Abstract
Vibrio cholerae is the causative agent of cholera, an acute diarrheal disease that remains endemic in many parts of the world. The mechanisms underlying immunity to cholera remain poorly defined, though it is increasingly clear that protection is associated with antibodies against lipopolysaccharide (LPS). Here we report that ZAC-3, a monoclonal antibody against the core/lipid A region of V. cholerae LPS is a potent inhibitor of V. cholerae flagellum-based motility in viscous and liquid environments. ZAC-3 arrested motility of the classical Ogawa strain O395, as well as the El Tor Inaba strain C6706. In addition, we demonstrate, in the neonatal mouse model, that ZAC-3 IgG and Fab fragments significantly reduced the ability of both V. cholerae strains O395 and C6706 to colonize the intestinal epithelium, revealing the potential of antibodies against the core/lipid A to contribute to immunity across biotypes, possibly through a mechanism involving motility arrest.
Keywords: cholera, mucosal, immunity, vaccine, colonization, enteric
Cholera remains endemic in many parts of the world, with sporadic outbreaks often occurring in association with natural disasters, such as the earthquake in Haiti in December of 2010, or displacement of large populations as the result of regional conflicts such as those ongoing in the Democratic Republic of the Congo [1]. The disease is caused by Vibrio cholerae, a Gram-negative bacterium that colonizes (but does not invade) the epithelium of the small intestine in a regiospecific manner, a process facilitated by the bacterium’s single polar flagellum [2–4]. The primary virulence factor associated with V. cholerae is cholera toxin (CT), an ADP- ribosylating enzyme that disrupts chloride homeostasis within intestinal epithelial cells and elicits a profuse watery diarrhea that can result in severe dehydration and death within hours [5, 6]. Although there are more than 200 known serogroups of V. cholerae, only O1 and O139 are associated with epidemic disease. The O-antigen or O-polysaccharide (OPS) of the O1 serogroup consists of (1->2)-linked moieties of 4-amino-4,6-dideoxy-alpha-D-mannopyranose (D-perosamine), in which the amino groups are acylated with 3-deoxy-L-glycero-tetronic acid [7]. Within the O1 serogroup, there are two serotypes, Ogawa and Inaba, which differ in the presence (Ogawa) or absence (Inaba) of methylation of the terminal non-reducing D-perosaminyl moiety of OPS. OPS is the distal component of lipopolysaccharide (LPS), which is anchored to the outer leaflet of V. cholerae’s outer membrane via a core/lipid A moiety that is common between O1 serotypes and among serogroups, including O139 [8].
OPS-specific secretory IgA (SIgA), as well as serum IgM and IgG, constitute a critical component of the protective immune response to V. cholerae [9]. In the neonatal mouse model, passive transfer of anti-OPS monoclonal IgA or IgG antibodies (MAbs) like 2D6 or polyclonal SIgA’s derived from the milk of vaccinated dams, reduces the number of viable bacteria recovered from intestinal tissues and protects animals against V. cholerae-induced death following experimental challenge [10–13]. Although the precise mechanisms by which antibodies limit the ability of V. cholerae to colonize the intestinal epithelium remains unknown, two groups have reported a correlation between the ability of OPS-specific IgA antibodies to arrest V. cholerae flagellum-based motility in vitro and protection in vivo [11, 12]. Indeed, we recently demonstrated that the Ogawa-specific IgA mAb, 2D6, which was shown to protect mice against classical O1 V. cholerae strain O395, is also an effective inhibitor of bacterial motility in semi-solid and liquid media [14].
Leitner and colleagues have suggested that V. cholerae motility arrest is a phenomenon specific to antibodies against OPS [12]. However, we recently reported that ZAC-3, a monoclonal antibody against the core/lipid A region of V. cholerae O1 LPS, abolishes migration of V. cholerae O1 strain O395 in a semi-solid agar motility assay, an observation that may have a number of implications for cholera vaccine development [15]. ZAC-3 was first isolated as a monoclonal IgA from a B cell hybridoma derived from mouse Peyer’s patches [16]. As the ZAC-3 hybridoma secretes very little antibody (K. Levinson and N. Mantis, unpublished results), we generated a recombinant ZAC-3 antibody in which the heavy (VH) and light (VL) chains of ZAC-3 IgA were cloned onto a human IgG1 framework [15]. Chimeric ZAC-3 IgG was successfully expressed in a Nicotiana benthamiana-based rapid antibody-manufacturing platform (RAMP), thereby ensuring a ready supply of antibody. By ELISA, chimeric ZAC-3 IgG reacted with the classical Ogawa strain O395, the El Tor Inaba strain C6706, as well as ATCC# 9459, an Inaba strain used in the Dukoral® cholera vaccine. ZAC-3 IgG did not, however, react with a V. cholerae O139 isolate, possibly because that particular strain is encapsulated, which may limit accessibility of the antibody to the core/lipid A region. Nonetheless, ZAC-3 IgG serves as a unique research tool to examine the capacity of an antibody against the core/lipid A region of LPS to interfere with the motility of classical and El Tor strains of V. cholerae O1.
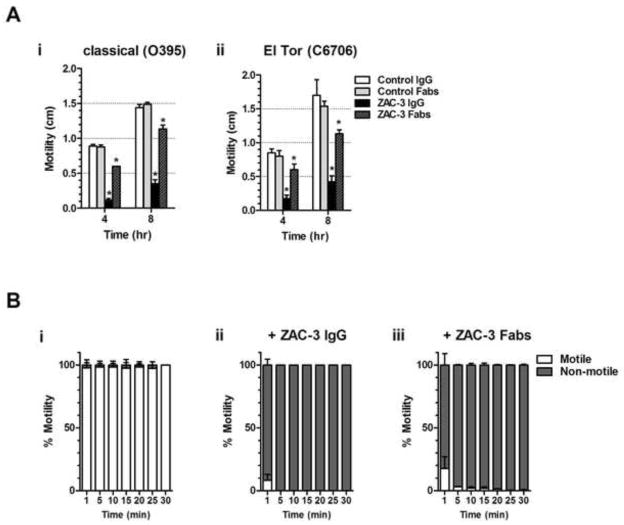
We first examined the effect of ZAC-3 IgG on flagella-based motility of classical V. cholerae when cultured in semi-solid medium. Strain O395 was inoculated into the center of 35 mm Petri dish containing LB agar (0.3%) supplemented with 9 μg/ml ZAC-3 IgG. The plates were incubated at 37°C and the diameter of bacterial migration away from the point of inoculation was determined at 4 and 8 h time points. When treated with PBS or an isotype antibody control, V. cholerae strain O395 had a migration diameter of ~0.8 cm at 4 h and 1.5 cm at 8 h. In the presence of ZAC-3 IgG, bacterial migration was reduced 4–10 fold (Figure 1Ai), which is consistent with our previous results [15]. The motility studies were then repeated with the El Tor Inaba strain C6706. ZAC-3 had a similar impact on the motility of the Inaba strain, as ZAC-3 IgG significantly reduced migration of C6706 at both the 4 (0.8 cm versus 0.2 cm) and 8 h (1.6 versus 0.4 cm) time points (Figure 1Aii). The effects of ZAC-3 on C6706 motility were dose-dependent, with a significant reduction in motility associated with >3 μg/ml of ZAC-3 IgG or Fab fragments (Figure S1).
Figure 1. ZAC-3 IgG and Fab fragments inhibit motility of V. cholerae classical and El Tor biotypes.
(A) V. cholerae motility assays in semi-solid LB agar (0.3%) containing IgG or Fabs of ZAC-3 or isotype controls, as described [14]. Isotype controls included recombinant human IgG1 MAbs directed against Salmonella O5 antigen (Sal4) (manuscript in preparation) or ricin toxin (PB10) [20]. A single colony of V. cholerae strain O395 (panel A.i) or C6707 (panel A.ii) was used to stab inoculate the center of LB agar plates, which were placed at 37°C. Bacterial migration away of the point of inoculation was assessed at 4 and 8 h time points. A minimum of three independent experiments was performed for each antibody treatment. The Student’s t test was used to determine statistical significance between ZAC-3 IgG and Fab fragment treatment compared to their respective controls (*, P<0.05, **, P<0.01). (B) Live digital microscopy of V. cholerae motility in liquid medium containing an isotype control (panel B.i), ZAC-3 IgG (panel B.ii) or ZAC-3 Fab fragments (panel B.iii), was performed as described [14]. For each antibody treatment, three independent videos containing 100 image sequences were acquired (7.14 frames per second) every 5 min for a total of 30 min. Within each 100 frame video, 3 sets of 6 frames were selected throughout the video and the number of bacteria observed moving between each frame and not moving between each frame was quantitated using Fiji software version 1.49f [21]. Percent motility was calculated using the average number of motile bacteria over the total number of bacteria seen in the selected frames. The mean (bars) and standard deviation (error bars) from the three videos for each treatment are shown in the graph. All IgG and Fab treatments presented in panels Bii and Biii resulted in a significant reduction in motility as compared to control samples (*P<0.0001, Student’s t test).
The effects of ZAC-3 IgG on V. cholerae motility were even more pronounced in a liquid medium. Mid-log phase cultures of strain O395 expressing GFP were mixed with ZAC-3 IgG then seeded on glass microscope slides and monitored by fluorescent digital video microscopy for 30 min, as described [14]. We found that ZAC-3 IgG treatment resulted in almost instantaneous motility arrest: >95% of the bacteria had stopped swimming within 2 min of antibody exposure (Figure 1Bii; Movies S1–2). In contrast, control cells remained >95% motile for the entire duration of the 30 min experiment (Figure 1Bi). ZAC-3 IgG had a similar effect on strain C6706, although we had difficulty quantitating motility of this strain because of its propensity to adhere to the glass surfaces (data not shown), possibly because of issues related to MshA pili-mediated attachment. Nonetheless, we conclude from these studies that an antibody directed against the core/lipid A region of V. cholerae LPS is highly effective at arresting motility of both classical (Ogawa) and El Tor (Inaba) biotypes.
We previously reported that MAb 2D6 inhibits the motility of strain O395 by antibody agglutination-dependent and -independent mechanisms [14]. To examine whether ZAC-3 can arrest motility independent of antibody-mediated agglutination we generated Fab fragments using the Pierce Fab preparation Kit (ThermoFisher Scientific, Waltham, MA) and tested them in semi-solid and liquid motility assays. Indeed, ZAC-3 Fab fragments significantly reduced motility of both V. cholerae O395 and C6706 strains in the semi-solid agar assay at the 4 and 8 h time points, although to a lesser degree than ZAC-3 IgG (Figure 1A; Figure S1B). In liquid medium, ZAC-3 Fab fragments effectively abolished motility of strain O395 within 10 min (Figure 1Biii; Movie S3), demonstrating that ZAC-3 can interfere with the swimming behavior of V. cholerae, independent of agglutination.
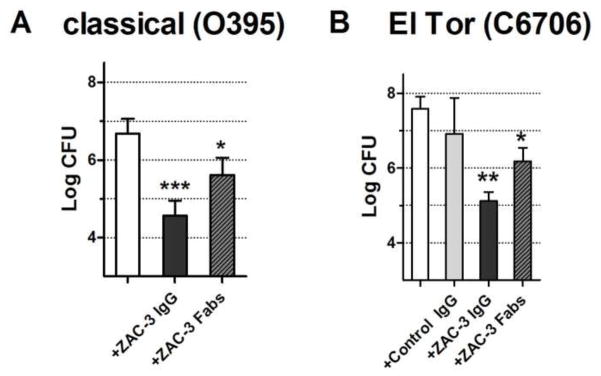
We next used a neonatal mouse model to determine whether ZAC-3 impacts the ability of V. cholerae to colonize the intestinal epithelium in vivo. The classical strain V. cholerae O395 (1 × 106 CFU), which is resistant to streptomycin, was mixed with ZAC-3 IgG (9 μg/ml), and administered by gavage to 3–4 day old BALB/c mice, following previously established protocols [17]. The pups were euthanized 24 h post-gavage and the entire length of the small and large intestines were homogenized and plated onto LB medium supplemented with streptomycin (100 μg/ml) and quantitated for CFUs. In intestinal homogenates from mice that received bacteria in saline, we recovered an average of ~5 × 106 V. cholerae CFUs (Figure 2A). In contrast, there was a two-log reduction in the number of CFUs recovered from intestinal homogenates of pups that received V. cholerae O395 co-administered with ZAC-3 IgG suggesting that ZAC-3 IgG limits intestinal colonization. We repeated these studies with the El Tor strain C6706. Administration of C6706 (1 × 106 CFU) in saline or an isotype control IgG to neonatal mice resulted in the recovery of ~3 × 107 CFUs from intestinal homogenates. Co-administration of ZAC-3 IgG (9 μg/ml) with strain C6706 resulted in a reduction (~2 log; P<0.01) in intestinal colonization, based on number of CFUs recovered from intestinal homogenates (Figure 2B), demonstrating for the first time that a MAb directed against the core/lipid A region of LPS is able to affect colonization of both classical and El Tor biotypes.
Figure 2. ZAC-3 IgG and Fab fragments reduce colonization of V. cholerae classical and El Tor biotypes in the neonatal mouse model.
Mid-log phase culture of V. cholerae O395 and C6706 were diluted (1:1,000) into phosphate buffered saline (PBS) containing blue food coloring (20% v/v) and ZAC-3 IgG (9 μg/ml) or Fabs (9 μg/ml). Three or four day old BALB/c mice were administered 100 μl of V. cholerae culture by gavage using a flexible pipette tip. Pups were maintained in a 38°C incubator with 40% humidity and then euthanized 24 h post-gavage. The entire length of the intestines were removed, ground through a 70 μm cell strainer (Corning, NY) and plated for CFUs on LB agar containing streptomycin (100 μg/ml). Treatments with ZAC-3 IgG or ZAC-3 Fab fragments led to a significant decrease in the log CFU of V. cholerae (A) O395 and (B) C6706, as compared to controls. A minimum of three pups was used for each antibody treatment and biotype. Due to limited litter size, an isotype control group was omitted from the experiment presented in Panel A. The bars represent the mean number (with standard deviations) of CFUs recovered from intestinal homogenates. The Student’s t test was used to determine significance (*, P<0.05; **, P < 0.003***, P < 0.0003).
As ZAC-3 Fab fragments were able to arrest V. cholerae motility in vitro, we next wanted to examine whether the Fab fragments also interfered with colonization in vivo. V. cholerae strains O395 and C6706 were mixed with ZAC-3 Fab fragments and then administered to neonatal mice by gavage. In both cases, treatment with ZAC-3 Fab fragments resulted in ~1 log (P<0.05) reduction in the number of bacteria that were recovered from intestinal homogenates (Fig. 2A,B), demonstrating agglutination- and Fc-independent effects of the antibody on intestinal colonization.
In summary, our results suggest that antibodies against a conserved epitope within the core/lipid A region of V. cholerae LPS can limit cholera intestinal colonization in vivo. One caveat to our study, however, is that colonization of V. cholerae in the mouse gut was inferred from CFUs recovered from intestinal homogenates. We did not directly assess the effect of ZAC-3 on the ability of either strain O395 or C6706 to physically associate with the epithelial surface. Nonetheless, it is tempting to speculate that ZAC-3 impacts V. cholerae’s fitness in the intestinal lumen by retarding flagellum-based motility. For example, the fold reduction in intestinal colonization associated with ZAC-3 treatment is consistent with what has been observed with strains that are genetically defective in motility [2].
One implication of our study is that anti-core/lipid A IgA and/or IgG antibodies, if present at appreciable levels in mucosal secretions, could play a role in immunity to V. cholerae infection across biotypes and serogroups, as the core/lipid A region is conserved across O1 and O139 serogroups [8]. However, there is little evidence to suggest that cross protection occurs in humans following natural infection or vaccination, which is why the Shanchol vaccine includes a mixture of O1 and O139 strains [9, 18]. Even in experimental animals models, the antibody response to whole cell or even OMV vaccines is predominantly against OPS, with little or no cross reactivity against core/lipid A [9, 11, 12]. However, as the ZAC-3 B cell hybridoma was originally isolated from a vaccinated mouse, it does indicate that the core/lipid A region of V. cholerae LPS is at least minimally immunogenic [16]. Based on our findings and the potential of core/lipid A-specific secretory antibodies to limit intestinal colonization of V. cholerae, a more in depth understanding of factors that dictate mucosal antibody production against components of LPS is warranted.
Finally, it should be underscored that the experiments presented in this study were conducted with recombinant chimeric form of ZAC-3 IgG, not ZAC-3 IgA. ZAC-3 was originally isolated as a monoclonal, dimeric IgA antibody from Peyer’s patch B cells from mice that had been immunized orally with heat killed V. cholerae cells (R. Weltzin, personal communication) [16]. Interestingly, the dimeric and SIgA forms of ZAC-3 bound LPS with much greater affinity than monomeric ZAC-3 IgA, which, if extrapolated to our study, would suggest that ZAC-3 in the form of SIgA would be much more effective at limiting bacterial colonization of the intestinal epithelium, as compared to ZAC-3 IgG. We are currently generating a recombinant human IgA version of ZAC-3 using tools previous developed in our laboratory in order to perform a direct comparison between different antibody isotypes in immunity to cholera [19].
Supplementary Material
HIGHLIGHTS.
ZAC-3 is a monoclonal antibody against a conserved Vibrio cholerae epitope
ZAC-3 is an inhibitor of V. cholerae flagellum-based motility
ZAC-3 IgG and Fab fragments reduce V. cholerae intestinal colonization in a mouse model
ZAC-3 reveals potential of antibodies to confer immunity across V. cholerae biotypes
Acknowledgments
We gratefully acknowledge the support of our collaborators in providing the bacterial strains used in this study. We thank Gabriela Kovacikova (Geisel School of Medicine, Dartmouth College) for providing the V. cholerae GFP strain (RT 4273) used in the digital fluorescent microscopy. The classical strain O395 was kindly provided by Dr. John Mekalanos (Harvard Medical School) and the El Tor strain C6706 was provided by Dr. Chris Waters (Michigan State University). We thank the Wadsworth Center’s Advanced Light Microscopy Core Facility for assistance with video and confocal microscopy. We thank Richard Cole and Dr. Jen Westfall for technical assistance and gratefully acknowledge veterinary science staff for assistance in establishing the neonatal mouse model. This work was supported by National Institutes of Health awards HD061916 and AI109275 to NJM. KL was the recipient of a Wadsworth Center’s Biodefense and Emerging Infectious Diseases training grant fellowship (5T32AI055429-08; McDonough). This manuscript is dedicated to the late Dr. Ronald Taylor, who advanced this project through his generosity and scientific insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Orata FD, Keim PS, Boucher Y. The 2010 cholera outbreak in Haiti: how science solved a controversy. PLoS Pathog. 2014;10:e1003967. doi: 10.1371/journal.ppat.1003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guentzel MN, Berry LJ. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975;11:890–7. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SH, Butler SM, Camilli A. Selection for in vivo regulators of bacterial virulence. Proc Natl Acad Sci U S A. 2001;98:6889–94. doi: 10.1073/pnas.111581598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millet YA, Alvarez D, Ringgaard S, von Andrian UH, Davis BM, Waldor MK. Insights into Vibrio cholerae Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria. PLoS Pathog. 2014;10:e1004405. doi: 10.1371/journal.ppat.1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop AL, Camilli A. Vibrio cholerae: lessons for mucosal vaccine design. Expert review of vaccines. 2011;10:79–94. doi: 10.1586/erv.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–76. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenne L, Lindberg B, Unger P, Gustafsson B, Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydrate research. 1982;100:341–9. doi: 10.1016/s0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee SN, Chaudhuri K. Lipopolysaccharides of Vibrio cholerae. I. Physical and chemical characterization. Biochim Biophys Acta. 2003;1639:65–79. doi: 10.1016/j.bbadis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, et al. Immune responses to O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 Ogawa in adult Bangladeshi recipients of an oral killed cholera vaccine and comparison to responses in patients with cholera. The American journal of tropical medicine and hygiene. 2014;90:873–81. doi: 10.4269/ajtmh.13-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apter FM, Michetti P, Winner LSd, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–85. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun. 2010;78:4402–20. doi: 10.1128/IAI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitner DR, Feichter S, Schild-Prufert K, Rechberger GN, Reidl J, Schild S. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect Immun. 2013;81:2379–93. doi: 10.1128/IAI.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winner Ld, Mack J, Weltzin R, Mekalanos JJ, Kraehenbuhl JP, Neutra MR. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infection & Immunity. 1991;59:977–82. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levinson KJ, De Jesus M, Mantis NJ. Rapid effects of a protective O-polysaccharide-specific monoclonal IgA on Vibrio cholerae agglutination, motility, and surface morphology. Infect Immun. 2015;83:1674–83. doi: 10.1128/IAI.02856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levinson KJ, Giffen SR, Pauly MH, Kim do H, Bohorov O, Bohorova N, et al. Plant-based production of two chimeric monoclonal IgG antibodies directed against immunodominant epitopes of Vibrio cholerae lipopolysaccharide. J Immunol Methods. 2015;422:111–7. doi: 10.1016/j.jim.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lullau E, Heyse S, Vogel H, Marison I, von Stockar U, Kraehenbuhl JP, et al. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. Journal of Biological Chemistry. 1996;271:16300–9. doi: 10.1074/jbc.271.27.16300. [DOI] [PubMed] [Google Scholar]
- 17.Krebs SJ, Taylor RK. Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model. J Bacteriol. 2011;193:5260–70. doi: 10.1128/JB.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddin T, Harris JB, Bhuiyan TR, Shirin T, Uddin MI, Khan AI, et al. Mucosal immunologic responses in cholera patients in Bangladesh. Clin Vaccine Immunol. 2011;18:506–12. doi: 10.1128/CVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantis NJ, Palaia J, Hessell AJ, Mehta S, Zhu Z, Corthesy B, et al. Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region. J Immunol. 2007;179:3144–52. doi: 10.4049/jimmunol.179.5.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim do H, et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol. 2014;21:777–82. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.