Abstract
BACKGROUND
Dermatoscopy is a non-invasive diagnostic tool used to examine skin lesions with an optical magnification. It has been suggested as a useful tool for monitoring therapeutic response in lentigo maligna patients treated with imiquimod.
OBJECTIVE
To examine the accuracy of dermatoscopy as a tool to monitor the therapeutic response of pigmented basal cell carcinoma treated with imiquimod.
METHOD
The authors designed a prospective study. Patients with pigmented basal cell carcinoma were included and data regarding the dermatoscopy features were collected following the Menzies criteria, prior to initiating the imiquimod treatment. Subsequent dermatoscopic evaluations were performed at weeks 4 and 8, following imiquimod discontinuation.
RESULTS
Twenty lesions were included. The most common pigmented dermatoscopy features were large blue-grey ovoid nests (80%), followed by blue-grey globules (50%) and leaf-like areas (30%). No spoke wheel areas were observed. In 17 out of 20 patients, a response was noted during the first evaluation at 4 weeks, while the clearance was noted at the second check-up after 8 weeks. In two patients, the clearance was found at the initial evaluation at 4 weeks, while in one patient, the response remained unchanged. Blue-grey globules were the fastest to exhibit clearance (50% at week 4), followed by leaf-like areas (15%) and large blue-grey ovoid nests (6.25%).
CONCLUSION
According to our results, dermatoscopic evaluation enhances the accuracy in the assessment of the clinical response to imiquimod in pigmented basal cell carcinoma.
Keywords: Basal cell nevus syndrome, Drug therapy, Skin neoplasms
INTRODUCTION
Basal cell carcinoma (BCC) is the most prevalent malignant skin cancer originating from the basal cell layer of the epidermis. It rarely metastasizes and a proportion of these tumors may contain pigment. Most of the histological subtypes of basal cell carcinoma can exhibit pigmented varieties but this is rarely observed in morphoeic and infiltrative subtypes.1 Histologically, melanin can be found in the tumour cells and surrounding stroma. Within the tumour mass, melanin is more often seen in the superficial component of the tumour, with melanosomes often confined to the melanocytes. However, they may be taken up by the surrounding malignant epithelial cells.2-4 In the stroma, melanin is typically found at the tumour shoulders, in melanophages, but small amounts may lie free. Occasionally, melanocytes are found in the deep tumour nodules or in the overlying epidermis.2
Because of its asymmetry of pigmentation and variety of growth patterns, pigmented basal cell carcinoma (PBCC) is included in the differential diagnosis of invasive melanoma, along with other benign, pigmented skin lesions.
Dermatoscopy is a non-invasive diagnostic tool used to examine skin lesions with an optical magnification. Using a polarized light, this technique allows a detailed examination of pigmented epidermis structures and the dermo-epidermal junction. Its use has become highly important among dermatologists in conducting a better clinical diagnosis of nearly all pigmented lesions.5-15
Regarding pigmented basal cell carcinoma (PBCC), dermatoscopy has proven to be an effective diagnostic technique2. Most basal cell carcinomas have < 50% of their area pigmented, and only 7% of lesions have > 75% of pigmented tumour area.16
Although dermatoscopy has already been used as a tool to control borders in the surgical management of BCC, to the authors' knowledge, it has not yet been used to control accurately the efficacy of topical treatments such as imiquimod.17 This drug is an imidazoquinoline amide, which modifies immune response with both antitumor and antiviral activity. Its use was first approved in 2004 by the US Food and Drug Administration to treat actinic keratosis and superficial basal cell carcinoma.18 In addition, nodular subtypes of basal cell carcinoma have been treated with this cream, entailing positive responses.19-23 In the current literature, only one study reports a case of large PBCC, which was successfully resolved after imiquimod therapy.24
This study aims to examine the accuracy of dermoscopy as a tool for monitoring the therapeutic response of PBCC treated with imiquimod.
METHOD
Study design
The authors designed a study, conducted between January 1st, 2011 and May 1st, 2011. It was a prospective, open-label trial. The Menzies criteria were followed to diagnose PBCC; Absence of pigment network and at least, one of the following features: leaf-like areas, spoke wheel areas, large blue-gray ovoid nests and multiple blue-gray globules).12 BCCs manifesting arborizing vessels and/or ulceration, with no pigmentation features, were excluded. The authors included adult patients with dermatoscopy-confirmed diagnosis of PBCC.
Imiquimod therapy
The treatment protocol for the lesions included in our study was the dosing frequency recommended by the manufacturer: one daily application 5 days a week for 6 weeks. Rest periods were used as necessary to manage local skin reactions, and continued for up to 6 weeks.
Assessment of dermatoscopic features
Prior to initiating treatment, the team scanned the lesion using the Fotofinder® device and collected data regarding the dermatoscopy features, following the above-mentioned criteria. Subsequent evaluations were performed at weeks 4 and 8 after imiquimod discontinuation. The team compared the images before and after to assess the changes in the dermatoscopy features. Responses were classified as: clearance (complete clearance of features), response (decrease in number and/or intensity of features), unresponsive (no changes in features) and worsening (increase in number and/or intensity of features).
The study's protocol was approved by the hospital's medical ethics committee, and all participants gave their written informed consent before enrollment.
RESULTS
Twenty lesions from 20 subjects (9 women and 11 men) were included in the study. The median age was 68.73 (±9.11) years. Ten lesions were superficial BCC, and ten were of the nodular type. The most commonly affected site was the temple (5 lesions, 25%) followed by the nose (4 lesions, 20%). Table 1 summarizes the epidemiological data from our sample.
Table 1.
Distribution of the sample with data regarding sex, BCC clinical type and location
| Patient | Age | Sex | Clinical Type (Superficial / Nodular) |
Location |
|---|---|---|---|---|
| 1 | 76 | M | S | Nose |
| 2 | 81 | M | S | Chest |
| 3 | 72 | F | N | Nose |
| 4 | 74 | M | N | Temple |
| 5 | 68 | F | S | Arm |
| 6 | 81 | M | N | Cheek |
| 7 | 74 | M | S | Temple |
| 8 | 81 | F | S | Forehead |
| 9 | 93 | M | S | Neck |
| 10 | 74 | F | N | Temple |
| 11 | 69 | M | S | Arm |
| 12 | 71 | M | N | Nose |
| 13 | 73 | F | S | Temple |
| 14 | 62 | F | N | Cheek |
| 15 | 58 | F | N | Trunk |
| 16 | 70 | F | N | Forehead |
| 17 | 71 | M | S | Ear |
| 18 | 67 | F | S | Temple |
| 19 | 55 | M | N | Nose |
| 20 | 67 | M | N | Trunk |
All patients completed the 6-week treatment cycle. Application site reactions were the most common adverse effects, seen in 5 patients (25%). No patient required discontinuation of treatment or a rest period for local site reactions. In 17 out of 20 patients, a response was observed at the first evaluation after 4 weeks, while the clearance was noticed at the second check-up after 8 weeks. In two patients, the clearance was found at the first evaluation after 4 weeks (Patients 7 and 8), while in one patient (Patient 6), the response remained unchanged at the evaluation after 8 weeks, in relation to the evaluation after 4 weeks. No worsening was observed in our sample.
The most common pigmented dermatoscopic features were large blue-grey ovoid nests (16 lesions, 80%), followed by blue-grey globules (10 lesions, 50%) and leaf-like areas (6 lesions, 30%). Arborizing vessels were found in 13 lesions (65%) and ulcerations in 8 lesions (40%). No spoke wheel areas were observed.
Regarding large blue-grey ovoid nests, complete dermatoscopic clearance was observed at week 8 in most lesions (14 out of 16, 87.5%). Only in one lesion (6.25%) was the clearance observed at week 4, while in another lesion (6.25%), the response remained unchanged.
In 5 out of 10 lesions (50%), the blue-grey globules exhibited complete dermatoscopic clearance at week 8, while in the other 5 lesions (50%), the clearance was observed at week 4.
Leaf-like areas cleared completely by week 8 in 5 out of 6 lesions (85%), while in other lesions (15%), clearance was noted at week 4.
As regards arborizing vessels, complete dermatoscopic clearance was observed at week 8 in most lesions (9 out of 13, 69%). In 3 lesions (23%), the clearance was observed at week 4. In one lesion (8%), the response remained unchanged.
With respect to ulcerations, complete dermatoscopic clearance was noted at week 4 in all lesions (100%).
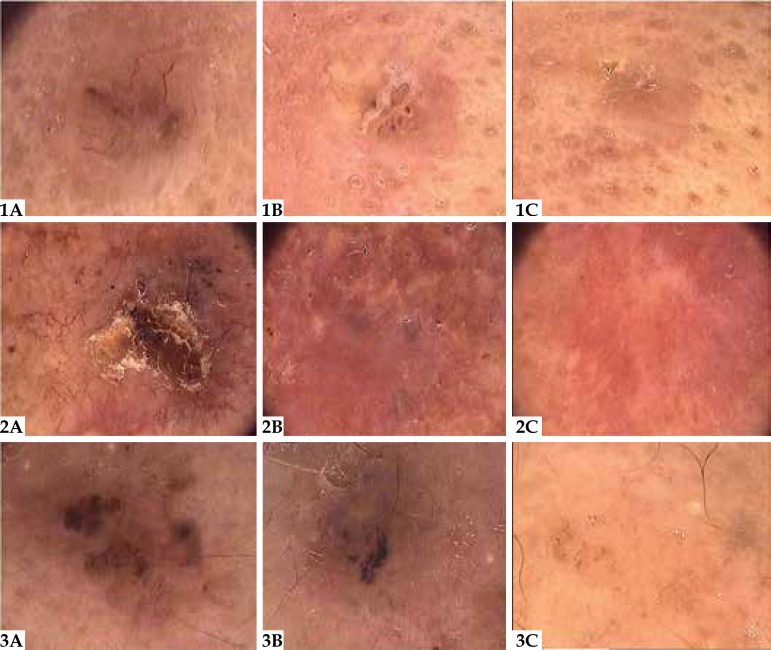
Figure 1 displays changes in dermatoscopic features in three of the lesions at the baseline, at 4 weeks and 8 weeks after treatment discontinuation. No PBCC relapses were observed in the follow-up period (12 months in most cases). Table 2 outlines the dermatoscopic features observed in the lesions and the response to imiquimod cream at weeks 4 and 8, respectively.
Figure 1.
Dermatoscopic findings in three lesions: (A) at baseline; (B) 4 weeks after treatment discontinuation, showing a decrease in number and/ or intensity of features; (C) 8 weeks after treatment discontinuation, showing complete clearance of features. (Original magnification x 20)
Table 2.
Dermatoscopic features of the 20 lesions prior to initiation of therapy and at weeks 4 and 8 after treatment with imiquimod
| ARBORIZING VESSELS | ULCERATION | BLUE-GRAY GLOBULES |
OVOID NESTS | LEAF-LIKE AREAS | SPOKE WHEEL AREAS |
|
|---|---|---|---|---|---|---|
| Patient 1 | + | - | + | + | - | - |
| W#4 | R | R | R | |||
| W#8 | C | C | C | |||
| Patient 2 | - | - | - | - | + | - |
| W#4 | R | |||||
| W#8 | C | |||||
| Patient 3 | + | - | + | + | - | - |
| W#4 | R | C | R | |||
| W#8 | C | C | C | |||
| Patient 4 | + | + | + | + | - | - |
| W#4 | C | C | C | R | ||
| W#8 | C | C | C | C | ||
| Patient 5 | - | + | + | + | + | - |
| W#4 | C | C | R | C | ||
| W#8 | C | C | C | C | ||
| Patient 6 | + | - | - | + | - | - |
| W#4 | R | R | ||||
| W#8 | R | R | ||||
| Patient 7 | + | - | - | + | - | - |
| W#4 | C | C | ||||
| W#8 | C | C | ||||
| Patient 8 | + | - | + | - | - | - |
| W#4 | C | C | ||||
| W#8 | C | C | ||||
| Patient 9 | - | + | + | + | - | - |
| W#4 | C | R | R | |||
| W#8 | C | C | C | |||
| Patient 10 | - | - | + | - | + | |
| W#4 | R | R | ||||
| W#8 | C | C | ||||
| Patient 11 | - | + | + | - | + | - |
| W#4 | C | R | R | |||
| W#8 | C | C | C | |||
| Patient 12 | + | - | + | + | - | - |
| W#4 | C | C | R | |||
| W#8 | C | C | C | |||
| Patient 13 | - | - | - | + | + | - |
| W#4 | R | R | ||||
| W#8 | C | C | ||||
| Patient 14 | + | - | - | + | + | - |
| W#4 | C | R | R | |||
| W#8 | C | C | C | |||
| Patient 15 | + | - | - | + | - | - |
| W#4 | C | R | ||||
| W#8 | C | C | ||||
| Patient 16 | + | + | + | + | - | - |
| W#4 | C | C | R | R | ||
| W#8 | C | C | C | C | ||
| Patient 17 | - | + | - | + | - | - |
| W#4 | C | R | ||||
| W#8 | C | C | ||||
| Patient 18 | + | - | - | + | - | - |
| W#4 | R | R | ||||
| W#8 | C | C | ||||
| Patient 19 | + | + | - | + | - | - |
| W#4 | C | C | R | |||
| W#8 | C | C | C | |||
| Patient 20 | + | + | - | + | - | - |
| W#4 | C | C | R | |||
| W#8 | C | C | C |
R: Response of features (decrease in number and/or intensity). C: clearance (complete clearance of features)
DISCUSSION
Dermatoscopy is a non-invasive technique of in vivo microscopy, used to diagnose non-pigmented and pigmented skin lesions by allowing the visualization of morphologic structures that are usually not discernible to the naked eye. In addition to this diagnostic role, it seems to be helpful in defining the margin of pigmented lesions such as lentigo maligna and it has been suggested as a useful tool for monitoring therapeutic response in PBCC patients treated with imiquimod.25-28 In this study, the authors evaluated the efficacy of dermatoscopy in monitoring the treatment of PBCC with imiquimod, based on the lesions' dermatological features. The changes in specific dermatoscopic features of BCC after treatment are of interest. Among these features, those with pigmentation according to the Menzies method are: large blue-gray ovoid nests, blue-grey globules, maple leaf-like areas and spoke wheel areas. The authors noted a rapid clearance of all pigmentation signs and signs of neovascularization and ulceration.
Ulceration and neovascularization were the first dermatoscopic features to undergo complete clearance in our study. Regarding pigmented dermatoscopic features, blue-grey globules were the fastest to exhibit clearance (50% at week 4), followed by leaf-like areas (15%) and large blue-grey ovoid nests (6.25%). Onan et al. have correlated the dermatoscopic findings of PBCC with their histological features.29 According to these authors, blue-grey globules correlate with small tumour nests localized in the papillary dermis, leaf-like areas correlate with multifocal tumour nests connecting each other localized in the papillary dermis, and blue-grey ovoid nests correlate with well-bordered tumour nests, with a few small buddings at the periphery, localized on the papillary and/or reticular dermis. Based on the findings of the study, the authors suggest that the onset and order of clearance of the pigmented dermatoscopic features are linked to the depth and size of the histological structures, with more intense and quicker imiquimod effects on the smaller structures localized at superficial layers. However, this is a purely morphological observation.
Clinical response does not always match the clearance of PBCC. The dermatoscopic assessment of clearance in the study suggests that dermatoscopy may have a place in monitoring the topical treatment of these lesions. At week 4, most dermatoscopic features had not undergone complete clearance, which may be interpreted as persistent PBCC when evaluated clinically. It was with the second dermatoscopic evaluation at week 8, when additional signs decreased and complete clearance of dermatoscopic features were apparent and the clearance was noticed. This outcome is of great interest to avoid clinical misdiagnosis with persistent PBCC. Dermatoscopic assessment provides more accuracy and precision to match the clearance of PBCC treated with imiquimod.
Topical, non-invasive, patient-administered treatment modalities continue to expand the options of dermatologists in managing a variety of skin conditions, including skin cancers. Less patient discomfort, favorable cosmetic outcomes and documented efficacy against BCCs make imiquimod an attractive treatment choice for managing PBCC. Imiquimod therapy plays a role in PBCC patients where other invasive treatment modalities are not recommended. Poor surgical candidates (i.e., patients who are elderly, anticoagulated or who have implanted cardiac pacemakers) would benefit from this non-invasive, self-administered topical therapy. In this context, dermatoscopy is a helpful tool for evaluating the clearance of the lesion after imiquimod without the need for assessing histological remission with risky incisional biopsies. Nonetheless, since dissociation between clinical and histological clearance has been reported, biopsies are still required to confirm remission. Indeed, the main limitation of our study is that no biopsies were performed to assess histological remission.30,31 However, a biopsy does not allow examination of the whole lesion because specimens are usually obtained from representative areas of a lesion to predict the histopathological condition. Hence, the authors suggest that dermatoscopic evaluation of treated lesions may enhance the accuracy of treatment response assessments: if dermatoscopic features remain on the treated lesion, a biopsy should be performed on that site. In this study, no signs of lesion recurrence were observed after one-year of follow-up and therefore no biopsies were needed.
Randomized controlled studies comparing dermatoscopic findings, as well as a histopathological evaluation of complete surgical excision, are required to confirm the real usefulness of dermatoscopy in monitoring imiquimod therapy for PBCC.
To the authors' knowledge, this is the first study to examine dermatoscopy as a therapeutic tool for monitoring PBCC treated with imiquimod. In conclusion, the data suggest that dermatoscopy is not only a diagnosis technique, but that it may also be useful in managing PBCC by facilitating the monitoring of response to topical treatments.
CONCLUSION
According to the findings, dermatoscopic evaluation enhances the accuracy in the assessment of the clinical response to imiquimod in PBCC.
Footnotes
Conflict of Interest: None
Financial Support: None
Work performed at the Hospital de Baza and Hospital San Cecilio – Granada, Spain.
References
- 1.Terstappen K, Larkö O, Wennberg AM. Pigmented basal cell carcinoma-comparing the diagnostic methods of SIAscopy and dermoscopy. Acta Derm Venereol. 2007;87:238–242. doi: 10.2340/00015555-0234. [DOI] [PubMed] [Google Scholar]
- 2.Maloney ME, Jones DB, Sexton FM. Pigmented basal cell carcinoma; investigation of 70 cases. J Am Acad Dermatol. 1992;27:74–78. doi: 10.1016/0190-9622(92)70160-h. [DOI] [PubMed] [Google Scholar]
- 3.Bleehen S. Pigmented basal cell epithelioma. Br J Dermatol. 1975;93:361–370. doi: 10.1111/j.1365-2133.1975.tb06509.x. [DOI] [PubMed] [Google Scholar]
- 4.Tezuka T, Ohkuma M, Hirose I. Melanosomes of pigmented basal cell epitheliomas. Dermatologica. 1977;154:14–22. doi: 10.1159/000251025. [DOI] [PubMed] [Google Scholar]
- 5.Krähn G, Gottlöber P, Sander C, Peter RU. Dermatoscopy and high frequency sonography two useful non-invasive methods to increase preoperative diagnostic accuracy in pigmented skin lesions. Pigment Cell Res. 1998;11:151–154. doi: 10.1111/j.1600-0749.1998.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 6.Steiner A, Pehamberger H, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. II. Diagnosis of small pigmented skin lesions and early detection of malignant melanoma. J Am Acad Dermatol. 1987:17584–17591. doi: 10.1016/s0190-9622(87)70240-0. [DOI] [PubMed] [Google Scholar]
- 7.Pehamberger H, Binder M, Steiner A, Wolff K. In vivo epiluminescence microscopy improvement of early diagnosis of melanoma. J Invest Dermatol. 1993;100:356S–362S. doi: 10.1111/1523-1747.ep12470285. [DOI] [PubMed] [Google Scholar]
- 8.Steiner A, Pehamberger H, Binder M, Wolff K. Pigmented Spitz nevi improvement of the diagnostic accuracy by epiluminescence microscopy. J Am Acad Dermatol. 1992;27:697–701. doi: 10.1016/0190-9622(92)70240-g. [DOI] [PubMed] [Google Scholar]
- 9.Binder M, Schwarz M, Winkler A, Steiner A, Kaider A, Wolff K, et al. Epiluminescence microscopy a useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Arch Dermatol. 1995;131:286–291. doi: 10.1001/archderm.131.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Nachbar F, Stolz W, Merkle T, Cognetta AB, Vogt T, Landthaler M, et al. The ABCD rule of dermatoscopy high prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30:551–559. doi: 10.1016/s0190-9622(94)70061-3. [DOI] [PubMed] [Google Scholar]
- 11.Pazzini C, Pozzi M, Betti R, Vergani R, Crosti C. Improvement of diagnostic accuracy in the clinical diagnosis of pigmented skin lesions by epiluminescence microscopy. Skin Cancer. 1996;11:159–161. [Google Scholar]
- 12.Binder M, Puespoeck-Schwarz M, Steiner A, Kittler H, Muellner M, Wolff K, et al. Epiluminescence microscopy of small pigmented skin lesions short-term formal training improves the diagnostic performance of dermatologists. J Am Acad Dermatol. 1997;36:197–202. doi: 10.1016/s0190-9622(97)70280-9. [DOI] [PubMed] [Google Scholar]
- 13.Carli P, De Giorgi V, Naldi L, Dosi G. Reliability and inter-observer agreement of dermoscopic diagnosis of melanoma and melanocytic naevi. Eur J Cancer Prev. 1998;7:397–402. doi: 10.1097/00008469-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Stanganelli I, Serafini M, Cainelli T, Cristofolini M, Baldassari L, Staffa M, et al. Accuracy of epiluminescence microscopy among practical dermatologists a study from the Emilia-Romagna region of Italy. Tumori. 1998;84:701–705. doi: 10.1177/030089169808400618. [DOI] [PubMed] [Google Scholar]
- 15.Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 16.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012–1016. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 17.Caresana G, Giardini R. Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1395–1399. doi: 10.1111/j.1468-3083.2010.03652.x. [DOI] [PubMed] [Google Scholar]
- 18.Jobanputra KS, Rajpal AV, Nagpur NG. Imiquimod. Indian J Dermatol Venereol Leprol. 2006;72:466–469. doi: 10.4103/0378-6323.29352. [DOI] [PubMed] [Google Scholar]
- 19.Ozolins M, Williams HC, Armstrong SJ, Bath-Hextall FJ. The SINS trial a randomised controlled trial of excisional surgery versus imiquimod 5% cream for nodular and superficial basal cell carcinoma. Trials. 2010;11:42–42. doi: 10.1186/1745-6215-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moehrle M, Breuninger H, Schippert W, Häfner HM. Letter Imiquimod 5% cream as adjunctive therapy for primary, solitary, nodular basal cell carcinomas before Mohs micrographic surgery: a randomized, double-blind, vehicle-controlled study. Dermatol Surg. 2010;36:428–430. doi: 10.1111/j.1524-4725.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 21.Gowda S, Tillman DK, Fitzpatrick JE, Gaspari AA, Goldenberg G. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878–881. doi: 10.1111/j.1600-0560.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- 22.Shulstad RM. Case study Imiquimod 5% cream in the treatment of a large nodular basal cell carcinoma. Dermatol Nurs. 2009;21:86–87. [PubMed] [Google Scholar]
- 23.Devirgiliis V, Panasiti V, Curzio M, Gobbi S, Rossi M, Roberti V, et al. Complete remission of nodular basal cell carcinoma after combined treatment with photodynamic therapy and imiquimod 5% cream. Dermatol Online J. 2008;14:25–25. [PubMed] [Google Scholar]
- 24.Mehta V, Balachandran C. Pigmented basal cell carcinoma successfully treated with 5% imiquimod cream. Indian J Dermatol. 2008;53:140–141. doi: 10.4103/0019-5154.43204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson JK. Use of digital epiluminiscence microscopy to help define the edge of lentigo maligna. Arch Dermatol. 2004;140:1095–1100. doi: 10.1001/archderm.140.9.1095. [DOI] [PubMed] [Google Scholar]
- 26.Costa MC, Abraham LS, Barcaui C. Lentigo maligna treated with topical imiquimod dermatoscopy usefulness in clinical monitoring. An Bras Dermatol. 2011;86:792–794. doi: 10.1590/s0365-05962011000400028. [DOI] [PubMed] [Google Scholar]
- 27.de Troya-Martín M, Frieyro-Elicegui M, Fúnez Liébana R, Aguilar Bernier M, Fernández-Canedo NI, Blázquez Sánchez N. Lentigo maligna managed with topical imiquimod and dermoscopy report of two cases. Dermatol Surg. 2008;34:1561–1566. doi: 10.1111/j.1524-4725.2008.34322.x. [DOI] [PubMed] [Google Scholar]
- 28.Micantonio T, Fargnoli MC, Peris K. Usefulness of dermoscopy to monitor clinical efficacy of imiquimod treatment for lentigo maligna. Arch Dermatol. 2006;142:530–531. doi: 10.1001/archderm.142.4.530-b. [DOI] [PubMed] [Google Scholar]
- 29.Tabanlioglu Onan D, Sahin S, Gököz O, Erkin G, Cakir B, Elçin G, et al. Correlation between the dermatoscopic and histopathological features of pigmented basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1317–1325. doi: 10.1111/j.1468-3083.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 30.Fleming CJ, Bryden AM, Evans A, Dawe RS, Ibbotson SH. A pilot study of treatment of lentigo maligna with 5% imiquimod cream. Br J Dermatol. 2004;151:485–488. doi: 10.1111/j.1365-2133.2004.05983.x. [DOI] [PubMed] [Google Scholar]
- 31.Stolz W, Schiffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20:276–278. doi: 10.1016/s0738-081x(02)00221-3. [DOI] [PubMed] [Google Scholar]