Abstract
Sudden, unexplained death during the perinatal period remains a major, longstanding challenge. Recent advances in diagnostic techniques and genetic testing has provided evidence that a significant fraction of these deaths may result from lethal cardiac arrhythmias. In this paper, we review current methods of diagnosing arrhythmia in the fetus and strategies for management of life-threatening arrhythmia throughout the perinatal period, including transitional care at the time of delivery.
Introduction
The risk of unexplained and unexpected death is especially high during the pre- and peri-natal periods(1). Knowledge and awareness of the different types of fetal and neonatal arrhythmias and conduction disorders, therefore, should be a concern of every neonatologist and perinatologist. In this review we give an update on fetal arrhythmias, focusing on those that have a greater potential for causing fetal death.
Sudden infant death syndrome (SIDS) is the third leading cause of infant death in the United States and the leading cause of death for infants aged 1 to 12 months. Similar unexplained deaths can occur in the apparently healthy, late-gestation fetus. Until recently, these deaths have been attributed to causes other than cardiac death.
A subset of SIDS may be linked to congenital long QT syndrome (LQTS)(2). In an Italian series of neonatal electrocardiographic recordings taken in the first week after birth (3, 4), Schwartz and colleagues found that those with QTc> 440 ms had a 41-fold increase in risk of SIDS, compared to normals. Fifty percent of all infants with SIDS in their series had prolonged-for-age QTc. Although these ion channelopathies are rare (1:2000 neonates would be expected to carry a known Class I mutation), the first hints of rhythm disturbances due to LQTS may be detectable during the last half of pregnancy.
Both genetic and acquired LQTS are mainly recognized for causing fetal sinus bradycardia (Figure 1)(5), but torsades de pointes ventricular tachycardia, T-wave alternans, and second-degree atrioventricular (AV) block can also occur prenatally. An acquired form of LQTS can also be seen with severe congenital hypothyroidism or exposure to QT prolonging maternal medications, especially in combination, or when coexisting, with chronic nutritional deficiencies (calcium, magnesium, potassium, and Vitamin D) common in pregnancy.
Figure 1.
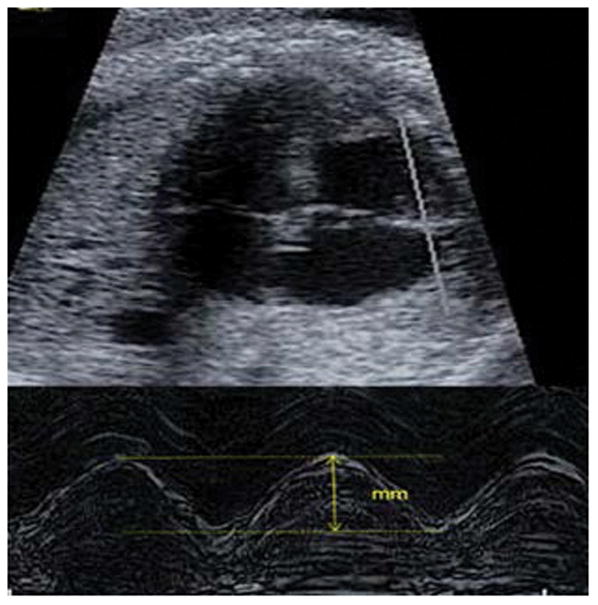
Four-chamber view by fetal echocardiography- TAPSE (tricuspid annular plane systolic excursion). Fetal TAPSE provides an estimate of vertical movement of the tricuspid annulus ventricular contraction, and provides another means of assessing systolic cardiac function.
In general, fetal arrhythmias account for about 20% of referrals to fetal cardiologists; however, obstetricians and neonatologists should be aware that this likely is a gross underestimate of the actual contribution of fetal and neonatal conduction and repolarization abnormalities leading to susceptibility to death. Markers of potentially life-threatening fetal arrhythmias may go unrecognized or may be believed to be harmless because of their subtlety. This is partly due to the fact that the most lethal changes in conduction (QRS and QT prolongation) may not change rate or rhythm and cannot be demonstrated by ultrasound or fetal echocardiography. In addition, it is not possible to routinely obtain a high-quality fetal electrocardiogram (ECG). Magnetocardiography, a new and highly sensitive fetal recording technique, may change this situation in the future; however, at this time the technique is available in only a few centers.
Fetal and neonatal arrhythmias can also be associated with cardiac diseases such as rhabdomyomas (6), ventricular aneurysms (7), myocarditis, and congenital heart disease. In many cases, however, fetal arrhythmias are idiopathic and resolve before one year of age.
Detection of fetal arrhythmias
Fetal Ultrasound
Ultrasound is the primary modality used by obstetricians to recognize fetal arrhythmias. It allows assessment of atrial and ventricular rate (regular or irregular) and contraction sequence. Obstetricians typically evaluate fetal growth, biophysical profile, and Doppler flow patterns of umbilical artery, middle cerebral artery, and ductus venosus. Referral to a maternal–fetal medicine (MFM) specialist is usually necessary to obtain a full targeted ultrasound for detection of congenital abnormalities, organ dysfunction, and acquired diseases, as well as to further define the specific type of arrhythmia(8).
Fetal Echocardiography
Fetal echocardiography is the mainstay of perinatal diagnostic tools for fetal heart evaluation. M-mode is still used to assess atrial and ventricular wall motion, cardiac function, and contraction pattern; however, Doppler techniques are more precise for evaluation of heart rhythm. Color M-mode is an often overlooked adjunct to quickly identify contraction sequence patterns. Recently, fetal tricuspid annular plane systolic excursion has been proposed as a surrogate for right ventricular function, which may be distinctively altered in certain arrhythmias (Fig 1). Pulsed Doppler is important for assessing mechanical PR interval, presence of AV valve regurgitation, venous flow reversal, cardiac output, and predicting VA or AV sequencing (9, 10). New tissue Doppler recording techniques provide accurate diagnosis of arrhythmia and are the most precise means of measuring mechanical PR interval. Sequencing may require simultaneous dual site display, which not all echocardiography units are capable of.
Although fetal echocardiographic methods have improved over time, there is still the fundamental limitation that fetal echocardiography measures the mechanical consequences of the arrhythmia rather than the electrical rhythm or conduction abnormality itself. Echocardiography is incapable of measuring QRS and QT interval. Further, echo/Doppler does not allow for prolonged continuous monitoring, such as with Holter or telemetry monitoring, and hence, can miss fleeting arrhythmias and subtle heart rate pattern changes that occur over time. These shortcomings argue for the need to continue developing new technologies, such as magnetocardiography.
Cardiotocography
Cardiotocography uses Doppler ultrasound to register baseline fetal heart rate, fetal heart rate variability, and uterine contractions. Cardiotocography is usually limited to pregnancies over 30 weeks’ gestation. It functions poorly, however, during fetal tachycardia or bradycardia. It can be used to monitor treatments, such as the response to transplacental medications, and to assess fetal heart rate variability as an adjunct to biophysical profile testing when sinus rhythm is the predominant rhythm.
Fetal Electrocardiography
The fetal ECG can detect QRS complexes in fetuses from as early as 17 weeks’ gestation; however, the technique is limited by the low amplitude of the fetal signal relative to noise and interferences. This is impacted by gestational age, maternal ECG interference, and the high electrical resistance of the fetal skin and vernix caseosa. Fetal ECG remains in relative disuse for antenatal monitoring due to its inconsistent quality.
Fetal Magnetocardiography
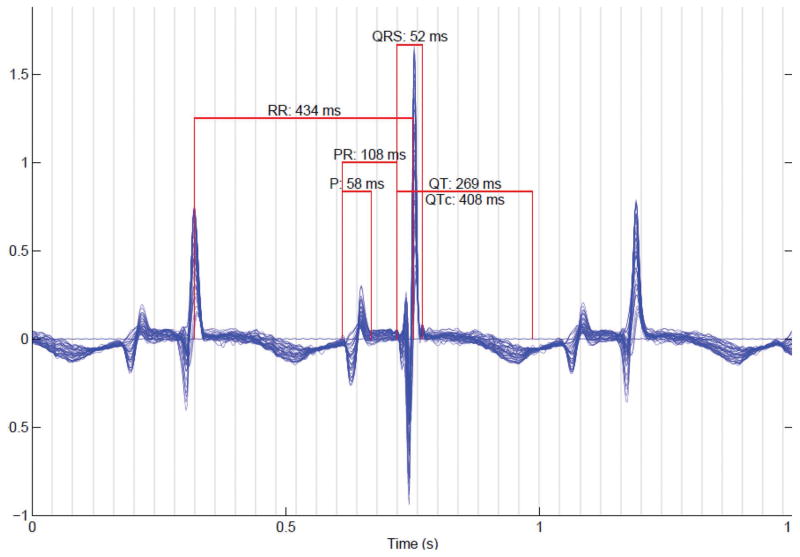
Fetal magnetocardiography (fMCG) is a new method which is more direct and precise than echocardiography for diagnosis of fetal arrhythmias and conduction abnormalities. At the moment, the method is very expensive, and therefore is currently available in a small number of laboratories worldwide; however, technical advances will likely allow greater access in the near future. The fetal magnetocardiogram is recorded using sensitive biomagnetometers, known as superconducting quantum interference devices (SQUIDs). Unlike magnetic resonance imaging, SQUIDs are passive sensors that do not emit energy or magnetic fields. The measurements must be performed within a magnetically shielded room which attenuates magnetic interference from environmental sources. FMCG allows assessment of cardiac waveform morphology and time intervals (P, QRS, and T waves; RR, PR, and QT intervals; Figure 4). The QRS complexes and other waveform components can be detected with greater consistency and signal-to-noise ratio than with fetal ECG. Over the past decade, fMCG has been shown to improve the accuracy and precision of diagnosis of LQTS, congenital AV block, and various tachyarrhythmias (Figure 5). FMCG has supported echocardiography in the evaluation of fetuses with structural cardiac defects, ventricular aneurysms, and cardiac tumors (11–13). FMCG has also led to changes in rhythm diagnosis and detection of unsuspected arrhythmias that have subsequently modified medical care, including drug therapy.(14)
Figure 4.
Signal averaged fetal magnetocardiogram of a healthy fetus
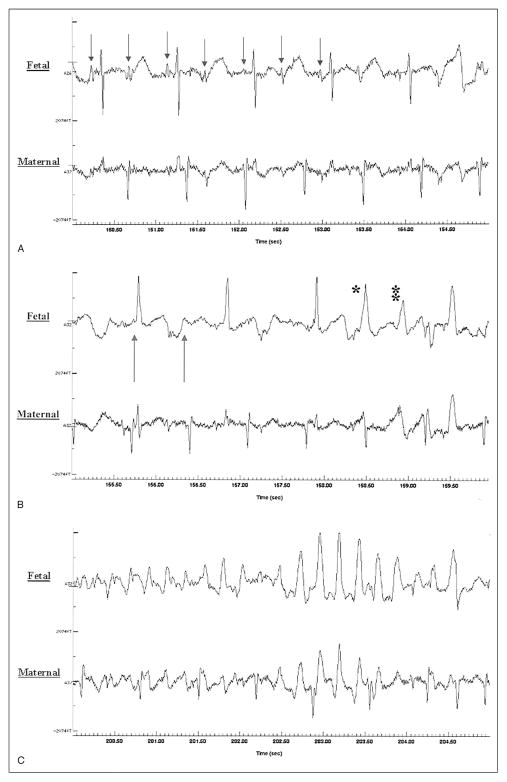
Figure 5.
FMCG tracings (A-C) showing second-degree AV block (A), marked QT prolongation (B), and onset of torsades de pointes ventricular tachycardia (B,C). Used with permission from the American Journal of Cardiology.
Update on Fetal arrhythmias
Bradycardia
Sinus Bradycardia
Fetal ventricular rate <110 beats/min is usually diagnosed as fetal bradycardia. The most common cause of non-persistent fetal bradycardia is sinus bradycardia which can be associated with fetal distress or vagal stimulation. The latter is often observed by obstetricians during routine ultrasound examinations at around 20 weeks’ gestation. As the fetal heart rate normally recovers quickly and it is believed that healthy fetuses have a large reserve, such transient decelerations are not life-threatening.(15)
More significant, however, is the persistent sinus bradycardia associated with cardiac ion channelopathies. These include LQTS and familial bradycardia syndromes caused by SCN5A, HCN4, and NKX2.5 gene mutations. Bradycardia can be present throughout pregnancy or may develop at around 15–20 weeks’. Often it does not meet the accepted definition of fetal bradycardia, in that the rates are low for gestational age but exceed 110 beats/min. Normative gestational age-based heart rates have been published and can be used to determine if the fetal heart rate is slower than expected (16, 17). For persistent fetal or neonatal bradycardia, a fetal magnetocardiogram or ECG, respectively, can determine whether QT prolongation is present.
Congenital heart disease, such as left atrial isomerism, can produce low atrial bradycardia. Maternal medications, such as beta blockers or sedatives, or Sjogren’s syndrome A (SSA) isoimmunization associated with maternal collagen vascular diseases are other etiologies. No specific treatment is required.
Long QT Syndrome
Fetal Arrhythmia Findings
Intrauterine fetal death or stillbirth occurs in approximately 1 of every 160 pregnancies. LQTS can be one cause (18). Crotti and colleagues(18) performed molecular autopsies on 97 fetuses, looking for ion channelopathies. They found that 3.3% had known Class I defects and 8.8% had channelopathies known to cause ion channel dysfunction. LQTS is associated with complex arrhythmias, including torsades de pointes and second-degree AV block. Low-for-gestational age heart rate is also strongly associated with fetal LQTS.
Perinatal care
It is important to check maternal vitamin D, magnesium, and calcium serum concentrations if QT lengthening is suspected, especially if the mother is experiencing muscle cramps. Since albumin levels vary during pregnancy and can impact electrolyte values, serum albumin testing will help determine if the electrolytes are low due to volume expansion. In addition we suggest reviewing the pregnant patient’s medication list for drugs that lengthen QT interval. Having more than one QT prolonging drug may have additive effects, depending on the mechanism at the cellular level. Many of these drugs inhibit L-type calcium channel currents. This may present risk to the mother as well as the fetus, and obtaining an ECG may be appropriate. Planning the delivery in a specialized center and communicating with a team (anesthesia, neonatology, pediatric cardiology) is essential.
Neonatal findings
Early recognition and treatment of LQTS in neonates has been shown to reduce mortality. If either the mother or father has a mutation in a known LQTS gene, the risk of the child having LQTS is 50% for the most common ion channelopathies. Currently, the standard of care is to test the offspring of parents with a mutation in a known LQTS gene for the parental mutation. If the offspring carries the mutation, he/she will be at lifelong risk of sudden cardiac death, require medical treatment from the time of diagnosis, may require device treatment if medications fail, and must be followed by a cardiologist his/her entire life. Medical therapy includes beta blockers and, for LQT3, mexiletine. Drugs that prolong QT interval and are known to be associated with torsades des pointes (www.Torsades.org) should be avoided in infants with LQTS. Propranolol is dosed TID at about 3–4 mg/kg/day. Transitioning to longer acting beta blockers such as atenolol or nadolol is recommended, if formulations are available.
Transitional Care
Genetic testing is performed on cord blood obtained at birth, or on the infant’s blood sample. Early conception testing and fetal cord blood testing can be performed.
Breast Feeding
QT prolonging drugs (http://www.sads.org.uk/drugs-to-avoid) should be avoided while breast feeding. Maternal use of beta blockers, when indicated, is permitted.
CPR Education for Parents
A video self-instruction course, combined with hands-on practice, can be an effective alternative to instructor-led basic life support. This should be completed prior to discharge of the infant.
Infant fever and illness
Events often occur when infants cannot keep down their short-acting beta blocker. Further efforts by drug companies to make sustained release infant preparations are needed. Fever can bring out Brugada-like ECG changes in some infants and should be promptly treated by parents.
AV Conduction abnormalities
Prenatal Arrhythmia Findings
AV conduction abnormalities are detected as first-degree (mechanical PR > 150 ms), second-degree, or complete AV block by fetal echocardiography. These conduction abnormalities are mainly associated with maternal autoantibodies and structural heart disease.
Neonatal findings
The causes of AV conduction abnormalities in newborns are multiple and include isoimunization, endocardial fibroelastosis, myocarditis, drugs, or congenital heart disease (left atrial isomerism, atrioventricular canal defect, or L-transposition of the great arteries). Strong vagal stimulation may also produce transient AV block that is usually benign.
Transitional Care
Infants should be observed in an intensive care setting. Most infants with heart rates over 55 beats/min will have no symptoms, but can develop occult acidosis; therefore, blood pH levels should be assessed every 4–6 hours in the first 1–2 days. The exception is the infant with congenital heart disease, where higher heart rates are often needed to maintain cardiac output. It is important to stabilize the metabolic milieu and hemodynamic status to improve the success of pacing. Temporary external pacing using defibrillator pads should be available for the hydropic baby, and can be attempted in the delivery room if necessary. Temporary transvenous balloon pacing can also be implemented. Epicardially-placed ventricular pacing wires can be used to bridge a very tiny premature infant to a size where permanent leads can be used if weight is >~2 kg. When ventricular function is poor, pacing slowly initially (70 beats/min) and then increasing the rate over time may allow myocardial recovery. Low heart rate variability in utero with ventricular rate < 55 beats/min is predictive of the need for neonatal pacing. For infants with endocardial fibroelastosis, rarely, sudden deterioration can occur due to rupture of the cordae tendinae or papillary muscles of the mitral or tricuspid valve.
Tachycardia
Fetal tachyarrhythmia (heart rates > 160 beats/min) is an uncommon condition that occurs in 0.4–0.6% of all pregnancies (19), and is an important cause of fetal morbidity and mortality. These fetuses are at risk for low cardiac output, hydrops, significant neurological morbidity, and fetal death (20).
Sinus tachycardia
Sinus tachycardia is often secondary to fetal systemic disease. It can be caused by maternal hyperthyroidism. Approximately 17% of fetuses who have maternal thyrotoxicosis develop significant thyrotoxicosis.(1) The antibody is responsible, but often is not measured when thyroid tests are obtained. Even when the mother is euthyroid, antithyroglobulin antibodies can produce fetal goiter and tachycardia.
Causes of sinus tachycardia
Fetal thyrotoxicosis
Infection
Myocarditis
Drugs
Primary cardiomyopathy
Fetal anemia
Management for fetal tachycardia: fetal /neonatal checklist
Avoidance of second hand exposure to caffeine, beta sympathomimetics, nicotine, illicit drugs or combinations (including through breastmilk)
Exclusion of maternal and fetal goiter, infection, and other treatable conditions
Blood levels of antiarrhythmics from cord blood if appropriate at time of birth
Adenosine readily available for neonatal SVT if appropriate. Dive reflex (ice application to the face for 15–20 seconds) can also be used
Awareness that fetal antiarrhythmic treatment in utero can lead to sinus node dysfunction following cardioversion (be prepared with external pacing if needed).
Supraventricular Tachycardia (SVT)
Prenatal Findings
Fetal reentrant SVT is usually characterized by a persistent tachycardia. Ventricular rates are 210–320 beats/min. Heart rhythm is regular with 1:1 AV conduction. It can start and stop abruptly and resume at the same rate. SVT usually presents around 28 to 30 weeks’ gestation. It leads rapidly to the development of hydrops fetalis when it is persistent for more than 12 hours or at rates of more than 230 beats/min (1). It is generally treated with one or more antiarrhythmic agents given to the mother, although, in infants over 35 weeks, delivery could be considered. Digoxin, sotalol, and flecainide are used, with no consensus on which is best.
Neonatal Findings
Narrow complex SVT is commonly present in the neonate. Treatment is recommended; however, it is important to obtain a 12 lead ECG or rhythm strip beforehand. During application of ice, administration of adenosine, or synchronized cardioversion, rhythm should be recorded continuously. In cases of fetal hydrops, multidrug exposition, prematurity or congenital heart disease, the management is more difficult. Due to the underlying problem or insufficient treatment there is also a high risk of death.
Transitional Care
Because newborns have impaired cardiac function and can have low cardiac output they should be delivered in a center capable of managing neonatal SVT, and they should be admitted to the intensive care unit even if their initial presentation is sinus rhythm. Most recurrences of fetal tachycardia are in the first 72 hours. There is an ongoing debate as to whether to treat an infant who remains in sinus rhythm after fetal tachycardia. A high percentage will not have recurrence, so it is our policy to allow the infant to “declare itself” before starting treatment. Parents should be taught CPR and ice application to reproduce the Dive reflex.
Dive Reflex technique:
Prepare as for full resuscitation, including having defibrillator at bedside and monitor attached and checked for paper supply. Begin running rhythm strip when ready to apply ice.
Fill quart-sized plastic bag partially full of crushed ice and add water. This allows a perspiration on the outside of bag that further cools the bag.
Apply over the upper 2/3 of face and forehead, including over the nose and mouth. Allow to stay in place for 20 seconds. Baby will struggle. Remove, then calm the infant, and if not converted, check for any signs of frost bite. If none, then repeat after about 1–2 minutes from last application.
Use full face application once if above is not successful, checking each time for frostbite. If unsuccessful after 2–3 attempts, consider adenosine intravenously.
WPW Syndrome
The Wolff-Parkinson-White (WPW) Syndrome is due to an abnormal accessory conduction pathway between the atrium and the ventricle. The pathway can mediate reentrant SVT, often triggered by a premature beat, with the forward limb over the AV node and retrograde limb over the accessory pathway, resulting in a narrow-QRS tachycardia.
Prenatal assessment
During routine ultrasound examination in sinus rhythm, it is important to look for a short mechanical PR interval (< 70 ms) to detect WPW syndrome. Structural abnormalities can accompany SVT and WPW syndrome (rhabdomyomas or congenital heart diseases, such as Ebsteins malformation of the tricuspid valve).
Neonatal findings
In case of a WPW syndrome, digoxin and verapamil are contraindicated because of the dependence of the infant on calcium transport for cardiac output. Digoxin is still used for fetal tachycardia as second line treatment even when WPW may be present, though first line treatment with sotalol or flecainide is preferred and usually effective.
Atrial flutter
Prenatal Findings
Fetal atrial flutter (AFl) accounts for up to a third of all fetal tachyarrhythmias. It is defined as rapid regular atrial rate of 300–600 beats/min accompanied by variable AV conduction (21). Experimental observations and the occurrence of atrial flutter in the third trimester of pregnancy is believed to support the hypothesis that atrial macro-reentry is the underlying mechanism of atrial flutter (21). Although most cases are isolated, cardiac malformations, such as atrioventricular septal defect, hypoplastic left heart syndrome and pulmonary atresia can be associated with fetal atrial flutter (22–30). Myocarditis or SSA/Ro antibody associations have also been described (10, 31, 32). Over 50% of fetuses and infants with atrial flutter have accessory AV connections. Authors report a relatively good prognosis, if conversion of fetal tachycardia was attained in utero. Sotalol and the combination of sotalol and digoxin were reported as having not only a high conversion rate, but also a low recurrence rate (19, 33)
Neonatal findings
Naheed and colleagues suggest that over 50% of fetuses will not have recurrence of arrhythmias after they are born (34). However, the most common approach to early neonatal management of sustained atrial flutter is synchronized cardioversion, or transesophageal overdrive pacing for those infants with sustained regular tachycardia. Treatment after conversion is controversial (28, 29). If sinus rhythm is achieved, recurrence is rare. Therefore, a wait-and-see policy may be reasonable. Naumburg and colleagues recommended a follow up period of 6 months due to late recurrences (27).
Ectopy
Prenatal Findings
Premature atrial contractions (PAC’s) are associated with good outcome and are ten times more common than premature ventricular contractions. In approximately 0.4 % of cases, ectopy may progress to runs of tachycardia and may even become persistent. It is therefore recommended that these patients be monitored weekly by their obstetricians while ectopy is active to check for new-onset SVT. PAC’s may also be blocked, producing a bradycardia known as blocked atrial bigeminy with ventricular rates of only 75–100 beats/min(35). With blocked atrial bigeminy the risk of SVT is much higher: approximately 10% in utero. M-mode echocardiography will show PACs not followed by a ventricular contraction (36, 37). The combination of conducted and blocked atrial beats can make monitoring of labor difficult (Figure 7B).
Figure 7.
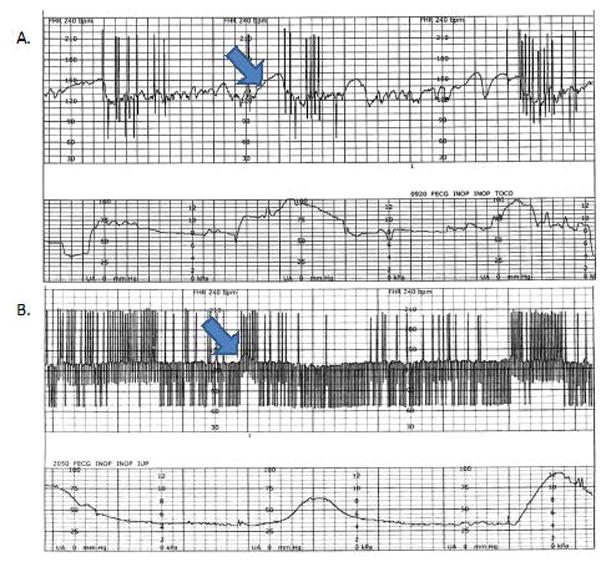
The top cardiotocography tracing shows single PAC’s stimulated by uterine contractions. In the bottom tracing, the blocked PAC’s (below the baseline, and with slower rates), and the conducted PAC’s make it difficult to monitor labor. The arrows denote heart rate accelerations, however, the majority of the lower tracing is nonreactive.
Summary of coordination of Care in a Fetus with Arrhythmia
The optimal care of the fetus and neonate with an arrhythmia depends on 1) early diagnosis, 2) a team with experience in management, and 3) expectant assessment in all phases. With these 3 principles, even the most dangerous arrhythmia can be managed.
Figure 2.
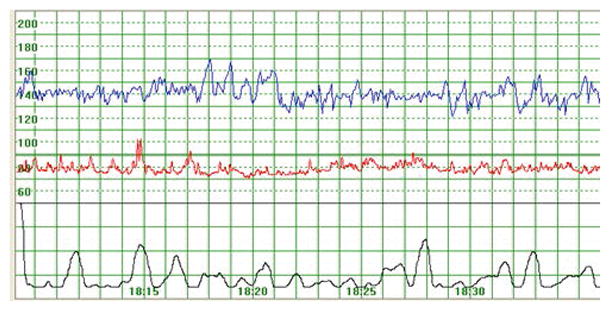
Example of a cardiotocography tracing (fetal heart rate, maternal heart rate, and uterine contractions)
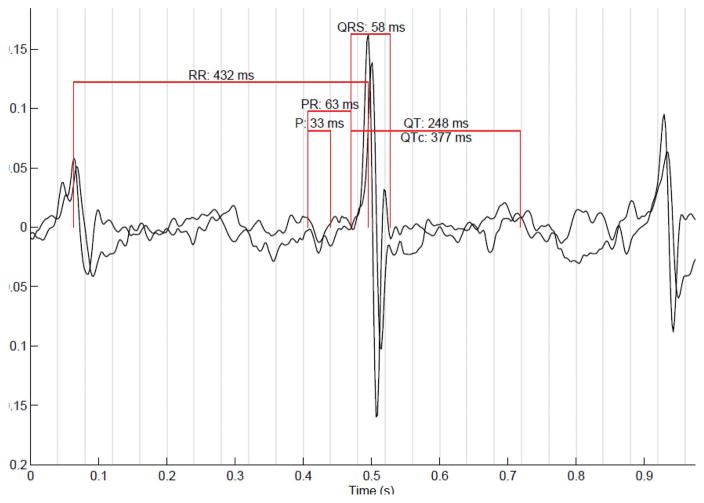
Figure 3.
Fetal electrocardiogram of a healthy fetus
Figure 6.
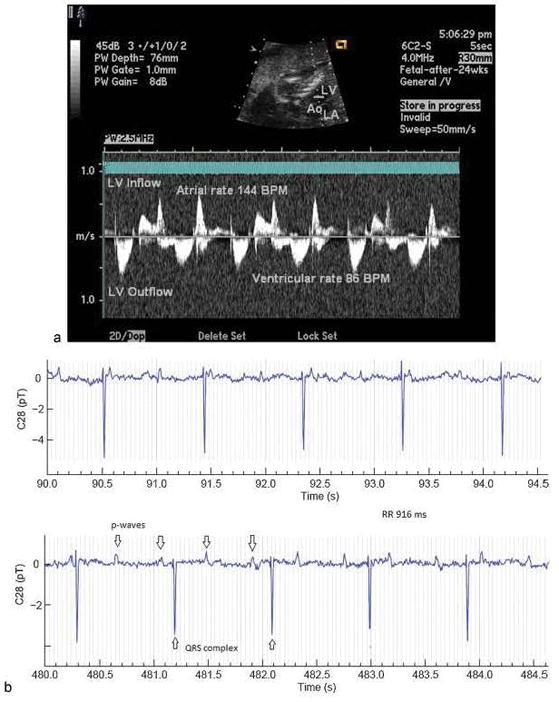
Complete AV block assessed by a) fetal echocardiography and b) fetal magnetocardiography. Some A waves are not seen because they occur at the time of ventricular contraction. Used with permission from the American Journal of Perinatology.
Acknowledgments
Grant Support: NIH R01HL63174 (Wakai)
References
- 1.Strasburger JF, Cheulkar B, Wichman HJ. Perinatal arrhythmias: diagnosis and management. Clinics in perinatology. 2007;34(4):627–52. vii–viii. doi: 10.1016/j.clp.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulze-Bahr E, Fenge H, Etzrodt D, Haverkamp W, Monnig G, Wedekind H, et al. Long QT syndrome and life threatening arrhythmia in a newborn: molecular diagnosis and treatment response. Heart. 2004;90(1):13–6. doi: 10.1136/heart.90.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ. Stillbirths, sudden infant deaths, and long-QT syndrome: puzzle or mosaic, the pieces of the Jigsaw are being fitted together. Circulation. 2004;109(24):2930–2. doi: 10.1161/01.CIR.0000133180.77213.43. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338(24):1709–14. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 5.Cuneo BF, Ovadia M, Strasburger JF, Zhao H, Petropulos T, Schneider J, et al. Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91(11):1395–8. doi: 10.1016/s0002-9149(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 6.Wacker-Gussmann A, Strasburger JF, Cuneo BF, Wiggins DL, Gotteiner NL, Wakai RT. Fetal arrhythmias associated with cardiac rhabdomyomas. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11(4):677–83. doi: 10.1016/j.hrthm.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters C, Wacker-Gussmann A, Strasburger JF, Cuneo BF, Gotteiner NL, Gulecyuz M, et al. Electrophysiologic features of fetal ventricular aneurysms and diverticula. Prenatal diagnosis. 2015;35(2):129–36. doi: 10.1002/pd.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacker-Gussmann A, Strasburger JF, Cuneo BF, Wakai RT. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol. 2014;31(7):617–28. doi: 10.1055/s-0034-1372430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Strasburger J. Overview of fetal arrhythmias. Curr Opin Pediatr. 2008;20(5):522–31. doi: 10.1097/MOP.0b013e32830f93ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters C, Wacker-Gussmann A, Strasburger JF, Cuneo B, Gotteiner N, Wakai RT. Electrophysiologic features of fetal ventricular aneurysms and diverticula. Cardiology in the Young. 2013;23(Suppl 1):S73. doi: 10.1002/pd.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wacker-Gussmann A, Strasburger JF, Cuneo B, Wiggins D, Gotteiner N, Wakai RT. Fetal Arrhythmias Associated with Cardiac Rhabdomyomas. Heart Rhythm. 2013 doi: 10.1016/j.hrthm.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Cuneo BF, Strasburger JF, Huhta JC, Gotteiner NL, Wakai RT. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51(1):77–84. doi: 10.1016/j.jacc.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuneo BF, Strasburger JF, Yu S, Horigome H, Hosono T, Kandori A, et al. In Utero Diagnosis of Long QT Syndrome by Magnetocardiography. Circulation. 2013;128(20):2183–91. doi: 10.1161/CIRCULATIONAHA.113.004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornberger LK, Sahn DJ. Rhythm abnormalities of the fetus. Heart. 2007;93(10):1294–300. doi: 10.1136/hrt.2005.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell JL, Cuneo BF, Etheridge SP, Horigome H, Weng HY, Benson DW. Fetal heart rate predictors of long QT syndrome. Circulation. 2012;126(23):2688–95. doi: 10.1161/CIRCULATIONAHA.112.114132. [DOI] [PubMed] [Google Scholar]
- 17.Serra V, Bellver J, Moulden M, Redman CW. Computerized analysis of normal fetal heart rate pattern throughout gestation. Ultrasound Obstet Gynecol. 2009;34(1):74–9. doi: 10.1002/uog.6365. [DOI] [PubMed] [Google Scholar]
- 18.Crotti L, Tester DJ, White WM, Bartos DC, Insolia R, Besana A, et al. Long QT syndrome-associated mutations in intrauterine fetal death. JAMA. 2013;309(14):1473–82. doi: 10.1001/jama.2013.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudijk MA, Ruskamp JM, Ververs FF, Ambachtsheer EB, Stoutenbeek P, Visser GH, et al. Treatment of fetal tachycardia with sotalol: transplacental pharmacokinetics and pharmacodynamics. J Am Coll Cardiol. 2003;42(4):765–70. doi: 10.1016/s0735-1097(03)00779-4. [DOI] [PubMed] [Google Scholar]
- 20.Mongiovi M, Pipitone S. Supraventricular tachycardia in fetus: how can we treat ? Current pharmaceutical design. 2008;14(8):736–42. doi: 10.2174/138161208784007725. [DOI] [PubMed] [Google Scholar]
- 21.Krapp M, Kohl T, Simpson JM, Sharland GK, Katalinic A, Gembruch U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89(8):913–7. doi: 10.1136/heart.89.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeggi E, Fouron JC, Drblik SP. Fetal atrial flutter: diagnosis, clinical features, treatment, and outcome. The Journal of pediatrics. 1998;132(2):335–9. doi: 10.1016/s0022-3476(98)70455-x. [DOI] [PubMed] [Google Scholar]
- 23.Azancot-Benisty A, Jacqz-Aigrain E, Guirgis NM, Decrepy A, Oury JF, Blot P. Clinical and pharmacologic study of fetal supraventricular tachyarrhythmias. The Journal of pediatrics. 1992;121(4):608–13. doi: 10.1016/s0022-3476(05)81156-4. [DOI] [PubMed] [Google Scholar]
- 24.van Engelen AD, Weijtens O, Brenner JI, Kleinman CS, Copel JA, Stoutenbeek P, et al. Management outcome and follow-up of fetal tachycardia. J Am Coll Cardiol. 1994;24(5):1371–5. doi: 10.1016/0735-1097(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 25.Soyeur DJ. Atrial flutter in the human fetus: diagnosis, hemodynamic consequences, and therapy. Journal of cardiovascular electrophysiology. 1996;7(10):989–98. doi: 10.1111/j.1540-8167.1996.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 26.Hajdu J, Szabo I, Papp C, Gorbe E, Cesko I, Papp Z. Management of hemodynamically significant fetal arrhythmias. Orvosi hetilap. 1997;138(37):2335–8. [PubMed] [Google Scholar]
- 27.Naumburg E, Riesenfeld T, Axelsson O. Fetal tachycardia: intrauterine and postnatal course. Fetal diagnosis and therapy. 1997;12(4):205–9. doi: 10.1159/000264469. [DOI] [PubMed] [Google Scholar]
- 28.Zielinsky P, Dillenburg RF, de Lima GG, Zimmer LP. Fetal supraventricular tachyarrhythmias. Experience of a fetal cardiology referral center. Arquivos brasileiros de cardiologia. 1998;70(5):337–40. doi: 10.1590/s0066-782x1998000500006. [DOI] [PubMed] [Google Scholar]
- 29.Krapp M, Baschat AA, Gembruch U, Geipel A, Germer U. Flecainide in the intrauterine treatment of fetal supraventricular tachycardia. Ultrasound Obstet Gynecol. 2002;19(2):158–64. doi: 10.1046/j.0960-7692.2001.00562.x. [DOI] [PubMed] [Google Scholar]
- 30.Jaeggi E, Fouron JC, Fournier A, van Doesburg N, Drblik SP, Proulx F. Ventriculo-atrial time interval measured on M mode echocardiography: a determining element in diagnosis, treatment, and prognosis of fetal supraventricular tachycardia. Heart. 1998;79(6):582–7. doi: 10.1136/hrt.79.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuneo BF, Strasburger JF, Niksch A, Ovadia M, Wakai RT. An expanded phenotype of maternal SSA/SSB antibody-associated fetal cardiac disease. J Matern Fetal Neonatal Med. 2009;22(3):233–8. doi: 10.1080/14767050802488220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacker-Gussmann A, Strasburger JF, Cuneo BF, Wakai RT. Diagnosis and Treatment of Fetal Arrhythmia. American journal of perinatology. 2014 doi: 10.1055/s-0034-1372430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasburger JF. Re: Sotalol as first-line treatment for fetal tachycardia and neonatal follow-up. L. B. van der Heijden, M. A. Oudijk, G. Manten, H. ter Heide, L. Pistorius and M. W. Freund. Ultrasound Obstet Gynecol 2013; 42: 285–293. Ultrasound Obstet Gynecol. 2013;42(3):254–5. doi: 10.1002/uog.12390. [DOI] [PubMed] [Google Scholar]
- 34.Naheed ZJ, Strasburger JF, Deal BJ, Benson DW, Jr, Gidding SS. Fetal tachycardia: mechanisms and predictors of hydrops fetalis. Journal of the American College of Cardiology. 1996;27(7):1736–40. doi: 10.1016/0735-1097(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 35.Wiggins DL, Strasburger JF, Gotteiner NL, Cuneo B, Wakai RT. Magnetophysiologic and echocardiographic comparison of blocked atrial bigeminy and 2:1 atrioventricular block in the fetus. Heart Rhythm. 2013;10(8):1192–8. doi: 10.1016/j.hrthm.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudijk MA, Visser GH, Meijboom EJ. Fetal tachyarrhythmia--part I: Diagnosis. Indian pacing and electrophysiology journal. 2004;4(3):104–13. [PMC free article] [PubMed] [Google Scholar]
- 37.Cuneo BF, Strasburger JF. Management strategy for fetal tachycardia. Obstetrics and gynecology. 2000;96(4):575–81. doi: 10.1016/s0029-7844(00)00996-0. [DOI] [PubMed] [Google Scholar]