Abstract
Intermediate-length CAG expansions (encoding 27–33 glutamines, polyQ) of the Ataxin2 (ATXN2) gene represent a risk factor for amyotrophic lateral sclerosis (ALS). Recently, it has been proposed that ≥31 CAG expansions may influence ALS phenotype. We assessed whether ATXN2 intermediate-length polyQ expansions influence ALS phenotype in a series of 375 patients of Sardinian ancestry. Controls were 247 neurologically healthy subjects, resident in the study area, age- and gender-matched to cases. The frequency of ≥31 polyQ ATNX2 repeats was significantly more common in ALS cases (4 patients vs. no control, p = 0.0001). All patients with ≥31 polyQ repeats had a spinal onset versus 73.3% of patients with <31 polyQ repeats. Patients with an increased number of polyQ repeats have a shorter survival than those with <31 repeats (1.2 vs. 4.2 years, p = 0.035). In this large series of ALS patients of Sardinian ancestry, we have found that ≥31 polyQ repeats of the ATXN2 gene influenced patients' phenotype, being associated to a spinal onset and a significantly shorter survival.
Keywords: ALS, Ataxin 2 gene, Genetic modifier
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by a progressive loss of cortical, bulbar, and spinal motor neurons, leading to the loss motor function up to death due to respiratory failure. About half of patients have also various degrees of cognitive impairment, going from an overt frontotemporal dementia (FTD) to milder forms of executive or nonexecutive impairment (Montuschi et al., 2015 and Phukan et al., 2012). In most populations, about 10% of ALS patients have a family history for ALS, FTD, or both (familial ALS), whereas in the remaining no family history is detectable (sporadic ALS). However, in ALS patients of Sardinian ancestry, the frequency of family history for ALS or FTD is higher than in most Caucasian populations (Borghero et al., 2014, Chiò et al., 2011 and Orrù et al., 2012), with the only exception of Finland and Northern Sweden (Andersen et al., 1995 and Majounie et al., 2012). Moreover, ~40% of Sardinian ALS cases carry a pathogenic mutation, with several cases carrying 2 different mutations (Borghero et al., 2014).
Among disease-modifying genes in ALS, Ataxin 2 (ATXN2) is one of the most validated. Intermediate-length (CAG) expansions (encoding 27–33 glutamines, polyQ) represent a risk factor for ALS, increasing the risk of about 10 fold, and are a modifier of ALS clinical presentation, being associated to a spinal phenotype and a more aggressive clinical course (Chiò et al., 2015).
The aim of this study was to assess the frequency of intermediate polyQ repeats in a series of patients of Sardinian ancestry and to analyze the clinical characteristics of these patients.
2. Methods
2.1. Patients
All ALS patients of Sardinian ancestry, defined as subjects with both parents of Sardinian origin, were eligible to be included in the study. Patients were identified between 2008 and 2013 through the SARDINIALS and ITALSGEN consortia (Borghero et al., 2014, Chiò et al., 2011 and Chiò et al., 2012). Clinical information, including cognitive status, was collected on all patients. ALS patients met the EL Escorial revised criteria for definite, probable, probable laboratory-supported, or possible ALS (Brooks et al., 2000).
2.2. Controls
The 247 control individuals, all of Sardinian origin, included 115 women and 132 men and had a mean age of 62.1 years (standard deviation [SD] 14.5) at the time of blood collection. They were recruited through the Department of Public Health, Clinical and Molecular Medicine, University of Cagliari and the staff of Multiple Sclerosis Center—Azienda Sanitaria Locale 8 di Cagliari, as subjects without known history of a neurological disorder, such as nonblood-related companions or spouses of patients.
2.3. Mutational screening
Genomic DNA was isolated from peripheral blood lymphocytes using a standard protocol. The ATXN2 CAG repeat in exon 1 (Ref Seq NM_002973.3) was amplified using a fluorescent primer and sized by capillary electrophoresis on an ABI 3500×L genetic analyzer (Applied Biosystem, Foster City, CA, USA) (Cancel et al., 1997). The size standard used was the GeneScan 500 ROX dye and for analysis the GenMapper v.4.0 software. Receiver operating characteristics analysis showed that a cutoff ≥31 polyQ repeats in ATXN2 had the greatest sensitivity and specificity for discriminating ALS patients versus controls.
The following exons and 50 base-pair flanking intron-exon boundaries were also screened for mutations by polymerase chain reaction amplification, sequencing using the Big-Dye Terminator v3.1 kit (Applied Biosystems Inc), and analysis on an ABI Prism 3130 genetic analyzer: (1) all 5 coding exons of SOD1, (2) exon 6 of TARDBP, and (3) exons 14 and 15 of FUS. These exons were selected as the vast majority of known pathogenic variants that lie within these mutational hotspots. A repeat-primed polymerase chain reaction assay was used to screen for the presence of the GGGGCC hexanucleotide expansion in the first intron of C9ORF72 (Dejesus-Hernandez et al., 2011 and Renton et al., 2011); a cutoff of ≥30 repeats combined with a typical sawtooth pattern was considered pathological.
2.4. Standard protocol approvals, registrations, and patient consents
The study design was approved by the ethical committees of all the involved centers. Patients and controls signed written informed consent. The study was conducted in-line with the Italian ethical rules for data collection for statistical or scientific purposes and for data protection.
3. Results
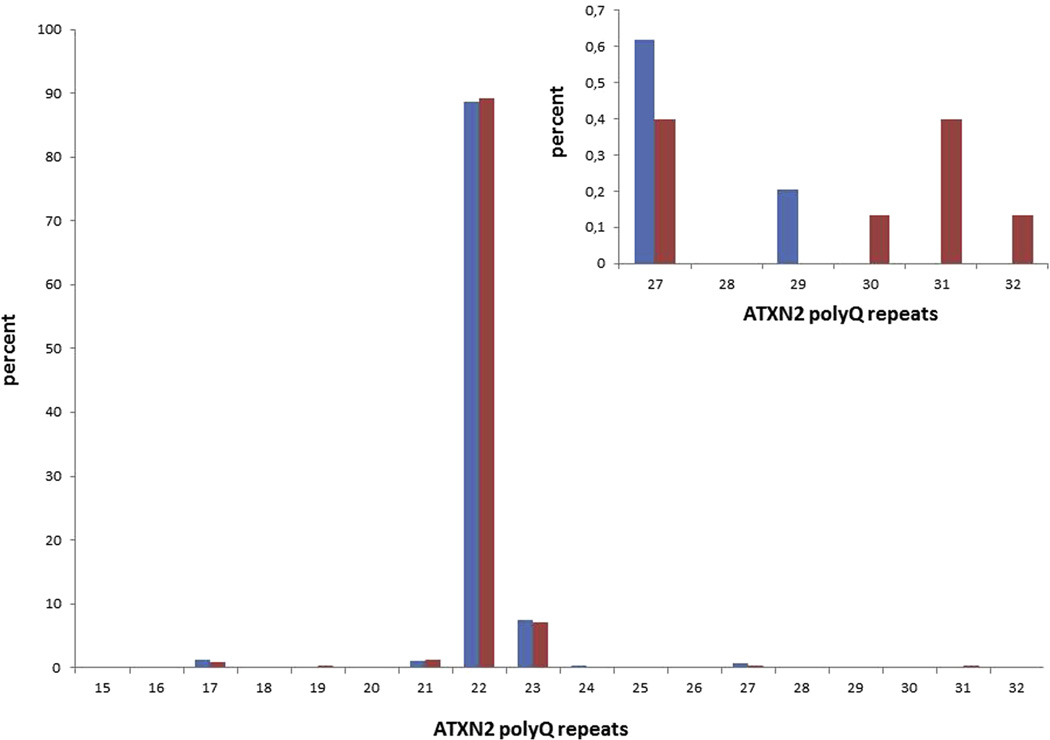
The clinical and genetic characteristics of the ALS patients included in this study have been previously described elsewhere (Borghero et al., 2014). The size of the ATXN2 repeats in ALS patients compared to the control group is reported in Fig. 1. The most common alleles (22 and 23) were identified in 98.9% of controls' chromosomes and 95.9% of cases' chromosomes. ATXN2 repeats ≤30 were similarly distributed between ALS cases and controls, whereas those ≥31 were significantly more common in cases (4 cases and no controls, p = 0.0001) (Fig. 1). The second allele in patients with ≥27 repeats was 22 in all cases. The overall frequency of cases with ≥31 polyQ repeats in this Sardinian series of patients was 1.1%.
Fig. 1.
Distribution of ATXN2 polyQ repeat lengths in amyotrophic lateral sclerosis (ALS) and control cases. In the insert, data bout cases and controls with ≥27 repeats are magnified. PolyQ lengths ≥31 are significantly more frequent in ALS cases (p = 0.0001) (red, ALS patients; blue, controls).
Overall, 155 patients (41.3%) carried a genetic mutation of one of the major ALS genes (Borghero et al., 2014). Three of 4 cases with ≥31 polyQ repeats were apparently sporadic and did not show any mutation of the examined ALS-related genes. The remaining case with ≥31 polyQ repeats was a familial ALS case who also carried a p.T622A missense mutation of the MATR3 gene (pedigree ITALS#10) (Johnson et al., 2014); her first degree cousin, who also carried the MATR3 mutation, was homozygous for 22-22 repeats of the ATXN2 gene; interestingly, the patient carrying the intermediate-length polyQ repeats died 33 months after ALS onset, whereas her cousin, who does not carry the repeat expansion, is still alive 50 months after onset, although on nocturnal noninvasive ventilation.
Of the 59 patients with a GGGGCC hexanucleotide repeat expansion of the C9ORF72 gene, 42 were homozygous for 22-22 repeats and 2 for 23-23 repeats, while the others were heterozygous (1 had 17–22 repeats, 1 had 21–22, and 13 had 22–23). None of them had >23 repeats.
3.1. Clinical characteristics of patients with ≥31 polyQ repeats
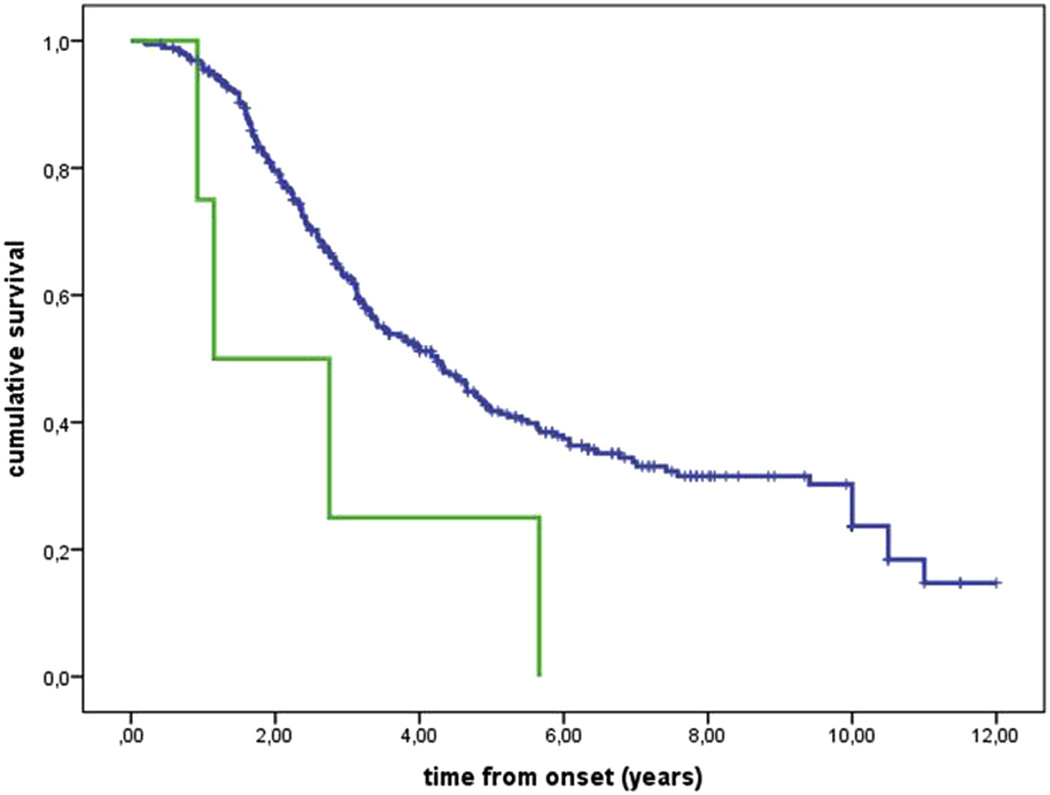
The mean age at onset of patients with ≥31 polyQ repeats was 59.5 years (SD 7.0), only slightly younger than that of patients with <31 polyQ repeats (61.2 years [SD 12.1]) (p = 0.78). All patients with ≥31 polyQ repeats had a spinal onset versus 73.3% of patients with <31 polyQ repeats (p = 0.58). The patients with ≥31 polyQ repeats had a significantly shorter median survival than that of patients with <30 polyQ repeats (1.2 years, interquartile range 0.9–2.7 vs. 4.2, interquartile range 2.2–10) (p = 0.035) (Fig. 2). The presence of polyQ repeats ≥31 remained independently significant as negative prognostic factor also at multivariable Cox analysis (data not shown).
Fig. 2.
Kaplan-Meier survival estimation from onset to death and/or tracheostomy. Blue line, <31 polyQ repeats; green line, ≥31 polyQ repeats. p = 0.035.
4. Discussion
In this large series of ALS patients of Sardinian ancestry, we have found that ≥31 polyQ repeats of the ATXN2 gene represent a significant risk for ALS. Moreover, ≥31 polyQ repeats influenced patients' phenotype, being associated to a spinal onset and a significantly shorter survival.
The frequency of intermediate-length polyQ repeats in Sardinian patients is lower than observed in continental Italy and southern Europe and similar to that of subjects of north European ancestry (Chiò et al., 2015). This finding is in keeping with the other genetic characteristics that differentiate Sardinians from continental Italians, such as the higher frequency of C9ORF72 mutations in ALS (Borghero et al., 2014) and the higher incidence of multiple sclerosis (Cocco et al., 2011), both comparable to the figures reported for northern Europe. Moreover, the frequency of SCA2 expansion patients in Sardinians is lower than in other Italian populations (unpublished data observed from the results of SCA2 genetic tests performed since 2006 in the laboratory of the Regional Multiple Sclerosis Center of Cagliari).
In this series, we did not find any patient carrying the C9ORF72 expansion to have ATXN2 intermediate-length polyQ repeats ≥27. This finding is in keeping with 2 cohorts of ALS patients from northern and central Italy (Chiò et al., 2015) but differs from another study (van Blitterswijk et al., 2014). It is possible that this difference is because of ethnic-based and highlights the importance of comparing large cohorts of patients of different origin.
The influence of ATXN2 intermediate-length polyQ repeats on ALS phenotype is intriguing. ALS cases with ≥31 polyQ repeats have more frequently a spinal onset and a have a significantly shorter survival than those without the expansion (Chiò et al., 2015). The mechanism of reduced survival in patients carrying ATXN2 intermediate-length polyQ repeats remains elusive. ATXN2 may act through several, nonmutually exclusive mechanisms, such as cytoplasmic aggregations of ATXN2 protein, aggregations of other proteins induced by abnormal polyQ expansions in ATXN2, defects in the endoplasmic reticulum-Golgi pathway, abnormal neuronal calcium signaling, and formation of nuclear RNA foci sequestering essential RNA-binding proteins (van den Heuvel et al., 2014).
We have shown that, in this cohort of Sardinian patients ATXN2 intermediate-length polyQ repeats represent a risk factor for ALS and negatively influence its prognosis. These findings support the idea that ATXN2 may represent a promising therapeutic target in ALS.
Acknowledgments
Adriano Chiò serves on a scientific advisory board for Biogen Idec, Cytokinetics, and Italfarmaco.
This work was in part supported by the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, 2010, grant RF-2010–2309489), the European Community's Health Seventh Framework Programme (FP7/2007–2013 under grant agreement 259867), the Joint Programme—Neurodegenerative Disease Research (Italian Ministry of Education and University) (Sophia and Strength Projects), the Agenzia Italiana per la Ricerca sulla SLA (ARISLA) (SARDINIALS project), the Associazione Piemontese per l’Assistenza alla SLA (APASLA), Torino, Italy, and the Fondazione Mario e Anna Magnetto, Alpignano, Torino, Italy. This work was supported by the Intramural Research Program of the National Institute on Aging (project Z01 AG000949-02), the National Institute of Neurological Disorders and Stroke, and the National Institute of Mental Health.
ITALSGEN Consortium: Francesco O. Logullo (Ancona); Isabella Simone (Bari); Giancarlo Logroscino (Bari and Tricase, LE); Fabrizio Salvi, Ilaria Bartolomei (Bologna); Margherita Capasso (Chieti); Claudia Caponnetto, Gianluigi Mancardi, Paola Mandich, Paola Origone (Genova); Francesca L. Conforti (Mangone, Cosenza); Gabriele Mora, Kalliopi Marinou, Riccardo Sideri (Milano, Maugeri Foundation); Christian Lunetta, Silvana Penco, Lorena Mosca (Milano, Neuromuscular OmniCenter and Niguarda Ca' Granda Hospital); Riva Nilo (Milano, San Raffaele Hospital); Giuseppe Lauria Pinter (Milano, Besta Neurological Institute); Massimo Corbo (Milano, Casa di Cura del Policlinico); Paolo Volanti (Mistretta, Maugeri Foundation); Jessica Mandrioli, Nicola Fini, Eleni Georgoulopoulou (Modena); Lucio Tremolizzo (Monza); Maria Rosaria Monsurrò, Gioacchino Tedeschi, Viviana Cristillo (Napoli); Vincenzo la Bella, Rossella Spataro, Tiziana Colletti (Palermo); Mario Sabatelli, Marcella Zollino, Amelia Conte, Marco Luigetti, Serena Lattante, Giuseppe Marangi (Roma, Catholic University of Sacred Heart); Marialuisa Santarelli (Rome, San Filippo Neri Hospital); Antonio Petrucci (Rome, San Camillo Forlanini Hospital); Fabio Giannini, Stefania Battistini, Claudia Ricci (Siena); Federico Casale, Giuseppe Marrali, Giuseppe Fuda, Irene Ossola, Stefania Cammarosano, Antonio Ilardi, Davide Bertuzzo (Torino), Raffaella Tanel (Trento); Fabrizio Pisano (Veruno, NO).
SARDINIALS Consortium: Emanuela Costantino, Carla Pani, Roberta Puddu, Carla Caredda, Valeria Piras, Stefania Tranquilli, Stefania Cuccu, Daniela Corongiu, Maurizio Melis, Antonio Milia (Cagliari), Anna Ticca (Nuoro), Angelo Pirisi, Patrizia Occhineri (Sassari), Enzo Ortu (Ozieri).
Footnotes
Disclosure statement
All other authors report no conflicts of interest.
Study concept and design was contributed by Borghero, Pugliatti, Traynor, Restagno, Chiò. Acquisition of data was done by Murru, Floris, Cannas, Parish, Occhineri, Cau, Loi, Ticca, Manera, Canosa, Miglia, Calvo, Barberis, Brunetti, Renton, Nalls. Analysis and interpretation of data was done by Borghero, Pugliatti, F. Marrosu, M.G: Marrosu, Murru, Renton, Nalls, Traynor, Restagno, Chiò. Drafting of the manuscript was done by Borghero, Pugliatti, Traynor, Chiò. Critical revision of the manuscript for important intellectual content was done by Borghero, Pugliatti, F. Marrosu, M.G Marrosu, Murru, Floris, Cannas, Parish, Occhineri, Cau, Loi, Ticca, Manera, Canosa, Moglia, Calvo, Barberis, Brunetti, Renton, Nalls, Traynor, Restagno, Chiò. Funding was obtained from Borghero, Pugliatti, Restagno, Chiò. Administrative, technical, and material support was from Murru, Floris, Cannas, Parish, Occhineri, Cau, Loi, Ticca, Manera, Canosa, Moglia, Calvo, Barberis, Brunetti, Renton, Nalls. Study supervision was done by Borghero, Pugliatti, Traynor, Restagno, Chiò. Adriano Chiò has full access to data. The corresponding author confirm that all authors have read and approved the final draft of the manuscript and given written permission to include their names in the manuscript.
References
- Andersen PM, Nilsson P, Ala-Hurula V, Keränen ML, Tarvainen I, Haltia T, Nilsson L, Binzer M, Forsgren L, Marklund SL. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat. Genet. 1995;10:61–66. doi: 10.1038/ng0595-61. [DOI] [PubMed] [Google Scholar]
- Borghero G, Pugliatti M, Marrosu F, Marrosu MG, Murru MR, Floris G, Cannas A, Parish LD, Occhineri P, Cau TB, Loi D, Ticca A, Traccis S, Manera U, Canosa A, Moglia C, Calvo A, Barberis M, Brunetti M, Pliner HA, Renton AE, Nalls MA, Traynor BJ, Restagno G, Chiò A ITALSGEN and SARDINALS Consortia. Genetic architecture of ALS in Sardinia. Neurobiol. Aging. 2014;35:2882.e7–2882.e12. doi: 10.1016/j.neurobiolaging.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Cancel G, Dürr A, Didierjean O, Imbert G, Bürk K, Lezin A, Belal S, Benomar A, Abada-Bendib M, Vial C, Guimarães J, Chneiweiss H, Stevanin G, Yvert G, Abbas N, Saudou F, Lebre AS, Yahyaoui M, Hentati F, Vernant JC, Klockgether T, Mandel JL, Agid Y, Brice A. Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum. Mol. Genet. 1997;6:709–715. doi: 10.1093/hmg/6.5.709. [DOI] [PubMed] [Google Scholar]
- Chiò A, Borghero G, Pugliatti M, Ticca A, Calvo A, Moglia C, Mutani R, Brunetti M, Ossola I, Marrosu MG, Murru MR, Floris G, Cannas A, Parish LD, Cossu P, Abramzon Y, Johnson JO, Nalls NA, Arepalli S, Chong S, Hernandez DG, Traynor BJ, Restagno G ITALSGEN, Consortium. A large proportion of ALS cases in Sardinia are due to a single founder mutation of the TARDBP gene. Arch. Neurol. 2011;68:594–598. doi: 10.1001/archneurol.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, Sendtner M, Brunetti M, Ossola I, Calvo A, Pugliatti M, Sotgiu MA, Murru MR, Marrosu MG, Marrosu F, Marinou K, Mandrioli J, Sola P, Caponnetto C, Mancardi G, Mandich P, La Bella V, Spataro R, Conte A, Monsurrò MR, Tedeschi G, Pisano F, Bartolomei I, Salvi F, Lauria Pinter G, Simone I, Logroscino G, Gambardella A, Quattrone A, Lunetta C, Volanti P, Zollino M, Penco S, Battistini S, Renton AE, Majounie E, Abramzon Y, Conforti FL, Giannini F, Corbo M, Sabatelli M ITALSGEN consortium. Clinical characteristics of familial ALS patients carrying the pathogenic GGGGCC hexanucleotide repeat expansion of the C9ORF72 gene. Brain. 2012;135:784–793. doi: 10.1093/brain/awr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A, Calvo A, Moglia C, Canosa A, Brunetti M, Barberis M, Restagno G, Conte A, Bisogni G, Marangi G, Moncada A, Lattante S, Zollino M, Sabatelli M, Bagarotti A, Corrado L, Mora G, Bersano E, Mazzini L, D’Alfonso S. PARALS ATXN2 polyQ intermediate repeats are a modifier of ALS survival. Neurology. 2015;84:251–258. doi: 10.1212/WNL.0000000000001159. [DOI] [PubMed] [Google Scholar]
- Cocco E, Sardu C, Massa R, Mamusa E, Musu L, Ferrigno P, Melis M, Montomoli C, Ferretti V, Coghe G, Fenu G, Frau J, Lorefice L, Carboni N, Contu P, Marrosu MG. Epidemiology of multiple sclerosis in south-western Sardinia. Mult. Scler. 2011;17:1282–1289. doi: 10.1177/1352458511408754. [DOI] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Pioro EP, Boehringer A, Chia R, Feit H, Renton AE, Pliner HA, Abramzon Y, Marangi G, Winborn BJ, Gibbs JR, Nalls MA, Morgan S, Shoai M, Hardy J, Pittman A, Orrell RW, Malaspina A, Sidle KC, Fratta P, Harms MB, Baloh RH, Pestronk A, Weihl CC, Rogaeva E, Zinman L, Drory VE, Borghero G, Mora G, Calvo A, Rothstein JD, Drepper C, Sendtner M, Singleton AB, Taylor JP, Cookson MR, Restagno G, Sabatelli M, Bowser R, Chiò A, Traynor BJ ITALSGEN Consortium. Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 2014;17:664–666. doi: 10.1038/nn.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Nicalou N, Waite A, Rollinson S, Chiò A, Restagno G, Simon-Sanchez J, van Swieten J, Abramzon Y, Johnson JO, Sendtner M, Pamphlett R, Orrell RW, Mead S, Houlden H, Rohrer JD, Morrison K, Talbot K, Ansorge O, Englund E, Borghero G, McCluskey L, Trojanowski JQ, van Deerlin VM, Schellenberg GD, Nalls MA, Drory V, Brice A, Drepper C, Williams N, Kirby J, Shaw P, Hardy J, Singleton A, Tienari PJ, Heutink P, Morris H, Pickering-Brown S, Traynor BJ The Chromosome 9-ALS/FTD Consortium; The ITALSGEN Consortium. Frequency of the 9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Lo Presti A, Cammarosano S, Canosa A, Chiò A. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J. Neurol. Neurosurg. Psychiatry. 2015;86:168–173. doi: 10.1136/jnnp-2013-307223. [DOI] [PubMed] [Google Scholar]
- Orrù S, Manolakos E, Orrù N, Kokotas H, Mascia V, Carcassi C, Petersen MB. High frequency of the TARDBP p.Ala382Thr mutation in Sardinian patients with amyotrophic lateral sclerosis. Clin. Genet. 2012;81:172–178. doi: 10.1111/j.1399-0004.2011.01668.x. [DOI] [PubMed] [Google Scholar]
- Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J. Neurol. Neurosurg. Psychiatry. 2012;83:102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ The ITALSGEN Consortium. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, Mullen B, Heckman MG, Baker MC, DeJesus-Hernandez M, Brown PH, Murray ME, Hsiung GY, Stewart H, Karydas AM, Finger E, Kertesz A, Bigio EH, Weintraub S, Mesulam M, Hatanpaa KJ, White CL, 3rd, Neumann M, Strong MJ, Beach TG, Wszolek ZK, Lippa C, Caselli R, Petrucelli L, Josephs KA, Parisi JE, Knopman DS, Petersen RC, Mackenzie IR, Seeley WW, Grinberg LT, Miller BL, Boylan KB, Graff-Radford NR, Boeve BF, Dickson DW, Rademakers R. Ataxin-2 as potential disease modifier in C9ORF72 expansion carriers. Neurobiol. Aging. 2014;35:2421.e13–2421.e17. doi: 10.1016/j.neurobiolaging.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel DM, Harschnitz O, van den Berg LH, Pasterkamp RJ. Taking a risk: a therapeutic focus on ataxin-2 in amyotrophic lateral sclerosis? Trends Mol. Med. 2014;20:25–35. doi: 10.1016/j.molmed.2013.09.001. [DOI] [PubMed] [Google Scholar]