small cohort of <0.10 and therefore may warrant further investigation in future studies. Another limitation of our study and any study looking at PH in BPD during the initial NICU stay, is the difficulty of diagnosing PH in the newborn period. Catheterization remains the gold standard for PH diagnosis in children and adults, however the morbidities and mortality of catheterization increase in neonates and therefore bedside echocardiography is currently the most commonly used test to diagnose PH in neonates (4).
Our findings suggest that genetic variations in DDAH, and specifically, DDAH1, may be associated with the development of PH in preterm infants who have BPD. Together with our previous work which found elevated levels of ADMA in BPD patients who went on to develop PH (11), the current study supports the role of the ADMA-DDAH axis in the pathogenesis of PH in BPD, and may provide important biomarkers to predict the onset of PH in BPD. PH in BPD remains the leading cause of mortality in this population, such that identifying novel targets and/or biomarkers that can speed the development of therapeutic approaches to prevent disease and improve outcomes is crucial for these patients.
Key Notes.
Bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) is a significant contributor to neonatal mortality, and biomarkers to predict which BPD patients will develop PH are needed to potentially prevent and diagnose disease.
In the present study, we found that the DDAH1 wildtype rs480414 is 92% sensitive and 53% specific for PH in patients with BPD.
The DDAH1 single nucleotide polymorphism (SNP) rs480414 was associated with a decreased risk of PH in an additive model of inheritance.
Acknowledgments
This study was funded by an intra-mural grant from the Center for Clinical and Translational Research at The Research Institute, Nationwide Children’s Hospital (CTSA grant UL1TR001070).
The authors would like to thank research staff of the Ohio Perinatal Research Network (OPRN).
Abbreviations
- ADMA
asymmetric dimethylarginine
- BPD
bronchopulmonary dysplasia
- DDAH
dimethylarginine dimethylaminohydrolase
- NO
nitric oxide
- PH
pulmonary hypertension
- rs
NCBI dbSNP number
- SNP
single nucleotide polymorphism
Footnotes
The authors declare no conflict of interest.
References
- 1.Baker CD, Abman SH, Mourani PM. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatric allergy, immunology, and pulmonology. 2014;27:8–16. doi: 10.1089/ped.2013.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 3.Strueby L, Thebaud B. Advances in bronchopulmonary dysplasia. Expert review of respiratory medicine. 2014;8:327–338. doi: 10.1586/17476348.2014.899907. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 5.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 6.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25:329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 7.Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. 2015;107:344–351. doi: 10.1159/000381129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelin LD, Moshin J, Thomas CJ, Sasidharan P, Dawson CA. The effect of inhaled nitric oxide on the pulmonary circulation of the neonatal pig. Pediatr Res. 1994;35:20–24. doi: 10.1203/00006450-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. Journal of the American Heart Association. 2015;4(6) doi: 10.1161/JAHA.115.001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boger RH. Asymmetric dimethylarginine: understanding the physiology, genetics, and clinical relevance of this novel biomarker. Proceedings of the 4th International Symposium on ADMA Pharmacological research: the official journal of the Italian Pharmacological Society. 2009;60:447. doi: 10.1016/j.phrs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Trittmann JK, Peterson E, Rogers LK, Chen B, Backes CH, Klebanoff MA, et al. Plasma Asymmetric Dimethylarginine Levels Are Increased in Neonates with Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003;4:33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 13.Pope AJ, Karuppiah K, Cardounel AJ. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacological research: the official journal of the Italian Pharmacological Society. 2009;60:461–465. doi: 10.1016/j.phrs.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 15.Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of pathology. 2013;229:242–249. doi: 10.1002/path.4127. [DOI] [PubMed] [Google Scholar]
- 16.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. The Biochemical journal. 1999;343(Pt 1):209–214. [PMC free article] [PubMed] [Google Scholar]
- 17.Abhary S, Burdon KP, Kuot A, Javadiyan S, Whiting MJ, Kasmeridis N, et al. Sequence variation in DDAH1 and DDAH2 genes is strongly and additively associated with serum ADMA concentrations in individuals with type 2 diabetes. PLoS One. 2010;5:e9462. doi: 10.1371/journal.pone.0009462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trittmann JK, Nelin LD, Zmuda EJ, Gastier-Foster JM, Chen B, Backes CH, et al. Arginase I gene single-nucleotide polymorphism is associated with decreased risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr. 2014;103:e439–443. doi: 10.1111/apa.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summar ML, Hall L, Christman B, Barr F, Smith H, Kallianpur A, et al. Environmentally determined genetic expression: clinical correlates with molecular variants of carbamyl phosphate synthetase I. Mol Genet Metab. 2004;81(Suppl 1):S12–19. doi: 10.1016/j.ymgme.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 21.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 22.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37:124–131. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 24.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci U S A. 2002;99:13527–13532. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamler J, Mendelsohn ME, Amarante P, Smick D, Andon N, Davies PF, et al. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circulation research. 1989;65:789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- 26.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, et al. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circulation research. 2000;87:999–1005. doi: 10.1161/01.res.87.11.999. [DOI] [PubMed] [Google Scholar]
- 27.Patrushev LI, Kovalenko TF. Functions of noncoding sequences in mammalian genomes. Biochemistry Biokhimiia. 2014;79:1442–1469. doi: 10.1134/S0006297914130021. [DOI] [PubMed] [Google Scholar]
- 28.Gallegos JE, Rose AB. The enduring mystery of intron-mediated enhancement. Plant science: an international journal of experimental plant biology. 2015;237:8–15. doi: 10.1016/j.plantsci.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Ravi S, Schilder RJ, Kimball SR. Role of Precursor mRNA Splicing in Nutrient-Induced Alterations in Gene Expression and Metabolism. The Journal of nutrition. 2015;145:841–846. doi: 10.3945/jn.114.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]