1. Introduction
Restless Legs Syndrome (RLS) is a movement disorder characterized by an urge to move the legs that is precipitated by rest, relieved by movement, and most pronounced in the evening or at night (Allen et al., 2003). RLS has gained substantial attention in recent years because of its clear links with not only sleep disruption, but also morbidity and mortality. Anecdotal reports and case series have suggested that RLS may be common among those experiencing opioid withdrawal (Ghosh & Basu, 2014; Park et al., 2010; Scherbaum, Stüper, Bonnet, & Gastpar, 2003). Moreover, opioids have been used successfully to treat RLS in patients who did not respond to first-line therapies (Trenkwalder et al., 2013; Walters et al., 1993). However, the prevalence of RLS among those experiencing opioid withdrawal is unknown, and whether the common appearance of this symptom is unique to opioids relative to other substance of abuse has not been studied. The aim of this study was to examine the prevalence of RLS during substance detoxification.
Primary RLS, which affects approximately 5–10% of the population, causes substantial sleep disruption and has been associated with cognitive impairment (Allen et al., 2005; Pearson et al., 2006). Patients with RLS have been shown to exhibit deficits similar in magnitude to those incurred by one night of sleep loss on cognitive tests of prefrontal cortical function, including verbal fluency and trail making (Pearson et al., 2006). RLS is also associated with a decrease in health-related quality of life to a degree similar to that observed in patients with chronic medical conditions such as type 2 diabetes mellitus, depression, and osteoarthritis(Allen et al., 2005). Moreover, RLS has been associated with increased risk for long-term adverse health consequences, including an approximately two-fold increase in the risk for cardiovascular disease (Winkelman, Shahar, Sharief, & Gottlieb, 2008) and overall mortality (Li et al., 2013).
Secondary RLS, which occurs as a result of other health problems, also has serious adverse consequences. For example, increased morbidity and mortality has been demonstrated in patients with RLS associated with end-stage renal disease, an established secondary cause of RLS(Lin et al., 2015; Winkelman, Chertow, & Lazarus, 1996). These data underline the importance of both primary and secondary RLS as causes of acute distress and predictors of adverse health and functional outcomes.
One case series found evidence of RLS in 13% of those detoxifying from opioids (Scherbaum et al., 2003). However, only those cases of RLS that had been spontaneously described by the patient and documented by the clinician were included. Patients may not report RLS symptoms to their physician in this situation because they may not identify the symptoms as part of a distinct medical condition. Furthermore, RLS symptoms are notoriously difficult for patients to articulate and can easily be misinterpreted by physicians as the more generalized restlessness associated with opioid withdrawal. Without direct questions aimed at identifying the symptoms that are specific to RLS, the distinction between nonspecific restlessness and true RLS can not be rigorously ascertained.
Distinguishing nonspecific restlessness from true RLS may be important not only to the clinical management of opioid detoxification, but also for managing the risk for relapse following detoxification. Sleep disruption during protracted withdrawal from opioids may confer risk for poor outcomes (Barta, Kurth, Stein, Tennen, & Kiene, 2009; Beswick et al., 2003); a case series suggested that RLS may also continue following acute withdrawal (Ghosh & Basu, 2014). Data from participants with alcohol use disorder suggest that ongoing sleep disruption may also increase risk for relapse, particularly among those who report using substances to aid with sleep (Brower, Aldrich, Robinson, Zucker, & Greden, 2001). Thus, identifying and treating RLS may also be important for mitigating the risk for relapse immediately following detoxification.
We conducted an observational study aimed at determining the prevalence of RLS among patients receiving detoxification from opioids or alcohol. To assess the specificity of RLS to opioid withdrawal syndrome, we compared the prevalence of RLS between patients experiencing opioid withdrawal and those experiencing alcohol withdrawal.
2. Methods
2.1 Participants
Participants were recruited for a survey of adults receiving treatment on an inpatient substance use disorder treatment unit. Adults aged 18 and older with a primary diagnosis of a substance use disorder were eligible to participate. Exclusion criteria included any current psychiatric or medical condition (e.g., active psychosis) that would preclude the ability to provide informed consent or complete a brief survey.
All participants were receiving medical detoxification. Potential participants were approached by a member of the study staff and offered the opportunity to participate. After providing written informed consent, participants completed a battery of questionnaires. Diagnosis of primary substance use disorder was retrieved from the medical chart. Of the 321 participants with either a primary opioid use disorder or alcohol use disorder, 304 completed a measure of RLS and are included in the analyses below. All procedures were approved by the local Institutional Review Board.
2.2 Detoxification protocol
Patients presenting to the inpatient unit with alcohol or opioid use disorder were assessed for withdrawal symptoms and treated according to the following protocols. For those with opioid use disorder, the Clinical Opioid Withdrawal Scale (COWS) and vital signs were assessed every 4 hours to identify signs of opioid withdrawal. Buprenorphine was administered once the patient exhibited signs of withdrawal (COWS score greater than 8). Once patients were in withdrawal according to this measure, they received 4 mg of buprenorphine up to 3 times per day on the first day with a reduction by 4 mg per day, tapering off completely prior to discharge. Patients presenting with alcohol use disorder received 50 mg of chlordiazepoxide as needed for signs of alcohol withdrawal in the first 24 hours with a taper by 25% per day over the next 3 days. Regular, ongoing monitoring of vital signs is conducted to identify the need for dose modifications.
2.3. Instruments
Demographic information was self-reported by participants, including gender, age, race, marital status, and employment status. Several clinical variables, including substance use disorder diagnosis, medication, and number of prior detoxifications were extracted from the medical chart.
The Brief Addiction Monitor is a self-report measure of substance use and functional consequences including ratings of physical and psychological well-being (Cacciola et al., 2013). This measure was administered and used to determine the number of days of substance use in the previous month.
The diagnosis of RLS was established based on a questionnaire modified from the tool used in the second examination of the Sleep Heart Health Study (SHHS-2), amended to ask about symptoms in the past day or since stopping the drug. The questions reflect the diagnostic criteria established by the International Restless Legs Syndrome Study Group and have been used in several large epidemiologic investigations (Allen et al., 2014; Budhiraja et al., 2012; Winkelman et al., 2008; 2009). The questions in Figure 1 reflect the required criteria for a diagnosis of RLS. Affirmative responses to questions 1a, 2, and 3 were required. For question 4, participants had to report that symptoms were present only “while resting, sitting, or lying down,” to avoid classifying leg discomfort from claudication or leg cramps as RLS. For question 5, RLS was defined as present if the participant responded “bedtime only” or “evening or nighttime.” If the response was “both day and night,” an affirmative response was required to question 6, “if both day and night, do they get worse at night?”
Figure 1.
Questionnaire for Diagnosis of Likely RLS
For further characterization of symptoms, participants were asked to rate the severity of their symptoms overall (none, mild, moderate, severe, or very severe) and their sleep disturbance due to these symptoms (none, mild, moderate, severe, or very severe). They were also asked to rate symptom severity on a scale from 0 (none) to 10 (maximum severity) at different times of day: “at bedtime,” “at night,” “during the day while resting,” and “during the day while active.”
2.3 Data Analysis
All variables were evaluated for skewness and univariate outliers to determine the appropriate statistical approach. First, the prevalence of RLS in opioid vs. alcohol use disorder was compared using a χ2 test. We then compared the opioid and alcohol use disorder groups on the severity of RLS symptoms using independent-samples t-tests. Given the use of multiple tests to compare symptoms between groups, the alpha was adjusted using the Sidak correction (alpha = .009).(Šidák, 1967) Finally, the difference in RLS prevalence in opioid vs. alcohol use disorder was tested in a logistic regression model controlling for sociodemographic variables, smoking status (current smoker vs. non-smoker), and current prescription for a serotonergic medication, which have been shown to be associated with RLS symptoms (Rottach et al., 2008). Alpha was set at .05 for the logistic regression.
3. Results
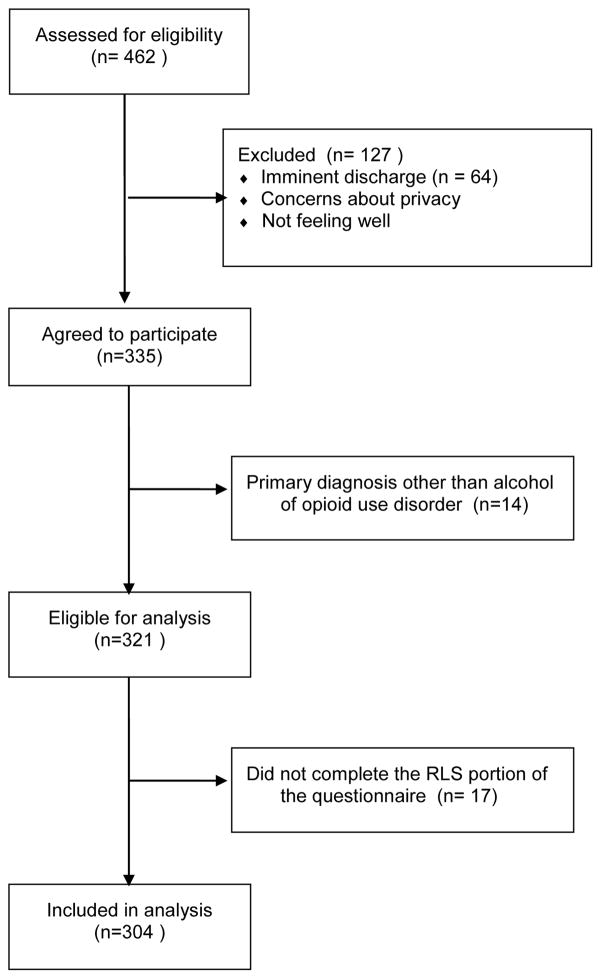
A total of 462 individuals were approached and offered participation (see Figure 2). Of these, 335 (72.5%) agreed to participate and enrolled in the study. The most common reasons for refusing participation were: not interested, concerns about privacy/confidentiality, imminent discharge, and feeling ill. One participant was ineligible and 64 expressed interest but were unable to enroll prior to discharge. For this analysis, those with either an alcohol or opioid use disorder as the primary disorder were included (n=321); of this subset 304 completed the RLS survey and were included in this study.
Figure 2.
Flow chart for recruitment of participants
The sample consisted of 124 adults with primary opioid use disorder and 180 with primary alcohol use disorder. The mean age was 37.7 years (SD=13.4), and the sample was 31.7% (n=96) female. Self-reported race was mostly White (94.1%). Employment status of the sample was self-reported as 42.6% unemployed, 45.3% employed, and a small percentage of other employment statuses (student, retired, or disabled). The majority of the sample was single (59.4%), followed by married (28.7%), and divorced (6.9%), with fewer than 5% separated, widowed, or partnered.
Sociodemographic and clinical characteristics of the sample categorized by primary substance use disorder and presence of RLS are presented in Table 1. There were several significant differences between those with opioid use disorder and alcohol use disorder. Those with opioid use disorder were younger (t[302]=−12.69, p <.001), more likely to be single (χ2[1, 303]=62.91, p<.001), more likely to be a current smoker (χ2[1, 301]=20.47, p<.001), more likely to have used substances more than 15 days in the previous month (χ2[1, 304]=13.41, p<.001) and less likely to have a current prescription for a serotonergic medication (χ2[1, 304]=7.03, p<.01). These variables were included as covariates in the logistic regression model.
Table 1.
Sample Characteristics by Primary Substance Use Disorder and Presence of Restless Leg Syndrome Diagnosis (% or mean (SD))
| Variable | Opioid Use Disorder (n=124) | Alcohol Use Disorder (n=180) | ||
|---|---|---|---|---|
| RLS (n=63, 51%) | No RLS (n=61, 49%) | RLS (n=39, 22%) | No RLS (n=141, 78%) | |
| Sociodemographic | ||||
| Agea | 26.27 (7.95) | 28.98 (11.72) | 40.21 (11.63) | 45.95 (12.47) |
| White | 95.2% | 93.4% | 92.3% | 95.7% |
| Singlea | 88.9% | 83.6% | 53.8% | 36.9% |
| Employed | 36.5% | 41% | 33.3% | 52.5% |
| Male | 69.8% | 77% | 69.2% | 63.1% |
| Substance Use | ||||
| Current smokera | 81% | 70.5% | 53.8% | 48.9% |
| >15 days of use in the past montha | 85.7% | 90.2% | 76.9% | 68.1% |
| Any opiate usea | 100% | 100% | 25.6% | 12.8% |
| Any heroin usea | 79.4% | 77% | 10.3% | 2.1% |
| Any prescription opioid usea | 69.8% | 70% | 23.1% | 11.3% |
| Number previous detox hospitalizations* | 4.09 (8.17) | 3.19 (7.12) | 1.55 (2.14) | 2.62 (7.02) |
| Other Clinical | ||||
| Chronic pain | 19.0% | 15.3% | 25.6% | 12.8% |
| Psychiatric disorder | 61.9% | 42.6% | 82.1% | 62.4% |
| Serotonergic medicationa | 11.1% | 9.8% | 23.1% | 22% |
Note.
Significant difference between alcohol use disorder and opioid use disorder groups as assessed by t-test (continuous variables) or chi-square test (dichotomous variables).
Data on number of prior hospitalizations was only available for 74.3% of the sample. Means reported reflect available data. For other variables, there was a small percentage of participants who declined to answer (full samples for each variable range from 298–304).
In the total sample, 33.6% met a likely RLS diagnosis. This included 50.8% of those with an opioid use disorder and 21.7% of those with an alcohol use disorder. This difference was statistically significant (χ2[1, 304]= 27.96 p < .001; see Figure 2). Moreover, among those with a primary alcohol use disorder, participants who reported any opioid use in the past 30 days (n=28) were more likely than those with no opioid use (n=151) to have RLS; however, this association was not significant, perhaps due to limited power to detect this effect (χ2 [1.179]= 3.78, p = .052).
The logistic regression model showed that opioid use disorder was significantly associated with RLS diagnosis, even controlling for the variables that were significantly different between the alcohol and opioid use groups (age, marital status, smoking status, and use of serotonergic medications). Diagnosis of opioid use disorder was associated with more than twice the likelihood of RLS diagnosis relative to diagnosis of alcohol use disorder (see Table 2).
Table 2.
Logistic Regression Predicting Restless Leg Syndrome Diagnosis (n=294)
| Variable | B | SEB | OR | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|---|---|
| Age | −.03 | .01 | 0.97 | 0.95 | 0.99 | .04 |
| Never Married | .18 | .37 | 1.20 | 0.58 | 2.47 | .63 |
| >15 Days of Use of Primary Substance in the Past 30 | .18 | .34 | 1.20 | 0.61 | 2.33 | .60 |
| Current Smoker | .20 | .29 | 1.22 | 0.69 | 2.16 | .50 |
| Serotonergic Medication | .24 | .36 | 1.27 | 0.63 | 2.57 | .50 |
| Primary SUD (reference=alcohol use disorder) | .72 | .33 | 2.05 | 1.08 | 3.92 | .03 |
Note. OR = Odds Ratio, CI = Confidence Interval, SUD = Substance Use Disorder.
Among those with RLS, symptom severity was significantly worse among those with an opioid use disorder relative to those with alcohol use disorder for all symptoms assessed as well as overall symptom severity (see Table 3).
Table 3.
Mean (SD) Restless Leg Syndrome Symptom Severity by Substance Use Disorder
| Severity variable | Opioid Use Disorder (n=63) | Alcohol Use Disorder (n=39) | t |
|---|---|---|---|
| Symptom severity (rated 0–4) | |||
| Overall | 2.54 (0.64) | 1.95 (0.73) | 4.25** |
| Sleep disturbance due to symptoms | 2.52 (0.98) | 1.74 (0.97) | 3.93** |
| Time (rated 0–10) | |||
| At bedtime | 7.63 (1.80) | 5.82 (2.41) | 4.34** |
| During the night | 7.13 (2.01) | 5.58 (2.43) | 3.40* |
| During the day while resting | 5.11 (2.38) | 3.53 (2.64) | 3.06* |
| During the day while active | 2.83 (2.52) | 1.47 (1.83) | 2.88* |
p<.01,
p<.001
4. Discussion
Restlessness is a well-known symptom of opioid withdrawal. It is included in the Clinical Opiate Withdrawal Scale (COWS), a commonly used measure of withdrawal severity (Wesson & Ling, 2003). Previous retrospective chart reviews and small case series have suggested that restlessness in a subset of opioid withdrawal patients may reflect the specific sensorimotor disorder RLS. We applied the accepted diagnostic criteria for RLS and established that approximately half of the patients receiving medical detoxification for opioid use disorder report the defining symptoms characteristic of RLS.
Importantly, we found that the characteristic circadian component of RLS – predominance of symptoms in the evening and at night – is present during opioid detoxification. To further distinguish this disorder from nonspecific discomfort or restlessness associated with substance withdrawal, we utilized data from patients withdrawing from alcohol as a comparison group. Although alcohol withdrawal may involve generalized physical and psychological discomfort and insomnia, this study demonstrates that patients in alcohol withdrawal experience symptoms that meet criteria for RLS with a much lower frequency than patients in opioid withdrawal.
A prior study of patients undergoing rapid opioid withdrawal provides preliminary evidence supporting opioid withdrawal as a cause of RLS (Freye, Levy, & Partecke, 2004). In that study, gabapentin, a drug known to be effective for primary RLS, provided substantial relief of these symptoms. Additionally, Ghosh et al. reported that RLS symptoms developing during opioid withdrawal in some cases persist beyond the duration of the opioid withdrawal syndrome, further supporting the idea that this syndrome is distinct from the withdrawal itself (Ghosh & Basu, 2014). Low-dose opioid medications have long been recognized as effective treatments for RLS and are included in current treatment guidelines for RLS, a fact that underscores the importance of this neurochemical system in the pathophysiology of RLS (Aurora et al., 2012).
The primary limitation of this study was our reliance on questionnaires rather than clinician interview to diagnose RLS. Although not formally validated and not previously use in patients with substance use disorders, this questionnaire has been employed in large population-based surveys on RLS and is derived from current RLS diagnostic criteria (Budhiraja et al., 2012; Winkelman et al., 2008; 2009). The fact that the symptoms meeting RLS criteria were more severe and more likely to predominate at night among opioid withdrawal patients compared to alcohol withdrawal patients lends further credibility to the diagnosis.
Another limitation is that the relationship of RLS symptoms to the administration of buprenorphine (in the opioid withdrawal group) or benzodiazepines (in the alcohol withdrawal group) could not be determined. It is possible that these medications influenced the appearance of such symptoms. Buprenorphine, a partial agonist at opioid receptors, may mitigate RLS symptoms. Alternatively, while benzodiazepines improve sleep, they have not been shown to decrease the severity of RLS (Aurora et al., 2012). In the population studied here, the patients with opioid use disorder received buprenorphine, while the patients with alcohol use disorder received benzodiazepines. Thus, any confounding introduced by these medications would be expected to decrease the observed association between opioid use disorder and RLS symptoms.
This study was also limited by inconsistency in timing of the assessment during the course of withdrawal and lack of data about RLS symptoms which may have been present prior to the detoxification. Our aim here was not to provide a longitudinal assessment of symptom severity, although this would also be a valuable area of future research. Instead, this study provides a controlled confirmation that RLS is common among opioid withdrawal patients and is not merely a generic symptom of withdrawal, e.g. from alcohol. Several other conditions are known to be precipitants of RLS (so-called secondary RLS), including end-stage renal disease, peripheral neuropathy, iron deficiency, and pregnancy. We believe that these data support the existence of a secondary form of RLS associated with opioid withdrawal. Future studies investigating the links between RLS and substance use disorder outcomes, as well as the course of RLS symptoms over time (i.e., whether these symptoms remit following remission of other acute withdrawal symptoms) are needed to better understand the impact of these symptoms in this population.
Although acute opioid withdrawal symptoms are typically time-limited, further research is needed to determine whether RLS symptoms continue past the initial detoxification period, including in patients on opioid maintenance therapy. In addition, it is possible that a subset of patients with opioid use disorder had pre-existing idiopathic RLS which triggered or perpetuated their opioid use disorder. Given the marked efficacy of opioid medications to treat RLS and the tendency for the condition to go unrecognized by medical providers, self-treatment of RLS symptoms is a plausible contributor to opioid seeking behavior. Future studies should collect historical data that could help make this distinction.
One point of clinical relevance of the present study is the suggestion that treating RLS symptoms may be a helpful addition to the treatment of opioid withdrawal. Effective medications are available for primary RLS, and several have also been used successfully in other forms of secondary RLS (Aurora et al., 2012). For instance, both dopamine agonists and gabapentin have demonstrated efficacy in RLS secondary to end-stage renal disease (Dauvilliers et al., 2016; Giannaki et al., 2013; Thorp, Morris, & Bagby, 2001). Future studies should assess whether treating RLS during opioid withdrawal effectively manages symptoms and contributes to reduction in relapse to opioid use.
Highlights.
RLS occurs in 51% of patients detoxifying from opioids.
RLS occurs in 22% of patients detoxifying from alcohol.
RLS is more severe in opioid detoxification compared to alcohol detoxification.
Footnotes
Conflicts of interest:
Dr. McHugh is supported by K23 DA035297.
Dr. Weiss has served on the Scientific Advisory Board for Indivior. He is supported by K24 DA022288, from the National Institute on Drug Abuse.
Dr. Winkelman is a consultant for UCB Pharma, Xenoport, Merck, and Flex Pharma. He has received research grants from Purdue, Xenoport, UCB Pharma, NeuroMetrix, and NIMH.
Drs. Mackie, Griffin, and McHugh have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RP, Picchietti DL, García-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Medicine. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. http://doi.org/10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Presented at the Sleep medicine. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Archives of Internal Medicine. 2005;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. http://doi.org/10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–1062. doi: 10.5665/sleep.1988. http://doi.org/10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta WD, Kurth ME, Stein MD, Tennen H, Kiene SM. Craving and self-efficacy in the first five weeks of methadone maintenance therapy: a daily process study. Journal of Studies on Alcohol and Drugs. 2009;70(5):735–740. doi: 10.15288/jsad.2009.70.735. [DOI] [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addiction Biology. 2003;8(1):49–57. doi: 10.1080/1355621031000069882. http://doi.org/10.1080/1355621031000069882. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. The American Journal of Psychiatry. 2001;158(3):399–404. doi: 10.1176/appi.ajp.158.3.399. http://doi.org/10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja P, Budhiraja R, Goodwin JL, Allen RP, Newman AB, Koo BB, Quan SF. Incidence of restless legs syndrome and its correlates. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 2012;8(2):119–124. doi: 10.5664/jcsm.1756. http://doi.org/10.5664/jcsm.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, Dephilippis D, Drapkin ML, Valadez C, Fala NC, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM) Journal of Substance Abuse Treatment. 2013;44(3):256–263. doi: 10.1016/j.jsat.2012.07.013. http://doi.org/10.1016/j.jsat.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Beneš H, Partinen M, Rauta V, Rifkin D, Dohin E, et al. Rotigotine in Hemodialysis-Associated Restless Legs Syndrome: A Randomized Controlled Trial. American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation. 2016 doi: 10.1053/j.ajkd.2015.12.027. http://doi.org/10.1053/j.ajkd.2015.12.027. [DOI] [PubMed]
- Freye E, Levy JV, Partecke L. Use of gabapentin for attenuation of symptoms following rapid opiate detoxification (ROD)--correlation with neurophysiological parameters-- Neurophysiologie Clinique = Clinical Neurophysiology. 2004;34(2):81–89. doi: 10.1016/j.neucli.2004.02.002. http://doi.org/10.1016/j.neucli.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Basu D. Restless legs syndrome in opioid dependent patients. Indian Journal of Psychological Medicine. 2014;36(1):85–87. doi: 10.4103/0253-7176.127262. http://doi.org/10.4103/0253-7176.127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannaki CD, Sakkas GK, Karatzaferi C, Hadjigeorgiou GM, Lavdas E, Kyriakides T, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrology. 2013;14:194. doi: 10.1186/1471-2369-14-194. http://doi.org/10.1186/1471-2369-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81(1):52–59. doi: 10.1212/WNL.0b013e318297eee0. http://doi.org/10.1212/WNL.0b013e318297eee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Sy HN, Chang HW, Liou HH, Lin CY, Wu VC, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. European Journal of Neurology. 2015;22(1):142–149. doi: 10.1111/ene.12545. http://doi.org/10.1111/ene.12545. [DOI] [PubMed] [Google Scholar]
- Park YM, Cho JH, Lim YS, Lee HJ, Kang SG, Kim L. The withdrawal from TDF therapy could induce transient RLS. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(2):419–420. doi: 10.1016/j.pnpbp.2009.12.006. http://doi.org/10.1016/j.pnpbp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Medicine. 2006;7(1):25–30. doi: 10.1016/j.sleep.2005.05.006. http://doi.org/10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Rottach KG, Schaner BM, Kirch MH, Zivotofsky AZ, Teufel LM, Gallwitz T, Messer T. Restless legs syndrome as side effect of second generation antidepressants. Journal of Psychiatric Research. 2008;43(1):70–75. doi: 10.1016/j.jpsychires.2008.02.006. http://doi.org/10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Scherbaum N, Stüper B, Bonnet U, Gastpar M. Transient restless legs-like syndrome as a complication of opiate withrawal. Pharmacopsychiatry. 2003;36(2):70–72. doi: 10.1055/s-2003-39047. http://doi.org/10.1055/s-2003-39047. [DOI] [PubMed] [Google Scholar]
- Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. Journal of the American Statistical Association 1967 [Google Scholar]
- Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation. 2001;38(1):104–108. doi: 10.1053/ajkd.2001.25202. http://doi.org/10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Beneš H, Grote L, García-Borreguero D, Högl B, Hopp M, et al. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurology. 2013;12(12):1141–1150. doi: 10.1016/S1474-4422(13)70239-4. http://doi.org/10.1016/S1474-4422(13)70239-4. [DOI] [PubMed] [Google Scholar]
- Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, Kavey N. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327–332. doi: 10.1093/sleep/16.4.327. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007. http://doi.org/10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation. 1996;28(3):372–378. doi: 10.1016/s0272-6386(96)90494-1. [DOI] [PubMed] [Google Scholar]
- Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32(6):772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. http://doi.org/10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]