Abstract
BACKGROUND
Surgical excision is the treatment of choice for basal cell carcinoma and micrographic surgery considered the gold standard, however not yet used routinely worldwide available, as in Brazil. Considering this, a previously developed treatment guideline, which the majority of tumors were treated by conventional technique (not micrographic) was tested.
OBJECTIVE
To establish the recurrence rate of basal cell carcinomas treated according to this guideline.
METHOD
Between May 2001 and July 2012, 919 basal cell carcinoma lesions in 410 patients were treated according to the proposed guideline. Patients were followed-up and reviewed between September 2013 and February 2014 for clinical, dermatoscopic and histopathologic detection of possible recurrences.
RESULTS
After application of exclusion criteria, 520 lesions were studied, with 88.3% primary and 11.7% recurrent tumors. Histological pattern was indolent in 85.5%, 48.6% were located in high risk areas and 70% small tumors. Only 7.3% were treated by Mohs micrographic surgery. The recurrence rate, in an average follow-up period of 4.37 years, was 1.3% for primary and 1.63% for recurrent tumors. Study limitations: unicenter study, with all patients operated on by the same surgeon.
CONCLUSION
The treatment guideline utilized seems a helpful guide for surgical treatment of basal cell carcinoma, especially if micrographic surgery is not available.
Keywords: Mohs surgery; Carcinoma, basal cell; Treatment outcome; Recurrence
INTRODUCTION
Incidence of basal cell carcinoma (BCC) is increasing. In the USA, more than 3.5 million new cases of nonmelanoma skin cancer were estimated for 2014, and it was also noted that the number of women under 40 diagnosed with BCC more than doubled in the last 30 years.1
Although less than 50% of the Brazilian population is Caucasian, nonmelanoma skin cancer is also prevalent in Brazil, representing 25% of all malignant tumors.2 For 2014 it was estimated 182,130 new cases of nonmelanoma skin cancer, and BCC corresponded to 70% of these diagnoses. 3
Surgical resection is the treatment of choice for BCC.4,5 Currently, in the USA, Mohs micrographic surgery (MMS) is indicated for all recurrent BCCs, except for superficial ones in low risk areas. For primary tumors, it is indicated for all aggressive tumors (except smaller than 0.5cm in low risk areas); for all nodular tumors in high and moderate risk areas and for those larger than 2cm in low risk areas; and for all superficial tumors in high risk areas and for those largest than 0.6cm in moderate risk areas.6
An increase of 400% in the use of MMS in the US from 1995 to 2009 was reported, and one in four skin cancers are treated this way.6 In Brazil, MMS was introduced in the 1980's and, although widely accepted, its application is still limited, mainly due to the small number of specialized services.7,8
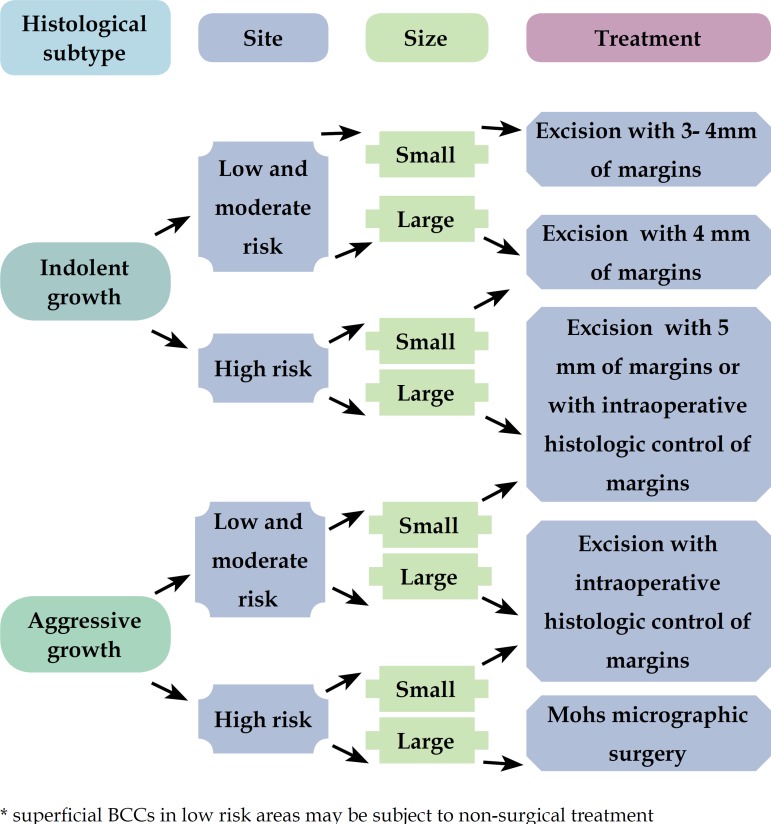
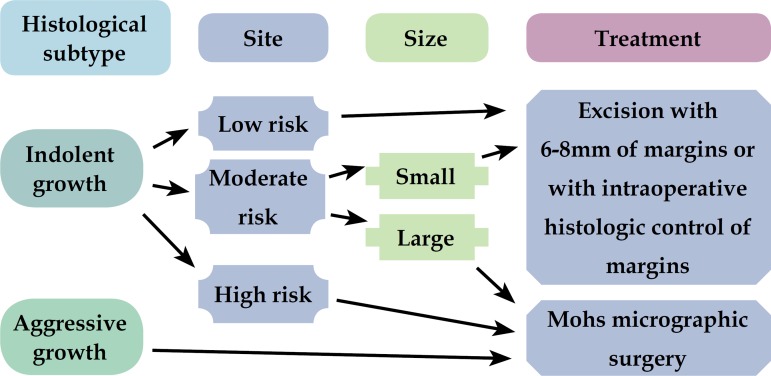
Because MMS is not yet widely and routinely used in many countries, the authors formulated in 2001 an algorithm to guide the surgical treatment of BCC, especially for places where there is still no broad access to micrographic techniques (Figures 1 and 2). 9
Figure 1.
Algorithm for surgical treatment of primary BCC
Figure 2.
Algorithm for surgical treatment of recurrent BCC
The objective of this study was to evaluate the cure rate of BCCs patients treated surgically according to this algorithm.9 Although non-surgical techniques are accepted for some cases of BCCs, they were not covered in this study.
METHODS
Patients diagnosed with BCC treated in a private treatment center skin cancer were analyzed. The study involved patients treated between May 2001 and July 2012.
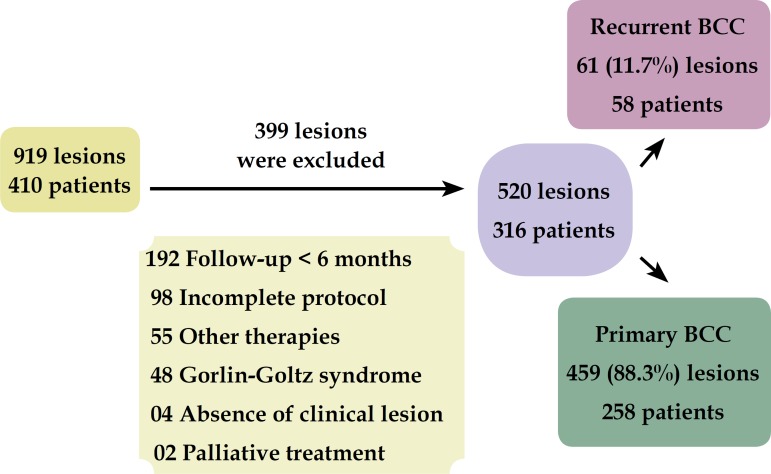
The study included: 1) lesions with histologic diagnosis of BCC treated surgically according to the proposed algorithm; and 2) patients who agreed to the proposed treatment. Exclusion criteria were: 1) patients with follow-up less than 6 months after treatment; 2) patients undergoing treatments other than surgery; 3) cases with no clinically visible lesion (previously treated by other doctors and referred to center after incomplete resection); 4) patients with Goltz-Gorlin syndrome; and 5) locally invasive lesions undergone palliative treatment (Figure 3).
Figure 3.
Exclusion criteria
The project was approved by the Ethics Review Board of the Universidade Federal Fluminense (document number: 155.435).
After initial classification of tumors in primary or recurrent, the pattern of histological growth was verified, according to previous biopsy. Presence of perineural invasion, metatypical sclerodermiform, infiltrative and micronodular subtypes were classified as aggressive growth; and nodular and superficial subtypes, as indolent growth, according to the Crowson classification.10
Thereafter, the location of the tumor was classified as high, moderate or low risk, according to the Huang and Boyce classification.11
The tumor size classification takes into account its location. Thus, we considered large lesions those greater than 1cm, located in high risk areas, those greater than 2cm in moderate risk areas and those greater than 4cm located in low risk areas.
After this stratification, the tumors were treated according to the algorithm (Figures 1 and 2). Some primary BCCs, superficial, located in low risk areas, were treated by non-surgical methods.12
All patients were treated by the same surgeon (FBL).
The demarcation of tumor margins was made after clinical skin degreasing with 70% alcohol, using surgical focus and maintaining the skin slightly stretched. From 2006, the polarized light dermoscopy (Dermalite II Pro®) started to be used to assist such delimitation.
In case of involvement of any of the margins in the histological analysis, new resections were made to confirm the total removal of the tumor.
From September 2013 to February 2014, patients were invited to attend the review consultation (with neutral observer), during which clinical signs and dermatoscopic relapse were sought. For patients who were unable to attend this consultation, telephone contact was made and data of the last visit made by the same surgeon were considered.
RESULTS
We evaluated 919 lesions in 410 patients. Of these, 301 met the exclusion criteria and 98 had incomplete registry data, preventing its inclusion. Thus, 521 lesions in 316 patients were effectively studied, 459 of these were primary tumors and 61 were recurrent tumors.
The general characteristics of the sample are shown in table 1.
Table 1.
Characteristics of the sample
| Total | Primary BCC | Recurrent BCC | |
|---|---|---|---|
| Nr. of lesions | 520 | 459 (88.3%) | 61 (11.7%) |
| Nr. of patients | 316 | 258 (81.6%) | 58 (18.4%) |
| Sex | |||
| Women | 171 (54.2%) | 139 (53.8%) | |
| Men | 145 (45.8%) | 119 (46.1%) | |
| Mean age | 68.83 years | 68.82 years | 67.77 years |
| (de 30 a 98) | |||
| Women | 70.16 years | 67.55 years | |
| Men | 67.36 years | 68.25 years | |
| Mean follow-up | 4.37years(±2.52) | 4.42 years | 3.98 years |
| 6 m/12y5m | 6 m/12y5m | 6 m/10y6m | |
| Minimum/Maximum | |||
| < 5 years | 331 (63.6%) | 288 (62.7%) | 44 (72.1%) |
| ≥ 5 years | 189 (36.4%) | 171 (373%) | 17 (27.9%) |
Histologic subtype of tumors
The nodular subtype was the most frequent, both in the group of primary and recurrent tumors (64.8% and 40.9%, respectively) (Table 2). When tumors were classified according to histologic growth pattern, the majority of primary and recurrent tumors were indolent (87.9% and 59.1%, respectively).
Table 2.
Histological subtype
| Histological subtype |
Primary BCC* N: 281 |
Recurrent BCC** N: 44 |
Histological subtype |
Primary BCC* N: 281 |
|---|---|---|---|---|
| Sclerodermiform | 10 (3.5%) | 4 (9.1%) | Indolent growth |
252 (89.7%) |
| Infiltrative | 3 (1.1%) | 6 ( 13.6%) | Aggressive growth |
29 (10.3%) |
| Metatypical | 1 (0.3%) | 2 (4.5%) | ||
| Mixed | 15 (5.3%) | 5 (11.4%) | ||
| Nodular | 182 (64.8%) | 18 (40.9%) | ||
| Micronodular | - | 1 (2.3%) | ||
| Superficial | 63 (22.5%) | 8 (18.2%) | ||
| Pigmented | 5 (1.8%) | - | ||
| Ulcerated | 2 (0.7%) | - |
Not reported: 178
Not reported: 17
Tumor site
The vast majority of primary (63.6%) and recurrent (85.2%) tumors were located in the head and neck. In the group of primary tumors, trunk was the most frequent isolated place (26.44%). In the group of recurrent, nose was the most commonly affected (36%), followed by the forehead (16.4%) (Table 3).
Table 3.
Tumor site
| Site | Primary BCC | Recurrent BCC | Site | Primary BCC | Recurrent BCC |
|---|---|---|---|---|---|
| Nose | 100 (21.8%) | 22 (36%) | High risk | 218 (47.5%) | 35 (57.4%) |
| Perioral | 40 (8.7%) | 2 (3.3%) | |||
| Temporal | 27 (5.9%) | 1 (1.7%) | |||
| Periocular | 26 (5.6%) | 2 (3.3%) | |||
| Ears and periauricular | 22 (4.8%) | 8 (13.1%) | |||
| Jaw 5 (1.1%) | 1 (1.6%) | ||||
| Forehead | 32 (7%) | 10 (16.4%) | Moderate risk | 77 (16.8%) | 19 (29.5%) |
| Scalp | 5 (1.1%) | 2 (3.3%) | |||
| Cheek | 24 (5.2%) | 4 (6.5%) | |||
| Neck | 11 (2.4%) | 0 | |||
| Upper limbs | 14 (3%) | 2 (3.3%) | Low risk | 164 (35.7%) | 8 (13.1%) |
| Lower limbs | 32 (7%) | 2 (3.3%) | |||
| Trunk | 121 (26.4%) | 5 (8.2%) |
In relation to risk areas, 47.5% of primary tumors and 57.4% of recurrent were located in high risk areas.
Tumor size
Considering the tumor size, 88.6% of primary and 73.2% of recurrent tumors had maximum diameter of 2cm (Table 4).
Table 4.
Tumor size
| Size | Primary BCC*
N: 280 |
Recurrent BCC**
N: 41 |
|---|---|---|
| ≤ 2cm | 248 (88.6%) | 30 (73.2%) |
| > 2cm | 32 (11.4%) | 11 (26.8%) |
| Small | 213 (76%) | 12 (29.3%) |
| Large | 67 (24%) | 29 (70.7%) |
Not reported: 179
Not reported: 20
Most primary (76%) and almost a third (29.3%) of recurrent tumors were small according to their location.
Treatment
The vast majority of primary and recurrent tumors were treated by conventional surgery (CS) (94.5% and 75.4%, respectively), with 62% of primary and 74% of recurrent tumors subjected to intraoperative histological analysis of their margins (Table 5).
Table 5.
Treatment
| Treatment | Primary BCC* | Recurrent BCC** |
|---|---|---|
| Conventional surgery | 94.5% (434)* | 75.4% (46)** |
| Postoperative
histological analysis of margins (paraffin) |
25.8% (112) | 13% (6) |
| Postoperative
histological analysis of margins (frozen section) |
62% (269) | 74% (34) |
| Mohs micrographic | 5.5% (25) | 24.6% (15) |
Not reported: 53
Not reported: 6
The rate of incomplete excision, among tumors treated by conventional surgery with intraoperative histological analysis of margins, was 4.1% (18 lesions) for primary and 4.3% (two lesions) for recurrent tumors. Among primary tumors, the lateral margin was involved in 13 cases, the deep margin in two, and both margins, lateral and deep, in three cases. Among the recurrent tumors, one case presented lateral margin and the other presented deep margin affected. All were called for subsequent surgery and all underwent additional resection until confirmation of complete tumor excision.
Among the 466 tumors with available information, the surgical repair occurred in 56.9% (265) for simple flap; 19.5% (91) by direct closure; 14% (65) by complex flap; 8.5% (40) graft; and 1% (5) by second intention.
Follow-up and recurrence of the algorithm
The mean follow-up was 4.37 years (minimum 6 months and maximum 12 years and 5 months), with a mean of 4.42 years among primary tumors and 3.98 years among recurrent tumors.
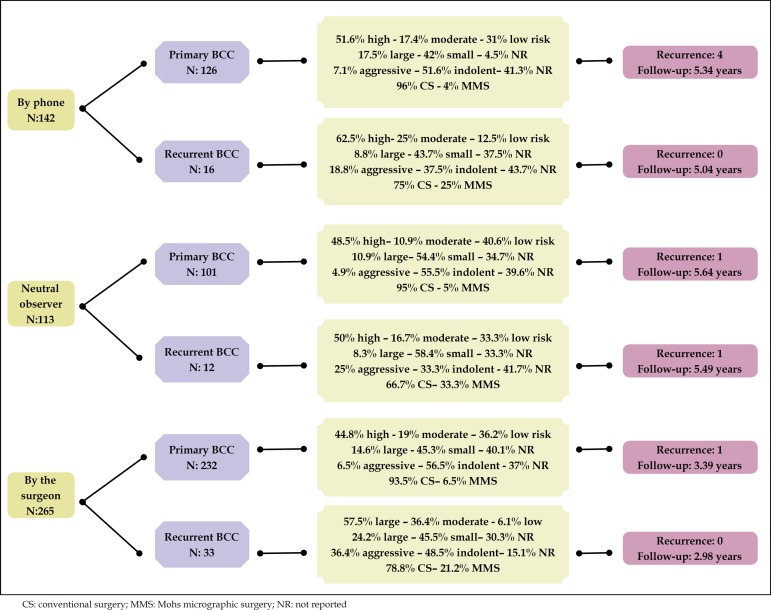
The review consultation was performed with 21.7% (113) patients by a neutral observer; 51% (265) by the same surgeon; and via telephone contact by neutral observer in 27.3% (142) of the cases. In the latter group, 28.2% (40) of the cases mentioned follow-up by another doctor (Figure 4).
Figure 4.
Characteristics of the sample according to the reviewer
The overall relapse rate was 1.34%, 1.3% (6) between primary and 1.63% (1) between recurrent tumors.
In two of the cases of recurrence, the lesions were located in the nose, and in two others, the lesions were clinically nodular (Table 6).
Table 6.
Characteristics of the cases of recurrence treated according to the algorithm
| Patient | Tumor status | Histological / clinical types | Site | Size | Treatment |
|---|---|---|---|---|---|
| 1 | Primary | Nodular/Nodular | Scalp | Small | CS with frozen section– 4mm margins |
| 2 | Primary | Sclerodermiform / Nodular | Nose | Large | CS with frozen section – 5mm margins |
| 3 | Recurrent | NR/NR | Nose | NR | MMS |
| 4 | Primary | NR/Nodular | Nose | Large | CS with frozen section – 4mm margins |
| 5 | Primary | NR/Nodular | Nose | NR | MMS |
| 6 | Primary | NR/ Sclerodermiform | Nose | Small | CS with frozen section – 3mm margins |
| 7 | Primary | Nodular/Nodular | Temporal | Small | CS with frozen section – 3mm margins |
CS: conventional surgery; NR: not reported; MMS: Mohs micrographic surgery
Most recurrences occurred in the nose and in tumors clinically classified as nodular.
With the exception of lesion 3, which has not yet been re-operated, other cases were reoperated according to the recurrent tumors algorithm and, so far, they didn't present a new relapse (Table 6 and Figure 2).
DISCUSSION
The discreet prevalence in women in this study was similar to previous reports, although high prevalence in men has also has been reported.13-16 Mean age was 65 years (minimum of 30 and maximum of 98 years) and was similar to previous reports.13,15,16
Similar to other reports, the nodular histologic subtype was the most frequent. There are reports showing the nose as the most affected site, but in this study, the trunk was the most common site among primary tumors (26.4%), followed by the nose (21.8%), which was the most frequent among the recurrent tumors (36%).14,17,18,19 Similar to other studies, approximately half of the primary and recurrent tumors were small (54% and 49.2%, respectively).13,18
Analyzing the cases according to the criteria adopted and tested in the algorithm (histologic growth pattern, risk areas and size according to location), 10.3% of primary tumors and 40.9% of recurrent tumors were aggressive; 47.5% of primary and 57.4% of recurrent tumors were located in high risk areas; and 24% of primary and 70.7% of the recurrent tumors were large.
Only 5.5% of primary and 24.6% of recurrent tumors were treated by MMS. If we applied the US indications of MMS6 to our sample, instead of the proposed algorithm, more than 70% of the treated cases would have indication for MMS.
More than half (63.1% - 303 of 480) of tumors treated by conventional surgery underwent intraoperative histological analysis of margins, and incomplete excision rate was 4.1% (20 of 480). Bariani et al. 14 obtained 8% of positive margins, excising well defined BCCs, smaller than 20 mm, with surgical margins of 3 mm, and poorly defined BCCs, larger than 20mm, with 5mm margins. Nagore et al. obtained 24% of positive margins excising BCCs with margins of 2 to 3 mm.20 Sherry et al. obtained incomplete excision rate of 3.2% excising primary BCCs with minimum margins of 3mm.16 Pichardo-Velazquez et al. obtained incomplete excision rate of 28.5%, excising high risk BCCs with surgical margins of 5mm. 21
The mean follow-up was 4.37 years (36.3% of the lesions were followed for more than 5 years), which is close to what is considered ideal by Gulleth et al.22
The overall recurrence rate of this study was 1.34%; 1.30% among primary tumors and 1.64% among recurrent tumors. Among the six cases of recurrence, which occurred in primary tumors, five were treated by conventional surgery, with complete removal of the tumor confirmed by the intraoperative histological analysis of margins, and one was treated by MMS. The only tumor that recurred in the group of previously treated lesions was located in high risk area and treated by MMS.
Although Fleischer et al. claim that the surgeon's experience does not affect the probability of incomplete resection, as all lesions were treated by the same surgeon, it would be interesting that the algorithm was tested in other centers.23
There are reports of recurrence in 5 years of 1% for primary BCCs treated by MMS and of 10.1% for those treated by conventional surgery; and of 5.6% for recurrent tumors treated by MMS and of 17.4% the ones treated by conventional surgery.24 Mosterd et al., in a prospective randomized study, obtained recurrence of 4.1% for primary BCCs treated by conventional surgery and of 2.5% for those treated by MMS, in a mean follow-up of 5 years.25 For recurrent tumors, they obtained recurrence of 12.1% for conventional surgery and of 2.4% for MMS. Cigma et al., excising BCCs with margins between 3 and 10 mm, according to their location, obtained recurrence of 2.6%.26 Wetzig et al. reported recurrence in 5 years of 0.5% for primary BCCs and of 2.9% for the excised recurrent BCCs with histologic control in paraffin.18 Rowe et al. reported recurrence in 5 years of 1% for primary BCCs and of 5.6% for recurrent tumors treated by micrographic techniques.27,28 Some studies involving periocular BCCs, excised with intraoperative histological analysis of margins, reported relapse of 2.15% and 9.7% for primary tumors and of 4.4% and 14.2% for recurrent tumors.29-31
Although the results of this study are similar to those obtained with MMS, we do not consider the sacrifice of healthy skin that, although not tested, is greater than with micrographic techniques. This observation has been demonstrated by Muller et al. that, in a randomized study, obtained a mean surgical defect of 111.6 mm² for small nodular BCCs treated by MMS versus 187.7mm² for conventional surgery. 32
Among the excluded cases, there was a recurrence in a patient with Gorlin-Goltz syndrome, treated by conventional surgery, one in a patient submitted to MMS for palliation and one in a patient treated by photodynamic therapy.
CONCLUSION
Complete excision is the key to surgical treatment of BCC. Accordingly, MMS has its indications well established in the literature and is the treatment of choice for most cases. However, considering the cure rate obtained in these studies, to sites where MMS is not yet widely available, the proposed algorithm can be a useful guide to direct the surgical treatment of basal cell carcinoma.
Footnotes
Conflict of interest: None
Financial Support: UFF Medical Science Masters Degree Scholarship (October 2013 to June 2014)
Study conducted at Centro de Cirurgia da Pele – Rio de Janeiro (RJ), Brazil.
References
- 1.Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 2.Brasil. Ministério do Planejamento, Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística. Censo Demográfico 2010 . Características Gerais da População, Religião e Pessoas com Deficiência. Rio de Janeiro: IBGE; 2010. [Google Scholar]
- 3.Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância . Estimativa 2014: Incidência de Câncer no Brasil. Rio de Janeiro: Inca; 2014. [Google Scholar]
- 4.Telfer NR, Colver GB, Morton CA. British Association of Dermatologists Line of actions for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 5.Gulleth Y, Goldberg N, Silverman RP, Gastman BR. What is the Best Surgical Margin for a Basal Cell Carcinoma A Meta-analysis of the Literature. Plast Reconstr Surg. 2010;126:1222–1231. doi: 10.1097/PRS.0b013e3181ea450d. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Dermatology. American College of Mohs Surgery. American Society for Dermatologic Surgery Association. American Society forMohs Surgery. Ad Hoc Task Force. Connolly SM, et al. AAD/ACMS/ASDSA/ASMS2012 Appropriate use criteria for Mohs Micrographic Surgery A report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582–1603. doi: 10.1111/j.1524-4725.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- 7.Kopke LFF, Schmidt SV. Carcinoma basocelular. An Bras Dermatol. 2002;17:249–282. [Google Scholar]
- 8.Cernea S. Remoção dos Carcinomas Basocelulares Considerações para a escolha do método terapêutico. J Soc Bras Cir Dermatol. 2001;29:8–8. [Google Scholar]
- 9.Luz FB, Ferron C, Cardoso GP. Surgical treatment of basal cell carcinoma an algorithm based on literature. An Bras Dermatol. 2015;90:377–383. doi: 10.1590/abd1806-4841.20153304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowson AN. Basal cell carcinoma biology, morphology and clinical implications. Mod Pathol. 2006;19:S127–S147. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- 11.Huang CC, Boyce SM. Surgical Margins of excision for basal cell carcinoma and squamous cell carcinoma. Semin Cutan Med Surg. 2004;23:167–173. doi: 10.1016/j.sder.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Aguayo-Leiva IR, Rios-Buceta L, Jaen-Olasolo P. Tratamiento quirúrgico vs no quirúrgico em el carcinoma basocelular. Actas Dermosifiliogr. 2012;101:683–692. [PubMed] [Google Scholar]
- 13.Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112:57–63. doi: 10.1097/01.PRS.0000067479.77859.31. [DOI] [PubMed] [Google Scholar]
- 14.Bariani RL, Nahas FX, Barbosa MV, Farah AB, Ferreira LM. Basal cell carcinoma an updated epidemiological and therapeutically profile of an urban population. Acta Cir Bras. 2006;21:66–73. doi: 10.1590/s0102-86502006000200003. [DOI] [PubMed] [Google Scholar]
- 15.Kyrgidis A1, Vahtsevanos K, Tzellos TG, Xirou P, Kitikidou K, Antoniades K, et al. Clinical histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma A 1062 patient-cohort study from a tertiary cancer referral hospital. Eur J Dermatol. 2010;20:276–282. doi: 10.1684/ejd.2010.0903. [DOI] [PubMed] [Google Scholar]
- 16.Sherry KR, Reid LA, Wilmshurst AD. A five-year review of basal cell carcinoma excisions. J Plast Reconstr Aesthet Surg. 2010;63:1485–1489. doi: 10.1016/j.bjps.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Chinem VP, Miot HA. Epidemiologia do carcinoma basocelular. An Bras Dermatol. 2011;86:292–305. doi: 10.1590/s0365-05962011000200013. [DOI] [PubMed] [Google Scholar]
- 18.Wetzig T, Woitek M, Eichhorn K, Simon JC, Paasch U. Surgical excision of basal cell carcinoma with complete margin control outcome at 5-year follow-up. Dermatology. 2010;220:363–369. doi: 10.1159/000300116. [DOI] [PubMed] [Google Scholar]
- 19.Bisson MA, Dunkin CS, Suvarna SK, Griffiths RW. Do plastic surgeons resect basal cell carcinoma too widely A prospective study comparing surgical and histological margins. Br J Plast Surg. 2002;55:293–297. doi: 10.1054/bjps.2002.3829. [DOI] [PubMed] [Google Scholar]
- 20.Nagore E, Grau C, Molinero J, Fortea JM. Positive margins in basal cell carcinoma relationship to clinical features and recurrence risk. A retrospective study of 248 patients. J Eur Acad Dermatol Venereol. 2003;17:167–170. doi: 10.1046/j.1468-3083.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 21.Pichardo-Velázquez P, Domínguez-Cherit J, Ma Vega-Memije, Moreno-Coutiño G, Proy H. Surgical option for nonmelanoma skin cancer. Int J Dermatol. 2004;43:148–150. doi: 10.1111/j.1365-4632.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 22.Gulleth Y, Goldberg N, Silverman RP, Gastman BR. What is the Best Surgical Margin for a Basal Cell Carcinoma A MetaAnalysis of the Literature. Plast Reconstr Surg. 2010;126:1222–1231. doi: 10.1097/PRS.0b013e3181ea450d. [DOI] [PubMed] [Google Scholar]
- 23.Fleischer Jr AB, Feldman SR, Barlow JO, Zheng B, Hahn HB, Chuang TY, et al. The specialty of the treating physician affects the likelihood of tumor-free resection margins for basal cell carcinoma results from a multi-institutional retrospective study. J Am Acad Dermatol. 2001;44:224–230. doi: 10.1067/mjd.2001.110396. [DOI] [PubMed] [Google Scholar]
- 24.Batra RS, Kelley LC. Predictors of extensive subclincal spread in nonmelanoma skin cancer treated with Mohs Micrographic Surgery. Arch Dermatol. 2002;138:1043–1051. doi: 10.1001/archderm.138.8.1043. [DOI] [PubMed] [Google Scholar]
- 25.Mosterd K, Krekels GA, Nieman FH, Ostertag JU, Essers BA, Dirksen CD, et al. Surgical excision versus Mohs´ micrographic surgery for primary and recurrent basal-cell carcinoma of the face a prospective randomized controlled trial with 5-year follow-up. Lancet Oncol. 2008;9:1149–1156. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 26.Cigna E, Tarallo M, Maruccia M, Sorvillo V, Pollastrini A, Scuderi N. Basal cell carcinoma 10 years of experience. J Skin Cancer. 2011;2011:476362–476362. doi: 10.1155/2011/476362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe DE, Carroll RJ, Day Jr CL. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 28.Rowe DE, Carroll RJ, Day Jr CL. Mohs' surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424–431. doi: 10.1111/j.1524-4725.1989.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 29.Khandwala MA, Lalchan SA, Chang BY, Habib M, Chakrabarty A, Cassells-Brown A. Outcome of periocular basal cell carcinoma managed by overnight paraffin section. Orbit. 2005;24:243–247. doi: 10.1080/01676830590952630. [DOI] [PubMed] [Google Scholar]
- 30.Conway RM, Themel S, Holbach LM. Surgery for primary basal cell carcinoma including the eyelid margins with intraoperative frozen section control comparative interventional study with a minimum clinical follow up of 5 years. Br J Ophthalmol. 2004;88:236–238. doi: 10.1136/bjo.2003.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong VA, Marshall JA, Whitehead KJ, Williamson RM, Sullivan TJ. Management of periocular basal cell carcinoma with modified en frozen section controlled excision. Ophthal Plast Reconstr Surg. 2002;18:430–435. doi: 10.1097/00002341-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Muller FM, Dawe RS, Moseley H, Fleming CJ. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma tissue-sparing outcome. Dermatol Surg. 2009;35:1349–1354. doi: 10.1111/j.1524-4725.2009.01240.x. [DOI] [PubMed] [Google Scholar]