Abstract
Possession of the Apolipoprotein E (APOE) gene ε4 allele is the most prevalent genetic risk factor for late onset Alzheimer’s disease (AD). Recent evidence suggests that APOE genotype differentially affects the expression of brain-derived neurotrophic factor (BDNF). Notably, aerobic exercise-induced upregulation of BDNF is well documented; and exercise has been shown to improve cognitive function. As BDNF is known for its role in neuroplasticity and survival, its upregulation is a proposed mechanism for the neuroprotective effects of physical exercise. In this pilot study designed to analyze exercise-induced BDNF upregulation in an understudied population, we examined the effects of ApoEε4 (ε4) carrier status on changes in BDNF expression after a standardized exercise program. African Americans, age 55 years and older, diagnosed with mild cognitive impairment participated in a six-month, supervised program of either stretch (control treatment) or aerobic (experimental treatment) exercise. An exercise-induced increase in VO2Max was detected only in male participants. BDNF levels in serum were measured using ELISA. Age, screening MMSE scores and baseline measures of BMI, VO2Max, and BDNF did not differ between ε4 carriers and non-ε4 carriers. A significant association between ε4 status and serum BDNF levels was detected. Non-ε4 carriers showed a significant increase in BDNF levels at the 6 month time point while ε4 carriers did not. We believe we have identified a relationship between the ε4 allele and BDNF response to physiologic adaptation which likely impacts the extent of neuroprotective benefit gained from engagement in physical exercise. Replication of our results with inclusion of diverse racial cohorts, and a no-exercise control group will be necessary to determine the scope of this association in the general population.
Keywords: Aerobic Exercise, BDNF, APOE, Oxygen Consumption, African Americans, Mild Cognitive Impairment
1. Introduction
Alzheimer’s disease (AD) is associated with many risk factors, advanced age being the strongest, with a doubling of risk every 5 years past the age of 65. African Americans (AA)s are also at higher risk for AD with estimated prevalence ranging from 14% to 100% higher than Caucasians [1–4]. Despite the disproportionately high incidence of AD within the AA population, this group remains severely underrepresented in clinical trials on AD [5]. Importantly, AAs also suffer from higher burdens of several diseases that increase the risk for AD including type-2 diabetes (T2DM) and cardiovascular disease (CVD).
Along-side investigations into putative treatments for AD, there is growing interest in the mechanisms underlying the health benefits of lifestyle adaptation, especially in light of its demonstrated impact on T2DM and CVD [6, 7]. In particular, aerobic exercise has been shown to improve cognitive function, in both young and elderly populations [8–10]. While exercise-induced upregulation of brain-derived neurotrophic factor (BDNF) is well documented [11–16], there are inconsistencies, with a few studies reporting either no change or lowered serum BDNF levels in exercised subjects [17–19]. These inconsistencies are poorly understood. Regardless, BDNF is critically important for neuronal differentiation, synaptic plasticity and neuron survival [20, 21]. In humans, increased BDNF levels have been linked to increased hippocampal volume and spatial memory performance [22], whereas decreased BDNF is linked to AD and other psychiatric disorders [22–27]. Thus, BDNF upregulation is a supported, proposed mechanism for the cognitive-enhancement triggered by lifestyle adaptation from physical exercise.
Recent evidence suggests that BDNF expression is differentially affected by variants of the apolipoprotein E (APOE) gene [28–30]. The APOE gene is polymorphic with three major alleles: ε2, ε3 and ε4. These gene variants produce proteins with differing physiologic properties and variable associations with health risk [31]. APOE is responsible for trafficking of lipoproteins, fat-soluble vitamins and cholesterol[32]. The ε4 allele has been associated with the development of atherosclerosis [33] and cardiovascular disease [34], both of which increase AD risk. In fact, hetero- and homozygosity for the ε4 allele incurs a 3 fold and 12 fold increased risk for AD, respectively, compared to those homozygous for the ε3 allele [35]. Though AAs are at higher risk of AD compared to Caucasian Americans, whether the APOE gene differentially influence exercise training-induced changes in BDNF levels in older AA, mild cognitively impaired (MCI) subjects has not be examined.
The aim of this pilot study was to examine the effects of a 6-month supervised and standardized exercise program, on serum levels of BDNF, in mild cognitively impaired elderly AAs. We hypothesized that an increase in aerobic capacity would result in a parallel increase in BDNF levels. Although, there was some expectation of baseline differences in serum BDNF levels based on APOE genotype, we anticipated an exercise-induced increase in serum BDNF across all APOE genotypes. Through our analyses, we identified an unexpected association between APOEε 4 status and 6-month changes in serum BDNF levels.
2. Methods and Materials
The protocols used in this investigation were approved by the Howard University Institutional Review Board. As required for studies involving human subjects, all participants completed a signed informed consent form prior to enrollment in the study. Participants were recruited mostly through newspaper advertisement, direct mailing, health fairs and hospital clinics.
2.1 Initial Eligibility Screening
Eighty-nine of the volunteers that were screened for eligibility met initial criteria and were tested for MCI and cardiovascular disease. All eligible participants fulfilled the following inclusion criteria: age >55 years; ability to exercise vigorously without causing harm to self (as determined by the cardiovascular disease screening, treadmill test and history of cardiovascular disease); diagnostic designation as MCI according to Petersen criteria[36] using education adjusted scores; have a committed study partner; be in good general health; and ability to undergo required medical and study related assessments. Based on the initial evaluation of medical history, volunteers with a history of the following conditions were excluded: head trauma, uncontrolled diabetes mellitus and hypertension; current chronic renal, liver, respiratory, musculoskeletal, or neurologic disorders; recent myocardial infarction (within the previous 6 months), unstable angina, and chronic alcohol or drug abuse. Persons using hormone replacement therapy (HRT) or medications that may affect memory (e.g., anticholinergics, sedative hypnotics, narcotics, and antiparkinsonian agents), and those starting new medications within 6 weeks of enrollment, were also excluded from the study.
2.2 MCI Diagnosis
Diagnosis of MCI was made using the following criteria: memory complaints, performance scores on the Wechsler Memory Scale Logical Memory II (adjusted for education), Clinical Dementia Rating Scale (CDR) rating of ≤ 0.5, Modified Hachinski Ischemic Score of ≤ 4, Geriatrics Depression Scale (GDS) rating of < 6, and education adjusted Mini-Mental State Examination (MMSE) scores (adjusted MMSE= raw MMSE−(0.471 × [education −12]) + (0.131 × [age−70]) of between 24–30 [37]. Subjects with probable dementia according to National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's disease and Related Disorders Association (NINCDS/ADRDA) criteria; and those having memory loss from medical, neurological or psychiatric conditions, medication effects, or delirium were excluded from the study.
2.3 CVD Screening
Qualified volunteers underwent a maximal treadmill exercise test using the Bruce protocol [38] to screen for cardiovascular disease (CVD). Blood pressure, heart rate, and ECG were recorded before the test, at the end of every exercise stage, and every 2 min for 6 min after discontinuing the test. Testing was discontinued when the subject unable to continue the required movement, or CVD signs and symptoms occurred. Signs of CVD included >2-mV ST-segment depression, significant ST segment elevation, extra systole, chest pain, arrhythmias, hypotension or dizziness [39–41]. Those showing signs of CVD were not continued in the study.
2.4 Blood Collection and Processing
Participants were instructed to fast (consume only water), refrain from smoking, and avoid use of any anti-inflammatory medications during the 24-hour period prior to the blood draw for baseline and 6-month testing. Additionally, subjects were instructed to refrain from exercise for 72 hours prior to testing, and to confirm that they had no infection in the week prior to testing. All blood samples were drawn using sterile techniques by personnel trained in phlebotomy. Blood samples were taken between 9:30am and 11:00am in order to minimize any possible circadian variations. Blood was drawn from the median cubital or cephalic vein.
For serum collection, blood samples were incubated at room temperature and allowed to coagulate for 45 minutes. After centrifugation at1000 × g for 10 minutes, in a refrigerated centrifuge, the overlying serum was collected, aliquoted and stored at −80°C until used for BDNF assays. For plasma and buffy coat collection, blood samples were collected in tubes containing anticoagulant. Samples were then centrifuged at 200 × g for 20 minutes (without brake). Plasma and buffy coat layers were then collected into cryotubes and stored at −80°C.
2.5 Dietary Guidelines and Randomization
Study partners and subjects were instructed that subjects should maintain their usual caloric intake during the study period. Participants were requested to maintain an American Heart Association Step 1 diet: consisting of less than 30% of energy from fat, approximately 55% from carbohydrate, approximately 15% from protein, and a cholesterol intake of less than 300 mg/day. Twenty-nine subjects were randomized into stretch (n=12) and aerobic (n=17) exercise groups prior to baseline measurements and genotyping.
2.6 VO2Max Testing
Maximal oxygen consumption (VO2Max) was determined at baseline and repeated after subjects completed the 6-month exercise program. VO2Max and endurance capacity were determined using a modified Bruce protocol and measured by a validated, customized telemetric gas analysis system (K4b2 by Cosmed, Chicago Illinois). Discontinuation criteria for this test were similar to those used for the CVD screening test.
2.7 Aerobic Exercise-Training protocol
Each subject's maximum heart rate was inferred from baseline VO2Max tests. Both aerobic exercise and stretch exercise group subjects participated supervised training 3 days/week. The aerobic exercise protocol complied with the American College of Sports Medicine Guidelines (ACSM) [40], and included treadmill walking or jogging, stair-stepping, and elliptical training. Subjects underwent a warm up period, followed by intensity targeted-training and an appropriate cool-down period. Initial training sessions lasted 20 min at an intensity targeted at 50% VO2Max. Recordings of exercise heart rate and duration were used to monitor and ensure protocol compliance. Training duration increased by 5 min. each week until subjects attained 40 min. of exercise at 50% VO2Max. Subsequently, training intensity was increased by 5% VO2Max weekly until achieving 70% VO2Max. Aerobic group participants were asked to include an unsupervised 45–60 min. lower intensity walk during the weekend after the first 4–6 weeks of exercise in order to ensure maintenance of acquired fitness levels as well as to maintain motivation and interest. Exercise-training lasted 6 months and took place at the Howard University Hospital, Clinical Research Unit, under the supervision of trained personnel.
2.8 Stretch Exercise-Training Protocol
Stretch activity consisted of static stretch of joints designed to increase flexibility and joint range. Stretch exercise positions produced a slight pull on the muscle without triggering pain and were maintained for 15–30 seconds. Using different positions for a total of about 40 min, each stretch was directed at muscles that are prone to tightness (e.g., hamstrings, hip flexors, calves and chest). Stretches were repeated slowly, 3–5 times on each body side, 3 days/week [40]. To more closely match the increasing intensity and duration (minutes) of aerobic-exercise, stretch training targeted an increasing number of muscles each week up to week 4 and was maintained thereafter. Training began with hamstrings during week-1; hamstring and hip flexors during week-2; hamstring, hip flexors and calves during week-3; and hamstring, hip flexors, calves and chest muscles during week-4 and continued until the end of the 6 month training period.
2.9 Measurement of Serum Levels of BDNF
Serum levels of BDNF were quantitatively determined by ELISA using the human BDNF ELISA kit (Abcam, Cambridge, MA USA). No significant cross-reactivity or interference has been observed using this assay. To minimize assay variance, baseline and 6-month serum samples from a particular participant were measured on the same ELISA plate. A second, separate assay of all baseline samples was also performed on the same ELISA plate, for baseline only comparisons. Protocols were performed according to the manufacturer’s instructions. Each serum sample was tested in duplicate and results were averaged. Intra-assay coefficient of variability (CV) was 8.79, according to the formula [SD/Mean]*100. Serum samples were diluted 1:20 and each ELISA plate contained no-serum controls as well as calibration controls for generation of standard curves. Measurements were expressed in ng/ml after correcting for sample dilution. The optical density of each well was measured using an automated microplate reader (BioTek, Winooski, VT USA).
2.10 APOE Genotyping
Subjects were genotyped for the APOE single nucleotide polymorphisms (SNPs) rs429358 and rs7412 (which define the ε2, ε3 and ε4 alleles). Genomic DNA was extracted from buffy coat samples using standard procedures and quantified using Nanodrop 8000 (ThermoScientific, Wilmington, DE USA). TaqMan (Applied Biosystems) methods were used for genotyping. Manufacturer's protocols and recommendations were followed. Whole genome amplified DNAs were added to SNP reaction mix in 96-well optical reaction plates. Each 96-well plate contained 2 control wells without DNA in order to detect possible contaminations. Endpoint fluorescence reading of TaqMan SNP assays was performed using the ABI Prism 7900HT Sequence Detection System.
2.11 Statistical Analyses
All data were analyzed using SPSS (SPSS Inc., Chicago, IL) and SigmaStat 4.0 (Systat software Inc.,) statistical software. The two-tailed hypothesis was tested with significance set at p ≤ 0.05. The Shapiro-Wilk Test was used to check the assumption of normality and Brown-Forsythe test was used to determine equality of variance. Standard t-tests and Two-Way ANOVAs were performed for analysis of parametric data. Non-parametric group comparisons were analyzed using the Mann-Whitney U Statistical test. Since Generalized Linear Regression Analysis (GLM) allows for the inclusion of additional factors and is most appropriate and often used for unbalanced designs [42, 43], we used GLM to analyze baseline versus 6 month levels of serum BDNF by exercise group, ApoE4 status and gender, while controlling for age. Regression analysis was also performed to determine associations between serum BDNF levels and change in VO2Max, as well as Baseline BMI and change in serum BDNF.
3. Results
Baseline Characteristics of Study Participants
The detailed demographic characteristics and baseline measurements of participants by exercise groups and ε4 carrier status are shown in Tables 1 and 2, respectively. Twenty-nine participants were randomized into exercise groups and 22 completed six months of exercise training. There were no significant differences in characteristics of age, gender, or education between Aerobic and Stretch participants or between non-ε4 carriers and ε4 carriers. Whereas, baseline BMI was significantly lower in the Aerobic- compared to the Stretch-group, baseline VO2Max and serum BDNF levels were similar between exercise groups and also ε4 status.
Table 1.
Baseline Characteristics of Participants by Exercise Group.
| Characteristics | Participants n=22 |
Stretch n=9 |
Aerobic n=13 |
P value |
|---|---|---|---|---|
| Age in years | 72.0 (7.2) | 70.41 (6.3) | 73.1 (7.8) | 0.393 |
| Female (%) | 15 (68.2) | 6(66.7) | 9 (69.2) | 0.900 |
| Body Mass Index, Kg/m2 | 28.9(5.5) | 32.8 (5.8) | 26.2(3.4) | 0.003 |
| Years of Education | 16.1(3.4) | 15.0 (3.1) | 16.8 (3.5) | 0.215 |
| BL VO2Max, mL/min/Kg | 23.9 (5.5) | 24.8 (8.1) | 23.2 (2.9) | 0.520 |
| BL BDNF, ng/mL | 72.9 (27.9) | 68.1 (28.3) | 76.3 (28.3) | 0.513 |
| APOEε4 Carrier | 9(40.9) | 2 (22.2) | 7 (53.8) | 0.367 |
All data are presented as mean (standard deviation) unless otherwise specified. A total 22 participants completed the 6-month exercise program. BL= baseline; VO2Max = maximum rate of oxygen uptake. P value indicates significance level of differences between aerobic and stretch groups.
Table 2.
Baseline Characteristics of Randomized Participants by APOEε4 Status
| Characteristics | Genotyped Participants n=21 |
Non- ε4 Carriers n=12 |
ε 4 Carriers n=9 |
P value |
|---|---|---|---|---|
| Age in years | 72.5(7.0) | 72.9(7.2) | 72.0 (7.1) | 0.76 |
| Female (%) | 14 (66.7) | 8 (66.7) | 6 (66.7) | 1.000 |
| Body Mass Index, Kg/m2 | 28.3 (4.9) | 28.7 (5.6) | 27.9 (4.1) | 0.716 |
| Years of Education | 16.3 (3.3) | 16.0 (2.6) | 16.7 (4.3) | 0.662 |
| BL VO2Max, mL/min/Kg | 23.9 (5.7) | 23.6 (7.3) | 24.2 (2.5) | 0.794 |
| BL BDNF, ng/mL | 73.3 (28.5) | 65.5 (22.4) | 83.6 (33.7) | 0.154 |
All data are presented as mean (standard deviation) unless otherwise specified. Twenty-one of the 22 participants, who completed the 6-month exercise program, were successfully genotyped. BL= baseline; VO2Max = maximum rate of oxygen uptake. P value indicates significance level of differences between ε4 carriers and non- ε4 carriers.
3.1 Distribution of APOE Genotypes
Twenty-one of the 22 participants who completed the 6 month exercise program were successfully genotyped at the APOE locus for the 3 major alleles. Those carrying two copies of the APOEε3 allele constituted the majority (11) of participants, followed by ε4/ε3 heterozygotes (6). There were two ε4/ε4 participants, one ε2/ε4, and one ε2/ε3. One participant’s APOE genotype remained undetermined. Within the stretch exercise group there were six non-ε4 carriers, two ε4 carriers and one undetermined. Within the aerobic exercise group there were six non-ε4 carriers and seven ε4 carriers.
3.2 Changes in VO2Max
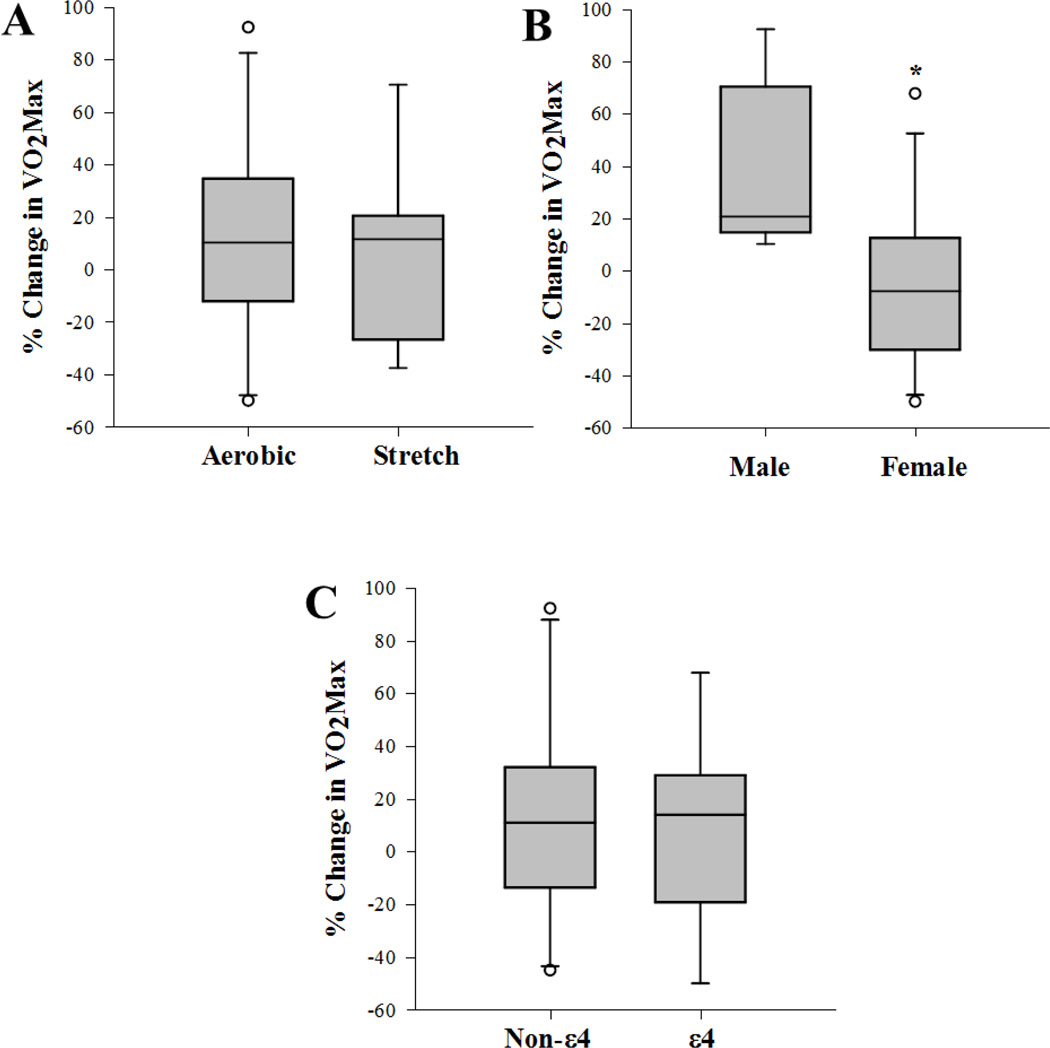
Compared to baseline, Stretch and Aerobic groups showed increases in VO2Max by 5.62% (P = 0.44) and 11.34% (P = 0.21), respectively, at the 6-month follow-up (Figure 1A.); but the differences were not statistically significant. Similarly, the percent change in VO2Max between Stretch and Aerobic groups was not significantly different (P= 0.667, t = 0.44) (Figure 1A). Since changes in VO2Max did not differ between Stretch and Aerobic groups, data from the two groups were combined for assessment of change from baseline by sex and ε4 carrier status. A significant difference in percent change in VO2Max between men and women (P= 0.024, t = 2.49) became evident (Figure 1B), and remained significant (P= 0.027, t=−2.43) after adjustment for age. Though both men and women were similar with respect to baseline VO2Max (P= 0.23, t = −0.75), men showed improvements (37.1%) in VO2Max, compared to a 4.7% decrease in women. In order to determine if the gender effect on exercise-induced changes in VO2Max was accounted for by changes in BMI, we also evaluated percent change in absolute values of VO2Max (VO2MaxAbs) which is not normalized to bodyweight. VO2MaxAbs also differed significantly between the genders (P=0.01, t = −2.48) but not by exercise groups (P=0.38, t=0.19,). Also, APOEε4 carrier status after adjustment for age showed no effect on change in VO2Max (relative P=0.857, t= −0.18, (Figure 1C); absolute P=0.32, t= 1.02).
Figure 1.
Box plot of percent (%) change in VO2Max from baseline values, after 6 months of exercise training (a) between exercise groups, (b) between genders, and (c) between non-ε4 and ε4 carriers. Middle line in box represents the median; lower box bounds the first quartile; upper box bounds the 3rd quartile. Whiskers represent the 95% confidence interval of the mean. Open circles are outliers from 95% confidence interval. *Significant difference between groups (P=0.024). Non-ε4 = non carriers of the APOE ε 4 allele, ε4 = carrier of the APOEε4 allele.
3.3 Changes in Serum BDNF
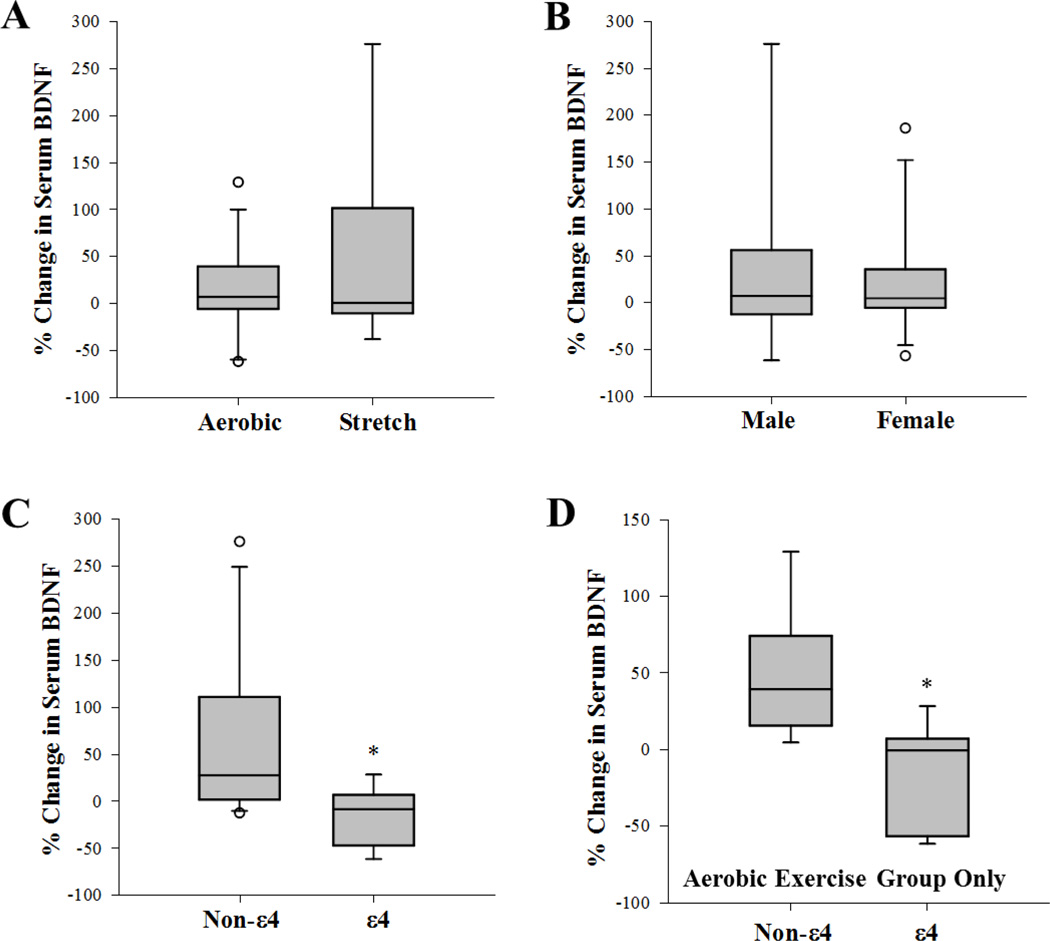
While both Stretch and Aerobic groups showed mean increases in serum BDNF (stretch=46.29%; and aerobic=15.12%) (Figure 2A), these increases were not significantly different from baseline values (stretch P=0.24; aerobic P=0.82), and median percent change in serum BDNF were similar for the two groups (stretch= 6.82; aerobic= 0.415; P= 0.950, U = 51). There were no gender effects on changes in serum BDNF levels (P=1.0, U=52.0) (Figure 2B). Linear Regression Analysis also revealed that there was no significant correlation between changes in BDNF and changes VO2Max (R=0.292, P=0.20). Baseline BMI and change in BMI also did not correlate with change in BDNF levels (R=0.028, P=0.900 and R=0.114, P= 0.623 respectively). Importantly, percent change in serum BDNF levels was significantly different between non-ε4 carriers and ε4 carriers (P= 0.012, U=18.0) (Figure 2C). With adjustment for age, these differences remained statistically significant (Non- ε4 carriers=27.2% versus ε4 carriers =−8.6%; P=0.019). Addition analyses using Generalized Linear Regression analysis was also performed on combined aerobic and stretch groups (Table 3) as well as the aerobics groups only (Table 4). Analyses of only aerobic exercise participants, showed a maintained, significant effect of ε4 carrier status on change in serum BDNF levels after accounting for gender, exercise group and age (P=0.021)
Figure 2.
Box plot of percent (%) change in serum levels of BDNF from baseline values, after 6 months of exercise training (a) between exercise groups, (b) between genders, (c) between non-ε4 and ε4 carriers and (d) between non-ε4 and ε4 carriers only within the aerobic exercise group. Middle line in box represents the median; lower box bounds the first quartile; upper box bounds the 3rd quartile. Whiskers represent the 95% confidence interval of the mean. Open circles are outliers from 95% confidence interval. *Significant difference between groups (P=0.016 and P=0.021 for C and D respectively). Non-ε4 = non carriers of the APOE ε 4 allele, ε4 = carrier of the APOEε4 allele.
Table 3.
Association of Change in Serum BDNF and ApoEε4 Carrier Status (Combined Stretch and Aerobic Participants)
| Parameter | Estimate | Standard Error | t Value | P-Value |
|---|---|---|---|---|
| Intercept | 144.86 | 101.80 | 1.42 | 0.174 |
| ApoE4 Status (C vs. N) | −47.04 | 17.38 | −2.71 | 0.016 |
| Age | −1.55 | 1.33 | −1.17 | 0.261 |
| Group (A vs. S) | 1.15 | 17.92 | 0.06 | 0.950 |
| Gender (F vs. M) | −6.85 | 18.97 | −0.36 | 0.723 |
Generalized Linear Regression analysis (6 months vs. Baseline); ApoE4 Status: C = Carriers, N = Non-carriers; Group: A = Aerobic, S = Stretch; Gender: F = Female, M = Male
Table 4.
Association of Change in Serum BDNF and ApoEε4 Carrier Status (Aerobic Participants Only)
| Parameter | Estimate | Standard Error | t Value | P-Value |
|---|---|---|---|---|
| Intercept | 198.06 | 133.58 | 1.48 | 0.172 |
| ApoEε 4 Status (C vs. N) | −59.44 | 21.37 | −2.78 | 0.021 |
| Age | −2.21 | 1.63 | −1.36 | 0.208 |
| Gender (F vs. M) | −2.37 | 24.83 | −0.10 | 0.926 |
Generalized Linear Regression analysis (6 months vs. Baseline); ApoE4 Status: C = Carriers, N = Non-carriers; Gender: F = Female, M = Male
4. Discussion
Our major finding suggests that APOE genotype was a crucial modulatory factor for BDNF upregulation in our aged cohort of 55–89 year-old AAs with MCI. In addition, changes in serum levels of BDNF did not parallel changes in VO2Max, suggesting that they occur through distinct physiologic pathways.
VO2Max, a measure of the maximum rate of oxygen consumption, is regarded as a standard measure of cardiorespiratory fitness. Physiologically, it is determined by several factors including red blood cell concentration, skeletal and cardiac muscle function, and oxygen transport. In our female participants, VO2Max response to stretch and aerobic exercise did not differ; however, a gender-based difference was noted. Exercise did not induce statistically significant increases in VO2Max of female subjects. In contrast, male participants showed an increased VO2Max. This result is a good indication that in older men, stretch exercise regimens may also be advantageous in elevating fitness levels, especially given the propensity for long term adherence. The significant gender-based difference in exercise-induced alteration in VO2Max denotes a role for sex distinct physiological characteristics in the regulation of this effect. A diminished VO2Max response to exercise training in women has been previously shown [44]. One suspected candidate for this gender effect is body composition. Previous studies have demonstrated that training-induced changes in VO2Max is at least partially explained by differences in body composition [45, 46], with increased lean mass positively affecting exercise-induced increase in fitness levels.
In our sample, we did not observe ε4-based differences in VO2Max or exercise-induced adaptations in VO2Max from baseline. This observation is not in concordance with that of Thompson and colleagues [47] who demonstrated a significant effect of APOE genotype on exercise-induced increases in VO2Max, with an enhanced effect in those carrying one ε3 and one ε4 allele compared to those carrying two ε3 alleles. A larger cohort may be necessary to reveal such an effect in our study.
Recently, a few studies have reported that ε4 carriers have lower serum levels of BDNF [28, 29, 48]. This is in accord with our observations of significant APOE gene-based and training-related increases in BDNF levels (P=0.012). At baseline, the ε4 carriers in our study had 21% lower mean level of serum BDNF compared to non-ε4 carriers, though the difference was not statistically significant (p= 0.15). Conversely, a lack of association has been reported by others. [49, 50]. Given its highly protective and reparative role in the brain, it is reasonable to suspect a critical role for BDNF in cognitive decline and neurodegenerative disease. Unfortunately, steady state levels of BDNF can vary drastically, obscuring potential differences. This study supports the use of an inducer of BDNF for revealing differences in BDNF regulation between groups.
BDNF is mainly produced in the brain with the highest concentrations found in the hippocampus [51, 52]. Correlation between brain and serum levels of BDNF has not been established; however, several studies suggest that serum levels of BDNF are positively correlative with levels found in brain [53–56]. Notably, exercise has been established as an up-regulator of BDNF in both animal and human subjects [14, 57–63], as well as in various states of cognitive decline including Alzheimer’s disease [11] for which lowered levels of BDNF have been reported [64–67]. Few studies have demonstrated direct positive correlations between exercise-induced BDNF upregulation and improved cognitive function in human subjects (Kimhy D 2015); however, the neuroprotective impact of BDNF upregulation has been well documented [68–70]. Together, these studies suggest that blood levels of BDNF are a likely reflection of brain levels; and the after-training levels may reflect training effects. In addition, BDNF upregulation through exercise or other means would be expected to have positive effects on brain health, particularly neuroplasticity and neuron survival.
Our data suggests that APOE genotype is an important factor for exercise-induced BDNF upregulation in elderly AA MCI subjects. Replication of our results in larger multi-racial cohorts will be necessary to determine the scope of this association within the general population. Weather this effect is also relevant for younger and cognitively normal populations should also be examined.
Our study is the first to offer evidence in support of a differential effect of APOEε4 carrier status, on exercise training-induced changes in BDNF levels. This result suggests that the ε4 allele of the APOE gene may influence the neuroprotective benefit gained from physical activity. Conversely, one group that examined the effects of exercise-induced BDNF production in ε4 compared to ε3 transgenic APOE-humanized mice [71], reported that the ε4 carrier mice had robust up-regulation of BDNF similarly to the ε3 mice, though the ε4 mice had lower baseline levels of BDNF. However, it remains possible that BDNF response may be under an alternate system of regulation in rodents compared to humans [72].
A few recent studies provide some insight into the mechanisms that may potentially explain our observations in this pilot study. BDNF expression is regulated by nine functional promoters [73]. It is now realized that exercise modulates BDNF expression through epigenetic mechanisms by causing demethylation of at least one BDNF promoter region[74]. For example, one study demonstrated that APOE functions as a transcription factor, and binds to various gene promoter regions including those regulating neurotropic factors [75]. Another study showed that BDNF promoter methylation was significantly higher in AD cases [76], and that APOE variants may differentially bind to and regulate BDNF promoter regions. Furthermore, it has been demonstrated that the APOEε4 variant increases translocation of histone deacetylases in human neurons, resulting in decreased BDNF expression [30]. Whereas, the amount of physiologic adaptation needed to improve BDNF levels is not immediately apparent in this study, collectively, these array of other evidence suggest that APOE may mechanistically explain differences in training-induced changes in BDNF.
4.1 Limitations
This paper presents analyses from a pilot study that was designed to primarily examine the effects of a standardized 6-month aerobic exercise regime on the mechanisms underlying changes in biomarkers of fitness and the enhancement of neuroprotection. Although there was some expectation of possible baseline differences in serum BDNF levels based on APOE genotype, we anticipated an exercise-induced increase in serum BDNF across all APOE genotypes. Therefore, APOE genotype was not incorporated into the study design, and was not factored into the randomization scheme. Consequently, the stretch group consisted of a larger proportion of non-ε4 carriers than the aerobic group. Conceivably, this may have accounted for the higher mean change in serum BDNF in our stretch group compared to the aerobic group. Since this was a highly supervised exercise intervention, and stretch participants not required to maintain exercise diaries or wear activity monitoring devices. As such, there may be some possibility that participates engaged in additional, unapproved and unrecorded exercise activity that may have contributed to our findings of no differences between stretch and aerobic groups. It may also be possible that males engaged in more outside exercise activities compared to women, which could have potentially contributed to gender differences found in our study.
Recruiting and retaining African American research participants is a highly recognized challenge within the scientific community [77, 78], which also impacted our sample size. Only 29 of the 89 volunteer that met initial criteria began the study, and 22 completed our six month exercise intervention. Our relatively small sample size is likely to have contributed to the lack of differences found between stretch and aerobics groups. Still, we believe that this study demonstrates an effect of APOEε4 on exercise-induced BDNF regulation, and that this effect may not be specific to AAs. Given the lack of significant training-induced differences in VO2Max or BDNF levels between exercise groups, and the absence of a “no-exercise control”, we cannot be certain that the increase in BDNF levels in non-ε4 carriers is definitively an exercise-induced response. However, we note with interest that a lack of direct correlation between changes in VO2Max and BDNF has been previously reported [79]. Importantly, our study is yet the most rigorous, supervised, and randomized controlled and mechanistic exercise study in mild cognitively impaired older AAs.
4.2 Conclusions
We believe we have identified a relationship between the APOEε4 allele and BDNF response to physical activity in a cohort of AAs with MCI. This observation provides a deeper understanding of the impact of the APOE gene variants on brain health, and the efficacy of exercise as a strategy for the upregulation of neuroprotective biomolecules. Potentially, this finding may have implications for the use of exercise-induced serum BDNF response as a diagnostic measurement for predicting disease progression in preclinical stages of Alzheimer’s disease. Our expectation that aerobic exercise would increase VO2Max and BDNF levels, in parallel, was unmet. Our subsequent, post hoc analyses led to unexpected results and thus, the generation of a new hypothesis: that the extent of exercise-induced up-regulation of serum levels of BDNF in humans is dependent on APOE genotype. This hypothesis should be tested in new studies specifically designed to examine APOEε4 effects on exercise-induced BDNF up-regulation.
Highlights.
Older adult men but not women demonstrate exercise-induced increases in VO2Max.
Exercise-induced up-regulation of BDNF varies by APOE genotype.
Muted BDNF response to physiological adaptations may explain ε4-related AD risk.
Acknowledgments
Funding:
This study was supported by the National Institute on Aging, NIH Grants, 5R01AG031517-2, 5R01AG045058-01A1, and in part, by UL1TR000101 from the National Center for Advancing Translational Sciences/NIH through the Clinical and Translational Science Award Program (CTSA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional contributions:
We are grateful to members of our research team who contributed many hours to this project including: Oludolapo Ogunlana, Saba Wolday, Graham Debra A. Ordor, Josephine R. Ezemobi, Linda L. Fletcher, Josephine Okomo-Awich, Yuanxiu Chen, Alice C. Ukaegbu.
REFERENCES
- 1.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- 2.Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, Rogers MA, Steffens DC. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009;5:445–453. doi: 10.1016/j.jalz.2009.04.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes LL, Bennett DA. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood) 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall KS, Gao S, Baiyewu O, Lane KA, Gureje O, Shen J, Ogunniyi A, Murrell JR, Unverzagt FW, Dickens J, Smith-Gamble V, Hendrie HC. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5:227–233. doi: 10.1016/j.jalz.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussbaum RL. Genome-wide association studies, Alzheimer disease, and understudied populations. JAMA. 2013;309:1527–1528. doi: 10.1001/jama.2013.3507. [DOI] [PubMed] [Google Scholar]
- 6.Gielen S, Laughlin MH, O'Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis. 2015;57:347–355. doi: 10.1016/j.pcad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 10.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 11.Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduroz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- 12.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Schmolesky MT, Webb DL, Hansen RA. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J Sports Sci Med. 2013;12:502–511. [PMC free article] [PubMed] [Google Scholar]
- 16.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 17.Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci Lett. 2014;566:304–308. doi: 10.1016/j.neulet.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Goda A, Ohgi S, Kinpara K, Shigemori K, Fukuda K, Schneider EB. Changes in serum BDNF levels associated with moderate-intensity exercise in healthy young Japanese men. Springerplus. 2013;2:678. doi: 10.1186/2193-1801-2-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, Church TS. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7:e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariga A, Zavadil J, Ginsberg SD, Chao MV. Withdrawal of BDNF from hippocampal cultures leads to changes in genes involved in synaptic function. Dev Neurobiol. 2015;75:173–192. doi: 10.1002/dneu.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilar M, Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lommatzsch M, Hornych K, Zingler C, Schuff-Werner P, Hoppner J, Virchow JC. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology. 2006;31:388–394. doi: 10.1016/j.psyneuen.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez A, Aleixandre M, Linares C, Masliah E, Moessler H. Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer's disease. J Alzheimers Dis. 2014;42:1347–1355. doi: 10.3233/JAD-140849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YH, Jiao SS, Wang YR, Bu XL, Yao XQ, Xiang Y, Wang QH, Wang L, Deng J, Li J, Zhou XF, Zhou HD, Wang YJ. Associations Between ApoEepsilon4 Carrier Status and Serum BDNF Levels--New Insights into the Molecular Mechanism of ApoEepsilon4 Actions in Alzheimer's Disease. Mol Neurobiol. 2015;51:1271–1277. doi: 10.1007/s12035-014-8804-8. [DOI] [PubMed] [Google Scholar]
- 30.Sen A, Nelson TJ, Alkon DL. ApoE4 and Abeta Oligomers Reduce BDNF Expression via HDAC Nuclear Translocation. J Neurosci. 2015;35:7538–7551. doi: 10.1523/JNEUROSCI.0260-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altenburg M, Johnson L, Wilder J, Maeda N. Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J Biol Chem. 2007;282:7817–7824. doi: 10.1074/jbc.M610712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez MF, Krastins B, Ning M. The role of apolipoprotein E in neurodegeneration and cardiovascular disease. Expert Rev Proteomics. 2014;11:371–381. doi: 10.1586/14789450.2014.901892. [DOI] [PubMed] [Google Scholar]
- 35.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 37.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- 38.Bruce RA, Hornsten TR. Exercise stress testing in evaluation of patients with ischemic heart disease. Prog Cardiovasc Dis. 1969;11:371–390. doi: 10.1016/0033-0620(69)90027-9. [DOI] [PubMed] [Google Scholar]
- 39.White RD, Evans CH. Performing the exercise test. Prim Care. 2001;28:29–53. vi. doi: 10.1016/s0095-4543(05)70006-3. [DOI] [PubMed] [Google Scholar]
- 40.American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 41.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 42.Buttenschon HN, Demontis D, Kaas M, Elfving B, Molgaard S, Gustafsen C, Kaerlev L, Petersen CM, Borglum AD, Mors O, Glerup S. Increased serum levels of sortilin are associated with depression and correlated with BDNF and VEGF. Transl Psychiatry. 2015;5:e677. doi: 10.1038/tp.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob Y, Cripps A, Evans T, Chivers PT, Joyce C, Anderton RS. Identification of genetic markers for skill and athleticism in sub-elite Australian football players: a pilot study. J Sports Med Phys Fitness. 2016 doi: 10.23736/S0022-4707.16.06647-0. [DOI] [PubMed] [Google Scholar]
- 44.Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B, Levine BD. Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985) 2015;119:37–46. doi: 10.1152/japplphysiol.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 46.Pribis P, Burtnack CA, McKenzie SO, Thayer J. Trends in body fat, body mass index and physical fitness among male and female college students. Nutrients. 2010;2:1075–1085. doi: 10.3390/nu2101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson PD, Tsongalis GJ, Seip RL, Bilbie C, Miles M, Zoeller R, Visich P, Gordon P, Angelopoulos TJ, Pescatello L, Bausserman L, Moyna N. Apolipoprotein E genotype and changes in serum lipids and maximal oxygen uptake with exercise training. Metabolism. 2004;53:193–202. doi: 10.1016/j.metabol.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Cui Y, Yang J, Zhang J, Yuan D, Wei Y, Li Y, Duo Y, Li S, Zhu W, Zheng L. Combining serum and urine biomarkers in the early diagnosis of mild cognitive impairment that evolves into Alzheimer's disease in patients with the apolipoprotein E 4 genotype. Biomarkers. 2015;20:84–88. doi: 10.3109/1354750X.2014.994036. [DOI] [PubMed] [Google Scholar]
- 49.O'Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R Texas Alzheimer's Research C. Brain-derived neurotrophic factor levels in Alzheimer's disease. J Alzheimers Dis. 2009;17:337–341. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nettiksimmons J, Simonsick EM, Harris T, Satterfield S, Rosano C, Yaffe K, Health ABCS. The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: a longitudinal study. PLoS One. 2014;9:e91339. doi: 10.1371/journal.pone.0091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 53.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 54.Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 55.Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- 56.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Song BK, So B, Lee O, Song W, Kim Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatry Res. 2014;220:792–796. doi: 10.1016/j.psychres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 59.Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 61.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 64.Costa A, Peppe A, Carlesimo GA, Zabberoni S, Scalici F, Caltagirone C, Angelucci F. Brain-derived neurotrophic factor serum levels correlate with cognitive performance in Parkinson's disease patients with mild cognitive impairment. Front Behav Neurosci. 2015;9:253. doi: 10.3389/fnbeh.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forlenza OV, Miranda AS, Guimar I, Talib LL, Diniz BS, Gattaz WF, Teixeira AL. Decreased Neurotrophic Support is Associated with Cognitive Decline in Non-Demented Subjects. J Alzheimers Dis. 2015;46:423–429. doi: 10.3233/JAD-150172. [DOI] [PubMed] [Google Scholar]
- 66.Kaur S, Gonzales MM, Tarumi T, Villalpando A, Alkatan M, Pyron M, Tanaka H, Haley AP. Serum Brain-Derived Neurotrophic Factor Mediates the Relationship between Abdominal Adiposity and Executive Function in Middle Age. J Int Neuropsychol Soc. 2016;22:493–500. doi: 10.1017/S1355617716000230. [DOI] [PubMed] [Google Scholar]
- 67.Khalil H, Alomari MA, Khabour OF, Al-Hieshan A, Bajwa JA. Relationship of circulatory BDNF with cognitive deficits in people with Parkinson's disease. J Neurol Sci. 2016;362:217–220. doi: 10.1016/j.jns.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 68.Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, Meeusen R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets. 2015;19:565–576. doi: 10.1517/14728222.2014.994506. [DOI] [PubMed] [Google Scholar]
- 69.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White LJ, Castellano V. Exercise and brain health--implications for multiple sclerosis: Part 1--neuronal growth factors. Sports Med. 2008;38:91–100. doi: 10.2165/00007256-200838020-00001. [DOI] [PubMed] [Google Scholar]
- 71.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theendakara V, Peters-Libeu CA, Spilman P, Poksay KS, Bredesen DE, Rao RV. Direct Transcriptional Effects of Apolipoprotein E. J Neurosci. 2016;36:685–700. doi: 10.1523/JNEUROSCI.3562-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang L, Wang Y, Ji H, Dai D, Xu X, Jiang D, Hong Q, Ye H, Zhang X, Zhou X, Liu Y, Li J, Chen Z, Li Y, Zhou D, Zhuo R, Zhang Y, Yin H, Mao C, Duan S, Wang Q. Elevation of peripheral BDNF promoter methylation links to the risk of Alzheimer's disease. PLoS One. 2014;9:e110773. doi: 10.1371/journal.pone.0110773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist. 2011;51(Suppl 1):S33–S45. doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally Competent Strategies for Recruitment and Retention of African American Populations into Clinical Trials. Clin Transl Sci. 2015;8:460–466. doi: 10.1111/cts.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]