Abstract
Cancer stigma has undergone an important transformation in recent decades. In general, this disease no longer fits squarely into Goffman’s classic taxonomy of stigmatized conditions. This review will demonstrate that, with important adaptations, an identity-threat model of stigma can be used to organize cancer stigma research post-Goffman. This adapted model postulates that one’s personal attributions, responses to situational threat, and disease/treatment characteristics can be used to predict identity threat and well-being of individuals with cancer. Implications for further research and clinical practice are discussed.
Keywords: cancer, identity threat, attributions, stigma
A society creates a system of categorizing individuals according to whether they possess attributes thought to be normal (Goffman, 1963). When individuals bear a distinguishing mark that is attributed to their disposition, they can be stigmatized (Jones et al., 1984). Stigmatization can discredit one’s social identity (Goffman, 1963) and can affect a person psychologically by influencing the processes related to identity threat (Major and O’Brien, 2005). Cancer once fit squarely into Goffman’s classification as a physical deformity or “abomination of the body.” However, scientific knowledge has since increased regarding the nature of the disease and its stigma. It is now more appropriate to examine cancer, and the stigmatization of cancer, as “complex and heterogeneous” (Else-Quest and Jackson, 2014: 165), varying by type of cancer across factors such as causes, treatments, and outcomes.
Evidence from the mid-20th century suggests that due to its association with death (Else-Quest and Jackson, 2014), cancer was often not discussed with patients (Holland, 2001). In 1961, nearly 90 percent of physicians at a US hospital reported that they preferred not to tell patients they had cancer, as it could cause them harm (Oken, 1961). In the 1970s, with the advent of psycho-oncology, it became more acceptable to discuss a cancer diagnosis (Holland, 2001). At present, practices vary. For example, in Qatar, most physicians report that they would inform patients of a cancer diagnosis, but 66 percent said they often made exceptions (Rodriguez Del Pozo et al., 2012). In Italy, 45 percent of physicians agree that patients should be given their diagnosis, but only 25 percent reported they did this consistently (Grassi et al., 2000). Finally, in China, it remains more acceptable to disclose a diagnosis to family members than to patients (Wuensch et al., 2013).
Most of the research in the 1980s and 1990s suggested that the stigma associated with cancer had decreased. The attribution–affect–help model explained that patients with HIV/AIDS were seen as more responsible for contracting the disease than those with cancer and thus experience more blame and less help (Weiner et al., 1988). For example, the moral worth of people with AIDS was rated to be lower than those with heart disease or cancer (Hayes et al., 2002). Furthermore, in South Africa, HIV-positive patients reported feeling more stigma than cancer patients (Idemudia and Matamela, 2012). Bloom and Kessler (1994) found that among women undergoing various surgeries, breast cancer patients experienced more emotional support. However, there is a need for more research on attribution, affect, and willingness to help that reflects changes in attitudes toward different types of cancer such as breast, lung, cervical, and colorectal (Else-Quest and Jackson, 2014).
A diagnosis of cancer no longer places an individual into a collectively understood stigmatized category. Rather, the potential stigmatization of cancer depends largely on whether a patient’s identity is threatened by the diagnosis. Stigma-induced identity threat can be understood as the extent to which one’s membership in a potentially stigmatized group is internalized to affect one’s sense of self. A modern understanding of cancer as a stigma should focus on the “targets’ understanding of how others view them, their interpretations of social contexts, and their motives and goals” (Major and O’Brien, 2005: 397). In other words, while past research focused on the disease itself as a stigma that affected all individuals the same way, it is now much more appropriate to examine factors surrounding cancer, such as type, visibility, and the likelihood that the disease will interfere with each individual’s ability to achieve personal goals or function in social contexts. For many, but not all, cancer patients, stigma is a central force in perceptions of the self (Fife and Wright, 2000) and is often based on distinguishing characteristics that vary by the type of cancer.
Psychologists have a longstanding interest in identity threat in stigmatized groups. Steele and colleagues examined stereotype threat, the tension that arises when people are in situations where negative stereotypes for their group apply (Steele, 1997). Crocker et al. (1998) have demonstrated the powerful relationships among attributions, identity threat, and social stigma. Else-Quest and Jackson (2014) recently synthesized research on cancer stigma, emphasizing important changes, and the need for more research that integrates these changes. This review aims to organize this important body of research to form a modified identity-threat model of stigma that can be used to guide contemporary empirical research on cancer stigma, taking into consideration the complexity of the disease and its stigmatization.1 Cancer affects so many that the National Cancer Institute (NCI) has recently prioritized applications for research aimed at reducing cancer-related stigma (NCI, 2013).
Major and O’Brien (2005) postulate that collective representations of stigma status, situational cues, and personal characteristics combine to affect the perception of stigma in various social contexts and, in turn, well-being (Major and O’Brien, 2005: 398). The effect of stigma is contingent upon the extent to which identity is threatened by stigmatized status and the resources to cope with it. While this model provides an excellent foundation for understanding the relationship between identity threat and stigma, not all types of cancer can be understood using it. This review suggests an adaptation of this model to address the contributors to identity threat and stigmatization of cancer across the ways in which individuals, situations, and the characteristics of the disease, and/or its treatments, vary (see Figure 1).
Figure 1.
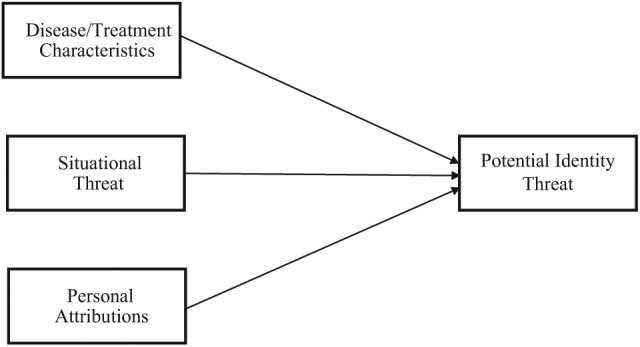
Contributors to identity-threat appraisals in cancer.
Source: Adapted from Major and O’Brien (2005: 398).
Disease/treatment characteristics
An important distinction between cancer patients and other stigmatized individuals is the collective awareness of being part of a stigmatized group. Major and O’Brien’s (2005) model includes collective representations, a shared understanding of being devalued. However, this may not apply to cancer, as it varies across several dimensions of stigma, such as controllability and visibility (Crocker et al., 1998). Patients with a cancer viewed as controllable (e.g. lung cancer), or those with visible treatment side effects, may be more likely to experience identity threat and stigma. Therefore, disease/treatment characteristics is a proposed modification of the model of contributors to identity threat in cancer (see Figure 1).
Perceived controllability
Some types of cancer are more likely to be considered controllable. People may use causal attributions to justify stigmatizing others, and stigmatized individuals may also internalize these attributions. Colorectal and cervical cancer patients report feeling stigmatized due to the perception that their illness resulted from unsavory sexual behavior (Else-Quest and Jackson, 2014). However, lung cancer appears to be most stigmatized as a result of its link with smoking (Marlow et al., 2010), and patients are often blamed for their condition (Chapple et al., 2004). The more controllable a stigma is judged to be, the less willing people are to help (Weiner et al., 1988). In a recent study, those who made causal attributions for cancer were less likely to prioritize funding for lung as opposed to breast cancer (Knapp-Oliver and Moyer, 2012). Reluctance to help may be due to the myth that illness is a deserved punishment (Peters-Golden, 1982). Cancer patients also wonder whether they deserve their illness (Colyer, 1996) and may look to less controllable factors such as environmental toxins (France et al., 2000).
This research has significant implications for anti-stigma interventions. Although the link between smoking and lung cancer is clear, the public may be less aware that there are less controllable risk factors for lung cancer (NCI, 2001).
Visibility
Crocker et al. (1998) also identify visibility as an important predictor of stigmatization that can affect interpersonal interactions and psychosocial well-being. Cancer can become visible as the disease progresses or treatment side effects become obvious (MacDonald and Anderson, 1984; Peters-Golden, 1982). Cancer survivors indicate a poor quality of life due to their appearance, particularly hair loss (McGarvey et al., 2001; Rosman, 2004), and individuals described as having a visible cancer in a context where stigmatization could be perceived as subtle were found to be least likely to receive help (Knapp-Oliver and Moyer, 2009).
Opportunities for cancer patients to take control of, and cope with, the visible consequences of cancer and its treatments require further research. However, the “Look Good, Feel Better” program delivered by volunteer beauty professionals (American Cancer Society (ACS), 2013) has had success. Interventions aimed at coping with the embarrassing side effects of treatment for gynecological and colorectal cancer also hold promise (Carmack et al., 2011; Schofield et al., 2013).
Situational threat
There are few specific situations under which stereotype threat is understood for all cancer patients, as with other social stigmas (e.g. African Americans students in standardized testing situations). However, cancer patients are likely to perceive and internalize stigma in situations where their disease may be a source of identity threat. Although blatant discrimination has become less socially acceptable, more subtle responses such as avoidance, isolation, barriers to treatment, and the possibility of being “outed” remain (Else-Quest and Jackson, 2014).
The workplace
Cancer patients once faced blatant discrimination when returning to the workplace, but better survival rates and new laws have decreased this (Hoffman, 2005. Nevertheless, they still struggle with issues related to functional ability and workplace climate (Compassion in the workplace? Discrimination against an employee with cancer, 2006). There is some support for the notion that side effects of cancer treatments can limit functioning with breast cancer survivors working 2.5 hours less over 2 weeks (Lavigne et al., 2008). However, although cancer patients may be stereotyped as being less productive in the workplace, there is little evidence for this (Feuerstein et al., 2007; Hansen et al., 2008).
When patients experience identity threat in the workplace, one dilemma is whether to reveal a health history to potential employers. Applicants are advised not to volunteer information about their cancer history, but maintain honesty, be aware of legal rights, and to keep the focus on present abilities (Hoffman, 2005). Individuals with cancer are encouraged to inform employers if they anticipate needing accommodations to perform required job tasks.
Although discrimination against cancer patients in the context of hiring has decreased due to legislation (Americans with Disabilities Act, Amendment Acts, 2008), there is still a need to improve the interactions between cancer patients and their coworkers, as well as other people in their social networks. Cancer patients might attempt to improve these relations by adjusting their self-presentation in ways that can increase the amount of social support they receive. Some patients may also find it useful to join support groups.
Interactions with medical providers
Cancer patients seek support from those close to them, but they also look for support from their medical providers. Some research has revealed a discrepancy between what cancer patients need from their providers and what they receive, which leads them to feel stigmatized. Physicians may not focus on side effects and underestimate the impact they will have on patients’ lives (Rosman, 2004). Some physicians are critical of self-help groups and may not inform cancer patients and their families of this resource (Muzzin et al., 1994). Lung cancer patients reported that stigma made it uncomfortable for them to ask their doctors for help (Chapple et al., 2004).
There is an opportunity for psychologists who study patient–provider interaction to make identity threat less likely for cancer patients in a medical setting. Psychosocial interventions may improve communication between medical providers and cancer patients, either by removing barriers to communication for patients (Sepucha et al., 2002) or by improving doctors’ communication skills (Back, 2006).
Interactions with caregivers
There has been a shift in care for cancer patients in recent years. Improvements in medicine and technology have resulted in individuals with cancer living longer, even with advanced-stage disease (Hazelwood et al., 2012). Coupled with the transfer in clinical care from inpatient to outpatient settings, these movements have brought the cancer caregiver to the forefront of the care team (Van Ryn et al., 2011).
Caregivers for cancer patients experience psychological, social, spiritual, and physical burden as a result of their role (Skalla et al., 2013). They report elevated rates of anxiety, depression, and guilt, as well as temporal and financial strain (Applebaum et al., 2013). Having to assume additional responsibilities such as domestic tasks and the patients’ activities of daily living often prevent caregivers from engaging in their own self-care leading to cardiovascular disease (Schneiderman et al., 2012), insomnia (Skalla et al., 2013), and even premature death (Christakis and Allison, 2006).
Caregiver burden can also have an effect on cancer patients and their internalization of stigma. More stigmatized types of cancer are linked to poorer quality of care; for example, caregivers of lung cancer patients who were smokers report being more likely to blame them for their current situation (Lobchuk et al., 2008 as cited in Else-Quest and Jackson, 2014). This blame, when internalized, could result in shame and identity threat for the cancer patient. More efforts should be made to prevent caregiver burden and improve the relationship between cancer patients and their caregivers.
Media and cultural/social group beliefs
The media can serve as a helpful resource for disease-related information. An increasing number of patients are turning to the Internet and social media for information and treatment decision-making (Hesse et al., 2005; Silk et al., 2013). For example, some cancer patients use their Twitter accounts to discuss treatment options and psychological support (Sugawara et al., 2012). However, patients from lower-income or education groups report that they do not trust or use the media for health information (Ramanadhan and Viswanath, 2006) and, compared to White patients, patients from ethnic minority backgrounds reported feeling anxious or confused after conducting searches or frustrated that services were not applicable to them when using the Internet for information on breast cancer (Littlechild and Barr, 2013).
The media can provide threatening information for people experiencing cancer and, unfortunately, this is difficult to monitor and control. Lung cancer patients reported that television advertisements perpetuated the link between lung cancer and smoking, causing them to feel “dirty and blameworthy” (Chapple et al., 2004). In newspaper articles, cancer was often found to be portrayed as a positive life event, and this idealized view may have negative implications for those undergoing debilitating treatments (Kromm et al., 2007). Furthermore, metaphors depicting cancer as a “battle” may result in more stigmatizing attitudes and perceptions of individuals with cancer than metaphors depicting cancer as a “race” or “journey” (Knapp-Oliver et al., 2010). Some suggest that training for journalists can help with reducing stigma (Daher, 2012).
Cancer patients might also encounter stigmatization from their cultural or social groups. African Americans were found to follow a code of silence about their diagnosis due to the stigma in their community (Shankar et al., 2002). Elderly cancer patients may receive less appropriate care as a result of stigmatization, putting them at a disadvantage physically and psychologically and contributing to the likelihood of a threatened identity. For example, daily pain has been found to go untreated, especially among older or minority cancer patients (Bernabei et al., 1998).
Messages in the media are difficult to change, but more research is being done on the ethics of public health communications. Guttman and Salmon (2004) suggest that ethical analyses be applied to public health communications to avoid the inadvertent labeling and stigmatizing of patients.
Personal attributions
Personal attributions for the contraction of, and coping with, cancer influence how stigma-related identity threat is appraised. Major and O’Brien (2005) identify stigma sensitivity, group identification, domain identification, and goals and motives as relevant personal characteristics. Cancer patients make attributions about the extent to which the control for their diagnosis, coping, or outcomes lie within themselves or their external environment. This can be applied within the context of the personal characteristics described by Major and O’Brien (2005).
Stigma sensitivity
Individuals differ in their sensitivity to being stigmatized (Major and O’Brien, 2005). Pinel (1999) characterizes this sensitivity as stigma consciousness, the tendency for potential targets of stigmatization to differ in their expectations of being stigmatized. These expectations then predict their behavior in social contexts; those with high stigma consciousness will expect and perceive more discrimination in their environments and will be less likely to attempt to break down stereotypes about their group than those with low stigma consciousness. While this phenomenon could protect an individual’s self-esteem in the short term (i.e. by attributing any fault to one’s stigma category rather than to the self), it also works to perpetuate these expected stereotypes (Pinel, 1999). For example, in a study where females with high stigma consciousness expected males to be prejudiced against them because of their gender, they acted negatively toward them, eliciting negative responses from the males, and perpetuating the females’ expectations and behavior toward the males in the study (Pinel, 2002).
While research is lacking in the area of stigma sensitivity among cancer patients, there is evidence that a similar relationship exists between expectations of stigma and behavior. This can be seen in cancer patients who lose their hair due to chemotherapy. Alopecia is one of the most identifiable signs that an individual has cancer (Else-Quest and Jackson, 2014). In a sample of breast cancer patients, some felt that their sense of self and identity was threatened by this side effect (Boehmke and Dickerson, 2005). Other patients embraced this indicator of their new identity as a “badge of honor.” Perhaps, the patients experiencing threat had a high stigma consciousness and responded to the perceived identity threat with fear and expectations of avoidance from others, while those who wore their baldness as a “badge of honor” had a low stigma consciousness and sought to break down the stereotypes of cancer patients as “sick” or “weak” due to the fact that they experience alopecia. Patients who have a higher sensitivity, or consciousness, about being stigmatized due to their cancer may experience more identity threat than those who are less sensitive or conscious.
It is also important to note that stigma sensitivity due to a physical illness such as cancer differs from other social stigmas. Those linked to race or gender are present at birth and are embodied for a lifetime. In contrast, cancer patients suddenly move to a stigmatized status upon diagnosis. Also, one’s race or gender is visually and cognitively salient, whereas cancer can often be concealed. These differences may represent unique challenges that cancer patients face when reacting to stigmatization.
Stereotype identification
Individuals whose stigmatized social identity is highly central to their self-identity are more likely to perceive discrimination (Major and O’Brien, 2005). As aforementioned, cancer is a unique stigma; there is no collective understanding that having cancer places one in a unified, stigmatized category or group. However, cancer patients differ in the extent to which they allow their social identity as a cancer patient to be internalized as part of their self-identity, and this may be dependent on well-established social stereotypes including cancer as fearful, cancer as fatal, and cancer as taking control over one’s life. Those who identify with these stereotypes may identify more with their social identity as a cancer patient and experience more identity threat.
Fear
Despite advances in understanding the causes, treatments, and outcomes of cancer, it remains one of the most feared illnesses (Else-Quest and Jackson, 2014). Cancer patients worry about how their life may change following diagnosis (Colyer, 1996), including changes in appearance (Reid, 1997) and the threat of recurrence (Atkinson et al., 2013). For example, some have observed the “Damocles Syndrome” in survivors of childhood cancer who have adjustment problems as adults due to their fear of cancer returning (Koocher and O’Malley, 1981; Muzzin et al., 1994). A meta-analysis found that cancer survivors reported low-to-moderate levels of fear of recurrence, and it was rated among the greatest concerns (Simard et al., 2013). Higher fear of recurrence predicted lower health-related quality of life (Van Liew et al., 2013).
Although social support is critical for cancer patients and survivors (Egestad, 2013), fear may often interfere with their ability to receive support. In a study investigating the role of social support in the cancer experience, Peters-Golden (1982) interviewed women with primary breast cancer and cancer-free individuals regarding their perceived (or anticipated) social support (if they had cancer). An intense fear of cancer resulted in avoidance and low social support for those with cancer. Fear of others’ negative reactions can result in maladaptive behaviors, such as a reluctance to discuss one’s diagnosis and seek social support or medical assistance when needed (Shankar et al., 2002). Similarly, fear of inducing anxious reactions from loved ones may cause patients to avoid divulging their fears of recurrence (Waldrop et al., 2011).
Cancer fatalism
Cancer fatalism is the belief that cancer will lead inevitably to death. Cancer fatalism can hinder engaging in cancer prevention practices and screening (Hall et al., 2008) and may be common in certain ethnic and socioeconomic status groups (Befort et al., 2013; Jun and Oh, 2013; Powe et al., 2009). These beliefs are prevalent in African American populations, and interventions have been designed to increase knowledge about cancer screening and decrease fatalistic beliefs (Morgan et al., 2010).
Loss of personal control
Cancer results in a loss of personal control (Björklund et al., 2008). Since cancer attacks one’s control over physical well-being, including one’s own mortality, some patients will exert control over their emotions in an attempt to control something (Muzzin et al., 1994). This phenomenon was also revealed in interviews with six men diagnosed with breast cancer (France et al., 2000). The feeling of shock at diagnosis was replaced with the impulse to “get on with it,” and focus on the practical and financial matters. Similarly, women with cancer in a sexual organ reported channeling the negative energy brought about by fear into “living creatively with dying” (Colyer, 1996: 498).
One area of research explores the extent to which patients attempt to gain personal control over the way that others perceive them through self-presentation. Silver et al. (1990) placed participants into conditions where they listened to scripted depictions of the same cancer patient. If the patient portrayed herself as a “balanced coper” or a “good coper,” participants responded more favorably to her as compared to the “poor coper.” Thus, interventions might teach patients to present themselves so as to minimize avoidance (Silver et al., 1990). Support groups are another way that patients attempt to increase personal control by supporting, and being supported by, others going through similar experiences. It is important to note that support groups are useful for many, but not all, cancer patients (Smith et al., 2013). Patients report entering support groups because they feel stigmatized and helpless, and believe that these groups provide a safe place with others who are going through similar challenges (Cella and Yellen, 1993). However, feelings of fear and uncertainty may arise when patients are exposed to others who are coping poorly (Helgeson and Cohen, 1996).
Domain identification
Individuals who identify strongly with domains in which their group is negatively stereotyped are more likely to see feedback in those domains as self-relevant and potentially threatening (Major and O’Brien, 2005). For example, studies on stereotype threat use situations where academic achievement is salient for African Americans (Steele, 1997). However, cancer, as of yet, has not been associated with specific situations where patients are stigmatized as a group. This is an area that could benefit from further research. Some situations that might be challenging for cancer patients include dating and planning for the future. For example, on a first date, cancer survivors might feel anxious about whether to disclose their health status. Similar to the effects of stereotype threat, such feelings may sabotage the development of a relationship.
Goals and motives
Goals and motives influence how individuals perceive and appraise situations (Major and O’Brien, 2005). People are more likely to perceive an evaluator as prejudiced against their group if they receive negative feedback from them (Major and O’Brien, 2005). This relates to two common reactions to people with cancer, ambiguity, and avoidance. People are also motivated to believe that the world is just and that people are fairly treated (Major and O’Brien, 2005). This relates to how likely those interacting with cancer patients are to stigmatize and also how likely cancer patients are to blame themselves for being stigmatized.
Ambiguity and avoidance
A cancer diagnosis creates ambiguity and uncertainty about how to behave around a person who has a new and unfamiliar identity (Goffman, 1963). For instance, friends and neighbors of children with chemotherapy-related hair loss feel embarrassed and unsure about how to be supportive (Reid, 1997). This discomfort can lead to avoidance or behaviors perceived to be unsupportive. In a study in women with early-stage breast cancer, overtly critical or avoidant responses by significant others were predictive of patients’ poor coping and distress (Manne et al., 2005). In contrast, in a study of patients with rectal cancer, half of the sample reported some degree of stigmatization, but only 4 percent reported that they felt others were avoiding them (MacDonald and Anderson, 1984). This inconsistency could be explained by the fact that the decision to avoid individuals with cancer varies according to how vulnerable an outsider may feel when faced with their own fear of cancer, and need to control to what happens in their own lives (Else-Quest and Jackson, 2014).
Beliefs in a just world and self-blame
People differ in their judgments of how fair the world is and the extent to which people get what they deserve (Rubin and Peplau, 1973). Just world beliefs held by others can be maladaptive to cancer patients. According to terror management theory, people are motivated to avoid facing their own vulnerability and mortality as a means to reduce anxiety and enhance self-esteem (Rosenblatt et al., 1989). Furthermore, people are reluctant to believe that they live in a tragic world and thus resort to blaming victims (Janoff-Bulman, 1992). For those motivated to believe in a just world, “if the victim can be blamed for what happened, then the world is not a random, malevolent, meaningless place” (Janoff-Bulman, 1992: 149). Unfortunately, this attempt at self-protection can result in a derogation of cancer patients.
Just world beliefs can also affect the way that individuals evaluate their own experience with cancer. Individuals who find themselves suddenly suffering from a disease or tragedy that cannot be explained through their own behavior, and thus cannot be explained by blaming oneself for the diagnosis, may feel helpless and/or powerless (Bloom and Spiegel, 1984). In a study on self-blame and its relationship to distress in breast cancer patients, both behavioral and characterological self-blame were related to distress for up to a year following diagnosis, suggesting that blaming oneself for a cancer diagnosis is maladaptive (Bennett et al., 2005). In a study assessing self-blame in a sample of men with colorectal cancer, 25 percent believed that it was at least a little bit true that they were responsible for their cancer. Furthermore, self-blame was significantly associated with depressive symptoms (Phelan et al., 2013). However, self-blame may at least help a patient to find meaning in their cancer. Buick (1997) explains that breast cancer patients consistently identify seven causal theories which include themselves, stress and extreme worrying, marital discord, lack of expressing emotions, change, and hazards in the environment. Such explanations are not necessarily maladaptive, but may actually provide a sense of control over cancer (Buick, 1997).
The personal attributions component may benefit the most from empirical studies using an identity-threat model of stigma. There are several clinical implications for applied use of these concepts, including various types of psychosocial interventions that aim to reduce depression, distress, and other potential negative psychological consequences of having cancer (Clark, 2010; Guo et al., 2013; Newell et al., 2002; Tatrow and Montgomery, 2006). Incorporating elements related to reducing fear of cancer, cancer fatalism, and loss of control related to cancer’s stigma in these interventions may provide further benefits.
Conclusion
Cancer is a label given to several different diseases with the potential to carry a stigma. A modified version of contributors to the stigma-induced identity-threat model created by Major and O’Brien (2005) provides a framework for understanding and organizing the ways in which the stigma associated with cancer differs from other social stigmas (see Figure 1). When describing the stigma attached to cancer, we suggest that considering disease/treatment characteristics, such as perceived controllability and visibility, is more applicable than the general collective representations component of Major and O’Brien’s model. Furthermore, the components situational threat and personal attributions allow individuals with 1 of 100 different types of cancer to vary in the way they appraise potentially threatening cues in various situations (e.g. in the workplace, social interactions/contexts, and the media) and the way that cancer causes, characteristics, and outcomes are attributed (i.e. through stigma sensitivity, goals and motives, group identification, or domain identification). Although the stigma attached to cancer is more subtle than it was in the past, it can still result in identity threat and holds the power to affect the well-being of cancer patients. Attention to stigmatizing aspects of cancer and its treatment could be fruitfully focused upon in future research and in the design of psychosocial interventions. Future research should prioritize the investigation of empirical studies to test the practical use of this theoretical model of cancer stigma.
Articles were located using primarily PubMed and EBSCOhost online research databases. Keywords included cancer AND stigma, self-blame, identity, attributions, media, visibility, hair loss, controllability, workplace disclosure, discrimination, fear, psychosocial interventions, and functioning.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- American Cancer Society (ACS) (2013) Look good feel better. Available at: http://www.cancer.org/treatment/supportprogramsservices/look-good-feel-better (accessed 13 February 2014).
- Americans with Disabilities Act, Amendment Acts (2008) ADA Amendments Act of 2008. Available at: http://www.eeoc.gov/laws/statutes/adaaa.cfm (accessed 13 February 2014).
- Applebaum AJ, Farran CJ, Marziliano AM, et al. (2014) Preliminary study of themes of meaning and psychosocial service use among informal cancer caregivers. Palliative & Supportive Care 12: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson TM, Noce NS, Hay J, et al. (2013) Illness-related distress in women with clinically localized cutaneous melanoma. Annals of Surgical Oncology 20: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back A. (2006) Patient-physician communication in oncology: What does the evidence show? Oncology 20: 67–74. [PubMed] [Google Scholar]
- Befort CA, Nazir N, Engelman K, et al. (2013) Fatalistic cancer beliefs and information sources among rural and urban adults in the USA. Journal of Cancer Education 28: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KK, Compas BE, Beckjord E, et al. (2005) Self-blame and distress among women with newly diagnosed breast cancer. Journal of Behavioral Medicine 28: 313–323. [DOI] [PubMed] [Google Scholar]
- Bernabei R, Gambassi G, Lapane K, et al. (1998) Management of pain in elderly patients with cancer. Journal of the American Medical Association 279: 1877–1882. [DOI] [PubMed] [Google Scholar]
- Björklund M, Sarvimäki A, Berg A. (2008) Health promotion and empowerment from the perspective of individuals living with head and neck cancer. European Journal of Oncology Nursing 12: 26–34. [DOI] [PubMed] [Google Scholar]
- Bloom JR, Kessler L. (1994) Emotional support following cancer: A test of the stigma and social activity hypotheses. Journal of Health and Social Behavior 35: 118–133. [PubMed] [Google Scholar]
- Bloom JR, Spiegel D. (1984) The relationship of two dimensions of social support to the psychological well-being and social functioning of women with advanced breast cancer. Social Science & Medicine 19: 831–837. [DOI] [PubMed] [Google Scholar]
- Boehmke MM, Dickerson SS. (2005) Symptom, symptom experiences, and symptom distress encountered by women with breast cancer undergoing current treatment modalities. Cancer Nursing 28: 382–389. [DOI] [PubMed] [Google Scholar]
- Buick DL. (1997) Illness representations and breast cancer: Coping with radiation and chemotherapy. In: Petrie KJ, Weinman JA. (eds) Perceptions of Health and Illness: Current Research and Applications. Amsterdam: Harwood Academic Publishers, pp. 379–409. [Google Scholar]
- Carmack CL, Basen-Enqquist K, Yuan Y, et al. (2011) Feasibility of an expressive-disclosure group intervention for post-treatment colorectal cancer patients. Cancer 117: 4993–5002. [DOI] [PubMed] [Google Scholar]
- Cella DF, Yellen SB. (1993) Cancer support groups: The state of the art. Cancer Practice 1: 56–61. [PubMed] [Google Scholar]
- Chapple A, Ziebland S, McPherson A. (2004) Stigma, shame, and blame experienced by patients with lung cancer: Qualitative study (Electronic version). BMJ 328: 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis NA, Allison PD. (2006) Mortality after the hospitalization of a spouse. New England Journal of Medicine 354: 719–730. [DOI] [PubMed] [Google Scholar]
- Clark PG. (2010) Decreasing psychological distress in cancer inpatients using FLEX Care®: A pilot study. Social Work in Health Care 49: 872–890. [DOI] [PubMed] [Google Scholar]
- Colyer H. (1996) Women’s experience of living with cancer. Journal of Advanced Nursing 23: 496–501. [DOI] [PubMed] [Google Scholar]
- Compassion in the workplace? Discrimination against an employee with cancer (2006) International Journal of Palliative Nursing 12: 554–556. [DOI] [PubMed] [Google Scholar]
- Crocker J, Major B, Steele C. (1998) Social stigma. In: Gilbert DT, Fiske ST, Lindzey G. (eds) Handbook of Social Psychology, vol. 2 (4th edn). Boston, MA: McGraw-Hill, pp. 504–553. [Google Scholar]
- Daher M. (2012) Cultural beliefs and values in cancer patients. Annals of Oncology 23: 66–69. [DOI] [PubMed] [Google Scholar]
- Egestad H. (2013) The significance of fellow patients for head and neck cancer patients in the radiation treatment period. European Journal of Oncology Nursing 17: 618–624. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Jackson TL. (2014) Cancer stigma. In: Corrigan P. (ed.) The Stigma of Disease and Disability. Washington, DC: American Psychological Association, pp. 165–182. [Google Scholar]
- Feuerstein M, Luff GM, Harrington CB, et al. (2007) Pattern of workplace disputes in cancer survivors: A population study of ADA claims. Journal of Cancer Survivorship 1: 185–192. [DOI] [PubMed] [Google Scholar]
- Fife BL, Wright ER. (2000) The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior 41: 50–67. [PubMed] [Google Scholar]
- France L, Michie S, Barrett-Lee P, et al. (2000) Male cancer: A qualitative study of male breast cancer. Breast 9: 343–348. [DOI] [PubMed] [Google Scholar]
- Goffman E. (1963) Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Grassi L, Giraldi T, Messina EG, et al. (2000) Physicians’ attitudes to and problems with truth-telling to cancer patients. Supportive Care Cancer 8: 40–45. [DOI] [PubMed] [Google Scholar]
- Guo Z, Tang H, Li H, et al. (2013) The benefits of psychosocial interventions for cancer patients undergoing radiotherapy. Health and Quality of Life Outcomes 11: 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N, Salmon CT. (2004) Guilt, fear, stigma and knowledge gaps: Ethical issues in public health communication interventions. Bioethics 18: 531–552. [DOI] [PubMed] [Google Scholar]
- Hall AG, Khoury AJ, Lopez ED, et al. (2008) Breast cancer fatalism: The role of women’s perceptions of the health care system. Journal of Health Care for the Poor and Underserved 19: 1321–1335. [DOI] [PubMed] [Google Scholar]
- Hansen JA, Feuerstein M, Calvio LC, et al. (2008) Breast cancer survivors at work. Journal of Occupational and Environmental Medicine 50: 777–784. [DOI] [PubMed] [Google Scholar]
- Hayes RA, Vaughan C, Medeiros T, et al. (2002) Stigma directed toward chronic illness is resistant to change through education and exposure. Psychological Reports 90: 1161–1173. [DOI] [PubMed] [Google Scholar]
- Hazelwood DM, Koeck S, Wallner M, et al. (2012) Patients with cancer and family caregivers: Management of symptoms caused by cancer or cancer therapy at home. Heilberufe Science 3: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Cohen S. (1996) Social support and adjustment to cancer: Reconciling descriptive, correlational, and intervention research. Health Psychology 15: 135–148. [DOI] [PubMed] [Google Scholar]
- Hesse BW, Nelson DE, Kreps GL, et al. (2005) Trust and sources of health information: The impact of the Internet and its implications for health care providers: Findings from the first Health Information National Trends Survey. Archives of Internal Medicine 165: 2618–2624. [DOI] [PubMed] [Google Scholar]
- Hoffman B. (2005) Cancer survivors at work: A generation of progress. CA: A Cancer Journal for Clinicians 55: 271–280. [DOI] [PubMed] [Google Scholar]
- Holland JC. (2001) Improving the human side of cancer care: Psycho-oncology’s contribution. Cancer Journal 7: 458–471. [PubMed] [Google Scholar]
- Idemudia ES, Matamela NA. (2012) The roles of stigmas in mental health: A comparative study. Curationis 35: E1–E8. [DOI] [PubMed] [Google Scholar]
- Janoff-Bulman R. (1992) Shattered Assumptions: Towards a New Psychology of Trauma. New York: Free Press. [Google Scholar]
- Jones E, Farina A, Hastorf A, et al. (1984) Social Stigma: The Psychology of Marked Relationships. New York: W. H. Freeman and Company. [Google Scholar]
- Jun J, Oh KM. (2013) Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. American Journal of Health Behavior 37: 145–154. [DOI] [PubMed] [Google Scholar]
- Knapp-Oliver S, Moyer A. (2009) Visibility and the stigmatization of cancer: Context matters. Journal of Applied Social Psychology 39: 2798–2808. [Google Scholar]
- Knapp-Oliver S, Moyer A. (2012) Causal attributions predict willingness to support the allocation of funding to lung cancer treatment programs. Journal of Applied Social Psychology 42: 2368–2385. [Google Scholar]
- Knapp-Oliver S, Marin S, Cokkinias C, et al. (2010) Metaphors influence perceptions of individuals with cancer. Poster presented at the Association for Psychological Science Annual Meeting, Boston, MA. [Google Scholar]
- Koocher GP, O’Malley JE. (1981) The Damocles Syndrome: Psychosocial Consequences of Surviving Childhood Cancer. New York: McGraw-Hill. [Google Scholar]
- Kromm EE, Smith KC, Singer RF. (2007) Survivors on Cancer: The portrayal of survivors in print news. Journal of Cancer Survivorship 1: 298–305. [DOI] [PubMed] [Google Scholar]
- Lavigne JE, Griggs JJ, Tu XM, et al. (2008) Hot flashes, fatigue, treatment exposures, and work productivity in breast cancer survivors. Journal of Cancer Survivorship 2: 296–302. [DOI] [PubMed] [Google Scholar]
- Littlechild SA, Barr L. (2013) Using the Internet for information about breast cancer: A questionnaire-based study. Patient Education and Counseling 92: 413–417. [DOI] [PubMed] [Google Scholar]
- Lobchuk MN, McClement SE, McPherson C, et al. (2008) Does blaming the patient with lung cancer affect the helping behavior of primary caregivers. Oncology Nursing Forum 35: 681–689. [DOI] [PubMed] [Google Scholar]
- MacDonald LD, Anderson HR. (1984) Stigma in patients with rectal cancer: A community study. Journal of Epidemiology and Community Health 38: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey EL, Baum LD, Pinkerton RC, et al. (2001) Psychological sequelae and alopecia among women with cancer. Cancer Practice 9: 283–289. [DOI] [PubMed] [Google Scholar]
- Major B, O’Brien LT. (2005) The social psychology of stigma. Annual Review of Psychology 56: 393–421. [DOI] [PubMed] [Google Scholar]
- Manne SL, Ostroff J, Winkel G, et al. (2005) Partner unsupportive responses, avoidant coping, and distress among women with early stage breast cancer: Patient and partner perspectives. Health Psychology 24: 635–641. [DOI] [PubMed] [Google Scholar]
- Marlow LAV, Waller J, Wardle J. (2010) Variation in blame attributions across different cancer types. Cancer Epidemiology, Biomarkers & Prevention 19: 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PD, Fogel J, Tyler ID, et al. (2010) Culturally targeted educational intervention to increase colorectal health awareness among African Americans. Journal of Health Care for the Poor and Underserved 21: 132–147. [DOI] [PubMed] [Google Scholar]
- Muzzin LJ, Anderson NJ, Figueredo AT, et al. (1994) The experience of cancer. Social Science & Medicine 38: 1201–1208. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) (2001) Report of the lung cancer progress review group. Available at: http://planning.cancer.gov/library/2001lung.pdf.
- National Cancer Institute (NCI) (2013) Research to characterize and reduce stigma. Available at: http://grants.nih.gov/grants/guide/pa-files/PA-13-246.html
- Newell SA, Sanson-Fisher RW, Savolainen NJ. (2002) Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. Journal of the National Cancer Institute 94: 558–584. [DOI] [PubMed] [Google Scholar]
- Oken D. (1961) What to tell cancer patients. A study of medical attitudes. Journal of the American Medical Association 175: 1120–1128. [DOI] [PubMed] [Google Scholar]
- Peters-Golden H. (1982) Breast cancer: Varied perceptions of social support in the illness experience. Social Science & Medicine 16: 483–491. [DOI] [PubMed] [Google Scholar]
- Phelan SM, Griffin JM, Jackson GL, et al. (2013) Stigma, perceived blame, self-blame and depressive symptoms in men with colorectal cancer. Psycho-oncology 22: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel EC. (1999) Stigma consciousness: The psychological legacy of social stereotypes. Journal of Personality and Social Psychology 76: 114–128. [DOI] [PubMed] [Google Scholar]
- Pinel EC. (2002) Stigma consciousness in intergroup contexts: The power of conviction. Journal of Experimental Social Psychology 38: 178–185. [Google Scholar]
- Powe BD, Cooper DL, Harmond L, et al. (2009) Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nursing 32: 412–417. [DOI] [PubMed] [Google Scholar]
- Ramanadhan S, Viswanath K. (2006) Health and the information nonseeker: A profile. Health Communication 20: 131–139. [DOI] [PubMed] [Google Scholar]
- Reid U. (1997) Stigma of hair loss after chemotherapy. Paediatric Nursing 9: 16–18. [DOI] [PubMed] [Google Scholar]
- Rodriguez Del Pozo P, Fins JJ, Helmy I, et al. (2012) Truth-telling and cancer diagnoses: Physician attitudes and practices in Qatar. The Oncologist 17: 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt A, Greenberg J, Solomon S, et al. (1989) Evidence for terror management theory: I. the effects of mortality salience on reactions to those who violate or uphold cultural values. Journal of Personality and Social Psychology 57: 681–690. [DOI] [PubMed] [Google Scholar]
- Rosman S. (2004) Cancer and stigma: Experience of patients with chemotherapy-induced alopecia. Patient Education and Counseling 52: 333–339. [DOI] [PubMed] [Google Scholar]
- Rubin Z, Peplau A. (1973) Belief in a just world and reactions to another’s lot: A study of participants in the national draft lottery. Journal of Social Issues 29: 73–93. [Google Scholar]
- Schneiderman N, Kim Y, Shaffer KM. (2012) Spouses of patients with cancer have an increased risk of cardiovascular disease: What do we know about this link? Circulation 125: 1721–1722. [DOI] [PubMed] [Google Scholar]
- Schofield P, Juraskova I, Bergin R, et al. (2013) A nurse- and peer-led support program to assist women in gynaecological oncology receiving curative radiotherapy, the PeNTAGOn study (peer and nurse support trial to assist women in gynaecological oncology): Study protocol for a randomized controlled trial. Trials 14: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepucha KR, Belkora JK, Mutchnick S, et al. (2002) Consultation planning to help breast cancer patients prepare for medical consultations: Effect on communication and satisfaction for patients and physicians. Journal of Clinical Oncology 20: 2695–2700. [DOI] [PubMed] [Google Scholar]
- Silk KJ, Perrault EK, Nazione S. (2013) Localized prostate cancer treatment decision-making information online: Improving its effectiveness and dissemination for nonprofit and government-supported organizations. Journal of Cancer Education 28: 709–716. [DOI] [PubMed] [Google Scholar]
- Silver RC, Wortman CB, Crofton C. (1990) The role of coping in support provision: The self-presentational dilemma of victims of life crises. In: Sarason BR, Sarason IG, Pierce GR. (eds) Social Support: An Interactional View. Oxford: John Wiley & Sons, pp. 397–426. [Google Scholar]
- Simard S, Thewes B, Humprhis G, et al. (2013) Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. Journal of Cancer Survivorship 7: 300–322. [DOI] [PubMed] [Google Scholar]
- Skalla KA, Smith EM, Li Z, et al. (2013) Multidimensional needs of caregivers for patients with cancer. Clinical Journal of Oncology Nursing 17: 500–506. [DOI] [PubMed] [Google Scholar]
- Smith AW, Parsons HM, Kent EE, et al. (2013) Unmet support service needs and health related quality of life among adolescents and young adults with cancer: The AYA Hope study. Frontiers in Oncology 3: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM. (1997) A threat in the air: How stereotypes shape intellectual identity and performance. American Psychologist 52: 613–629. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Narimatsu H, Hozawa A, et al. (2012) Cancer patients on Twitter: A novel patient community on social media. Biomedical Central Research Notes 5: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatrow K, Montgomery GH. (2006) Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: A meta-analysis. Journal of Behavioral Medicine 29: 17–27. [DOI] [PubMed] [Google Scholar]
- Van Liew JR, Christensen AJ, Howren MB, et al. (2013) Fear of recurrence impacts health-related quality of life and continued tobacco use in head and neck cancer survivors. Health Psychology 33: 373–381. [DOI] [PubMed] [Google Scholar]
- Van Ryn M, Sanders S, Kahn K, et al. (2011) Objective burden, resources, and other stressors among informal cancer caregivers: A hidden quality issue? Psycho-oncology 20: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop DP, O’Connor TL, Trabold N. (2011) Waiting for the other shoe to drop: Distress and coping during and after treatment for breast cancer. Journal of Psychosocial Oncology 29: 450–473. [PubMed] [Google Scholar]
- Weiner B, Perry RP, Magnusson J. (1988) An attributional analysis of reactions to stigmas. Journal of Personality and Social Psychology 55: 738–748. [DOI] [PubMed] [Google Scholar]
- Wuensch A, Tang L, Goelz T, et al. (2013) Breaking bad news in China—The dilemma of patients’ autonomy and traditional norms: A first communication skills training for Chinese oncologists and caretakers. Psycho-oncology 22: 1192–1195. [DOI] [PubMed] [Google Scholar]


