Summary
Langerhans cell histiocytosis (LCH), the most common histiocytic disorder, is characterized by the accumulation of CD1A+/CD207+ mononuclear phagocytes within granulomatous lesions that can affect nearly all organ systems. Historically, LCH has been presumed to arise from transformed or pathologically activated epidermal dendritic cells called Langerhans cells. However, new evidence supports a model in which LCH occurs as a consequence of a misguided differentiation programme of myeloid dendritic cell precursors. Genetic, molecular and functional data implicate activation of the ERK signalling pathway at critical stages in myeloid differentiation as an essential and universal driver of LCH pathology. Based on these findings, we propose that LCH should be re-defined as an inflammatory myeloid neoplasia. Increased understanding of LCH pathogenesis will provide opportunities to optimize and personalize therapy through improved risk-stratification, targeted therapy and assessment of therapy response based on specific molecular features and origin of the pathological myeloid cells.
Keywords: Langerhans cell histiocytosis, BRAF V600E, extra-cellular signal-regulated kinase signalling pathway, misguided myeloid differentiation, inflammatory myeloid neoplasia
Clinical overview
Histiocytic disorders include diseases involving aberrant ‘histiocytes’. While histiocyte translates to ‘tissue cell’, histiocytic disorders include all diseases associated with cells of the mononuclear phagocyte system, which is comprised of haematopoietic cells with mononuclear morphology and phagocytic activity (van Furth & Cohn, 1968; Favara et al, 1997). Langerhans cell histiocytosis (LCH) is the most common histiocytic disorder, arising in approximately five children per million, similar in frequency to paediatric Hodgkin lymphoma and acute myeloid leukaemia (AML) (Guyot-Goubin et al, 2008; Stalemark et al, 2008; Salotti et al, 2009). The median age of presentation is 30 months, though LCH is reported in adults in approximately one adult per million, both as unrecognized chronic paediatric disease and de novo disease (Baumgartner et al, 1997). There are occasional reports of affected non-twin siblings and multiple cases in one family, though it is not clear if this is significantly more frequent than one would expect by chance (Arico et al, 2005).
The clinical presentations of LCH vary from clinically trivial lesions that resolve spontaneously or with curettage to life-threatening disseminated disease that requires systemic chemotherapy and, in some cases, haematopoietic stem cell transplantation. The wide spectrum of clinical manifestations that overlap with more common paediatric conditions make diagnosis of LCH challenging. In an institutional study, we found that the time from clinical symptoms of skin LCH lesions to diagnostic biopsy was a median of 3 months, and in some cases diagnosis was delayed by over 5 years (Simko et al, 2014a). However, once biopsy is performed, diagnosis of LCH is straightforward: LCH is defined by CD1A+/CD207+ histiocytes with an inflammatory background, including variable numbers of lymphocytes (enriched for regulatory T cells), macrophages and eosinophils (Favara et al, 1997; Chikwava & Jaffe, 2004; Senechal et al, 2007).
Traditionally, therapy for patients with LCH is determined by the extent and location of the lesions. The optimal therapeutic approaches for LCH remain incompletely defined as patients with skin-limited lesions, single-bone lesions and isolated diabetes insipidus have not been studied on prospective clinical trials. Standard of care for LCH has evolved from serial clinical trials based on empirically-derived therapeutic strategies. Patients with liver, bone marrow and/or spleen involvement are at highest risk for mortality. In the most recent Histiocyte Society trial, LCHIII, 5-year overall survival for patients with risk-organ disease was 84%, compared to 99% for patients without risk-organ disease (Gadner et al, 2013). The current standard of care, based on LCHIII, is 1 year of therapy with vinblastine and prednisone, plus mercaptopurine for patients with high-risk disease. Response to initial therapy is also an important prognostic factor for patients with high-risk LCH: Survival was 95% for patients with good response after the first 6 weeks of treatment on LCHIII, 83% for patients with intermediate response and 57% for patients who progressed on therapy (Gadner et al, 2013).
Langerhans cell histiocytosis may involve the central nervous system (CNS) with mass lesions (brain tumours), pituitary lesions resulting in diabetes insipidus (DI) and/or a neurodegenerative syndrome. A large international series observed that risk of development of DI was decreased in patients with bone lesions in mastoid, temporal, orbital and skull base who received systemic chemotherapy (Grois et al, 2006). Therefore, systemic therapy is now considered standard of care for patients with ‘CNS risk’ skull and pituitary lesions. A devastating consequence of LCH is development of a progressive CNS neurodegenerative syndrome that may develop years after the apparent resolution of LCH (Grois et al, 2010). Reports of treatment for the CNS neurodegenerative syndrome have been limited to case series and pilot studies, and include corticosteroids, cladribine, all-trans retinoic acid, intravenous immunogobulin and cytarabine (Idbaih et al, 2004; Dhall et al, 2008; Imashuku et al, 2008; Allen et al, 2010a).
While mortality in patients with high-risk LCH is improving, and survival is near universal in patients with low-risk LCH, ‘reactivation’ (a term for relapse intended to remain neutral on LCH as immune versus a malignant disorder) remains a significant problem. Over half of all patients are refractory to vinblastine/prednisone or develop recurrent lesions (Minkov et al, 2008; Gadner et al, 2013). Optimal ‘salvage’ strategies have not been defined. In a prospective Histiocyte Society trial, cladribine (5 mg/m2 daily × 5 days per month) had high response rates, but rarely resulted in cure in a prospective trial (Weitzman et al, 2009). A much more intense strategy with cladribine (9 mg/m2) and cytarabine (1 g/m2 daily × 5 days per month) was tested in a small trial and resulted in a high rate of cure, but also a very high rate of treatment-related mortality (Bernard et al, 2005). Clinical series suggest that clofarabine, when dosed ~50% below the maximum tolerated dose, used in AML and other paediatric malignancies (25–52 mg/m2/day × 5 days per month) may be effective with modest toxicity in patients with disease refractory to other salvage regiments (Rodriguez-Galindo et al, 2008; Abraham et al, 2013; Simko et al, 2014b). This efficacy might further be improved by higher doses of clofarabine in refractory cases, but clinical experiences are limited to single cases (Simko et al, 2014b).
Permanent consequences and late effects of disease as well as therapy remain significant challenges, arising in the majority of patients with LCH. The risk of developing complications is related to extent of disease at presentation as well as the duration of active, uncontrolled disease (Willis et al, 1996; Haupt et al, 2013). Complications include endocrinopathies (posterior and anterior pituitary), neurodegenerative syndrome, decreased pulmonary function, orthopaedic problems and liver failure due to progressive sclerosing cholangitis.
Historical concepts of LCH pathogenesis (1900s–2010)
Langerhans cell histiocytosis has suffered from several historical identity crises, many of which persist today. We hypothesize that difficulty in defining the biology of LCH relative to other neoplastic disorders has left it (and patients with LCH) on the sidelines of scientific and haematology/oncology clinical research cooperative group agendas.
Evolution of the LCH brand
The first reports of patients now recognized as having LCH were reported in the early 1900s, describing children with bone lesions, mucosal lesions and diabetes insipidus (Hand-Schüller-Christian disease) or infants with hepatosplenomegaly and histiocytic infiltration of the bone marrow (Letterer-Siwe disease) (Arceci, 1999). Hashimoto-Pritzker syndrome describes spontaneously-resolving skin lesions in infants (Hashimoto & Pritzker, 1973). ‘Eosinophilic granuloma’ is also a frequently used nomenclature for bone LCH. Despite the spectrum of clinical presentations, the histological appearance of an LCH lesion is relatively consistent. In 1953, Dr. Lichtenstein proposed that the various clinical conditions with the shared histopathology probably represent a common condition, which he proposed to be collectively named ‘Histiocytosis X’, with the ‘X’ as an indication of incomplete understanding of the cell of origin (Lichtenstein, 1953). Twenty years later, the Birbeck granule, a cytoplasmic structure associated with langerin (CD207), thought to have some function in antigen processing, was discovered by electron microscopy in the pathological mononuclear phagocytic cells of LCH lesions. As epidermal Langerhans cells (LCs) were the only cells at that time known to contain Birbeck granules, ‘Histiocytosis X’ was hypothesized to arise from epidermal LCs, and has since been rebranded as ‘Langerhans cell histiocytosis’ (Nezelof et al, 1973). For the next four decades, models of LCH were primarily based on the assumption that LCH arises from epidermal LC, with the ongoing question: Does LCH arise from a pathological activation or neoplastic transformation of the epidermal LC?
Inflammation/immune dysregulation in LCH
Langerhans cell histiocytosis lesions have a median of only 8% pathological ‘LCs’ (Berres et al, 2014). The remainder of the lesion is composed of a diverse inflammatory infiltrate. The impressively inflammatory character of LCH lesions led to the investigation of infection and immune dysregulation as mechanisms of pathogenesis. Many viruses have been hypothesized to play roles in LCH pathogenesis, but none have been convincingly validated (Jeziorski et al, 2008). Most recently, an association with the relatively ubiquitous Merckel cell polyomavirus and LCH has been reported (Murakami et al, 2014), however the significance of this observation remains uncertain.
Immunologically, the lesional cells share some features with resting epidermal LCs, including high levels of CD207+ and CD1A+ expression, but also possess features of activated LCs, including expression of T cell co-stimulatory molecules and pro-inflammatory cytokines, creating a ‘cytokine storm’ (Geissmann et al, 2001; Laman et al, 2003; Allen et al, 2010b). Interestingly, CD4+ CD25+ CTLA4+ regulatory T cells are enriched in LCH lesions, and patients with LCH may have impaired skin delayed-type hypersensitivity responses (Senechal et al, 2007; Allen et al, 2010b). Moreover, a causal role for LCH pathogenesis from elevated IL17a expression by LCH cells has also been proposed, but these results have not been confirmed by other groups (Coury et al, 2008; Allen & McClain, 2009; Peters et al, 2011; Makras et al, 2012).
Despite the inflammatory character of LCH lesions, infectious or autoimmune causes for LCH pathogenesis remain to be proven. Furthermore, the contributions of inflammation to clinical manifestations of LCH remain to be defined.
LCH as a neoplastic disorder
The alternative model to LCH pathogenesis caused by dysfunctional immune activation has traditionally been neoplastic transformation of epidermal LCs (Nezelof et al, 1973). Langerhans cell histiocytosis lesions do have many classical features of malignancy (Hanahan & Weinberg, 2011). Based on non-random X-inactivation, the CD1A+ cells of LCH lesions were determined to be clonal (Willman et al, 1994; Yu et al, 1994). Moreover, lesions display malignancy-associated mechanisms, e.g., immune evasion with enriched regulatory T cell populations, tumour-promoting inflammation with increased local and systemic pro-inflammatory cytokines, expression of metalloproteases potentially promoting invasion and metastasis (Hayashi et al, 1997; da Costa et al, 2005; Allen et al, 2010b) and overexpression of BCL2L1, which may contribute to resistance to cell death (Schouten et al, 2002; Amir & Weintraub, 2008; Allen et al, 2010b). However, despite clonality and other malignant hallmarks, LCH cells are characterized by a ‘benign’ morphology and mitoses are observed at very low and similar rates as in normal epidermal LCs. Clonality is essential for malignancy, but physiological LCs in mice may also have regional clonality, arising from a common precursor (Merad et al, 2002; Waskow et al, 2008). Karyotypes from LCH biopsies are typically normal and gross chromosomal lesions have not been described (da Costa et al, 2009). Langerhans cell histiocytosis cells do not survive long in cell culture, and no successful xenograft model has been reported, further supporting a malignant character of LCH.
Redefining LCH in the molecular era
Somatic gene mutations/MAPK activation in LCH
Analysis of genomic alterations in LCH had traditionally been challenging due to the cellular heterogeneity of the lesions. The progress in sequencing technology amplifying the depth of sequencing coverage by next-generation sequencing techniques recently allowed the identification of a recurrent somatic mutation in the gene encoding for the protein kinase BRAF (Badalian-Very et al, 2010). The high prevalence of the BRAF V600E point mutation in LCH has been validated in several independent cohorts and attributed to the histiocytes within the lesions (Haroche et al, 2012; Sahm et al, 2012; Satoh et al, 2012; Berres et al, 2014). A recent meta review comprising 653 patient samples assessed an overall frequency of 48 5% for the BRAF V600E point mutation in LCH (Bubolz et al, 2014). However, we speculate the actual frequency is higher as methods other than quantitative polymerase chain reaction may be insufficiently sensitive to identify BRAF V600E in cases of lesions with sparse infiltration by pathological LCH cells.
BRAF is a central kinase of the RAS/RAF/MEK pathway, which is essentially involved in numerous cell functions including cell proliferation and migration and is frequently mutated in various cancer cells (Davies et al, 2002). The BRAF V600E mutation results in a constitutive, RAS-independent activation of the downstream kinases extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase (MAPK)/ERK kinase (MEK) (Maurer et al, 2011). Of note, both downstream kinases are highly activated in LCH cells with BRAF V600E mutation, supporting the potential functional relevance of the mutation in LCH (Badalian-Very et al, 2010; Chakraborty et al, 2014). Interestingly, ERK signalling has been highly implicated in myeloid cell differentiation and maturation under physiological conditions. While hyperphosphorylation of ERK drives differentiation of dendritic cell (DC) progenitors (Miranda et al, 2005; Hamdorf et al, 2011), sustained phosphorylation inhibits DC maturation upon stimulation with Toll-like receptor ligands or tumour necrosis factor, as recently demonstrated (Puig-Kroger et al, 2001; Aguilera-Montilla et al, 2013). This role of ERK activation in myeloid cell differentiation and maturation during homeostatic conditions might also have valuable implications on the origin and functional alterations of histiocytes in LCH.
Besides the frequent BRAF V600E mutation, single case reports have described additional mutations/polymorphisms within the BRAF gene locus with potential functional consequences, including the somatic mutations BRAF V600D, BRAF 600DLAT and the germline mutation/poylmorphism BRAF T599A (Satoh et al, 2012; Kansal et al, 2013). Moreover, a complex compound somatic mutation in ARAF with enhanced kinase activity in vitro has also been described in a single patient (Nelson et al, 2014). However, extended studies are necessary to elucidate the frequency and significance of these additional RAF mutations in LCH.
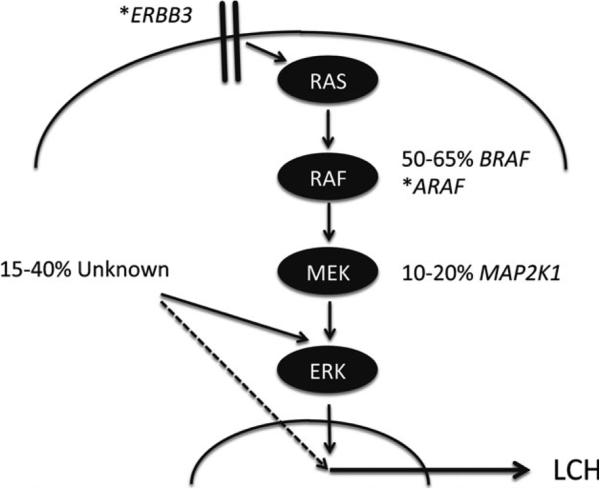
Using whole exome sequencing on matched LCH lesions and peripheral blood tissue samples obtained from 41 patients, we recently set out to analyse the broader genomic landscape of LCH. The overall rate of somatic mutations was remarkably low, at 0·03 mutations per Mb, or a median of 1 somatic mutation within exons per patient. In this study, we identified a second recurrent mutated gene locus in LCH, MAP2K1 (encoding mitogen-activated protein kinase kinase 1 [MAP2K1, alternatively termed MEK1], also a member of the RAS/RAF/ERK signalling pathway) with a frequency of 33% in lesions with wild-type BRAF (Chakraborty et al, 2014). A second study identified mutations through targeted sequencing of the second and third exons of MAP2K1, in 50% of wild-type BRAF cases (Brown et al, 2014). Interestingly, both independent studies could only identify mutually exclusive mutation of BRAF and MAP2K1. Functionally, all mutations described within the MAP2K1 gene resulted in the expression of a hyperactive kinase and subsequent constitutive phosphorylation of the downstream targets MAPK3 (also termed ERK1) and MAPK1 (also termed ERK2) in vitro comparable to the effects observed by the expression of BRAF V600E (Chakraborty et al, 2014). In addition to recurrent somatic mutations in BRAF and MAP2K1, we observed single cases of mutations of other genes that transcribe MAPK pathway proteins, specifically in ERBB3 and again within the ARAF locus (Chakraborty et al, 2014) in our study, though their frequency and functional significance remains to be proven in larger series (Fig 1).
Fig 1.
Routes to ERK activation in LCH. Model of MAPK pathway activation resulting from serial phosphorylation from cellular receptors through RAS, RAF, MEK and, ultimately, ERK. Estimates of frequency of somatic mutations of BRAF and MAP2K1 are illustrated. (*) indicates genes with individual case reports of somatic mutations. ‘Unknown’ indicates ERK activation by mechanisms that have not yet been defined. While activated ERK has been identified in all lesions studied to date, there remains the possibility (dashed line) that Langerhans cell histiocytosis (LCH) may arise from alternative mechanisms in some cases.
In addition to genetic analysis, imaging flow cytometry and Western blotting was used to analyse activation of MEK and ERK proteins in CD207+ cells from LCH lesions. MAP2K1 was highly phosphorylated in samples with BRAF V600E and MAP2K1 mutations. However, MAP2K1 phosphorylation was minimal in cases with no detectable mutation in the MAPK pathway. In every case tested, MAPK3/MAPK1 was highly phosphorylated independent of the mutation or MAP2K1 phosphorylation status (Chakraborty et al, 2014). Similarly, the original BRAF V600E report by Badalian-Very et al (2010) also demonstrated ERK activation in BRAF wild-type lesions by immunohistochemistry. These reports support a common critical role of hyperactivation of the ERK signalling pathway in LCH pathology independent of the specific underlying mutation. Deciphering the mechanisms that drive ERK activation in the cases with undiscovered MAPK pathway mutations will require extended studies including complimentary methods (e.g. whole genome sequencing, RNA sequencing and assessment of epigenetic modifications) to fully assess the causative spectrum of ERK activation in LCH.
Activation of ERK may be universal to LCH pathogenesis, but like other diseases driven by MAPK pathway hyperactivity, it is likely that specific somatic mutations will be associated with certain unique clinical features. Although genotype-based risk assessment will require prospective studies in several independent cohorts, data so far suggest that the genotype does not correlate with extent of disease (high versus low-risk) or survival (Badalian-Very et al, 2010; Berres et al, 2014; Bubolz et al, 2014; Chakraborty et al, 2014). However, in our institutional series, the BRAF V600E mutation status correlated significantly with increased risk of initial treatment failure e.g. refractory disease or recurrence (Berres et al, 2014). In addition to the potential relevance of the genotype for clinical risk stratification, it might also have therapeutic implications given that mutations within the MAPK pathway predicted response to specific MAPK pathway inhibitors in vitro. While CD207+ cells with BRAF V600E and MAP2K1 mutation had predictable responses to BRAF and MEK inhibition, responses of BRAF wild-type/MAP2K1 wild-type lesional cells were highly variable (Chakraborty et al, 2014). Therefore, assessment of the mutation status will probably become an important clinical feature of LCH therapy to personalize and optimize therapeutic regimens.
LCH as an inflammatory myeloid neoplasia
The identification of the frequent BRAF V600E mutation in 2010 tipped the scales of the historical LCH debate to favour the classification of LCH as a neoplastic disorder. The mutation can be detected in various neoplastic diseases and seems to play a pivotal role in their pathology. The impact of BRAF V600E appears thereby to depend on the cellular context as it is frequently observed in more benign conditions, such as epidermal nevi and colon polyps, as well as in highly aggressive malignancies, such as malignant melanoma (Cantwell-Dorris et al, 2011; Pratilas et al, 2012).
Discovery of BRAF V600E within the lesions provided an important foothold from which to understand pathogenesis of LCH, however, the specific impact of BRAF V600E on LCH pathogenesis remained elusive. To analyse the potential function of BRAF V600E mutation as an essential driver of LCH pathology, we recently crossed mice expressing the conditional BRAF V600E allele with transgenic mice expressing the cre-recombinase under control of the langerin promoter to enforce expression of BRAF V600E in langerin-expressing DC (differentiated epidermal LCs and langerin-expressing lymphoid and non-lymphoid DCs). To determine the significance of the stage during DC differentation at which the somatic mutation occurs, we also crossed mice with the conditional BRAF V600E allele to mice expressing cre-recombinase under the control of CD11c, which is already expressed in DC progenitors and immature DCs (for further details please see (Berres et al, 2014). Mice of both strains spontaneously developed classical granuloma-like lesions characterized by the accumulation of CD207+ histiocytes by 2–6 months of age. The accumulation of histiocytes was associated with increased expression of specific cytokines and chemokines and the recruitment of additional inflammatory cell e.g. macrophages, multi-nucleated giant cells, B cells and T cells (including T regulatory cells), comparable to human LCH lesions. Interestingly, induction of BRAF V600E expression in circulating blood and bone marrow resident DC progenitors using the CD11c promoter system resulted in a much more severe and accelerated course, approximating the human high-risk LCH phenotype. Taken together, these results provide evidence that expression of oncogenic BRAF V600E in cells within the mononuclear phagocytes system is sufficient to induce LCH-like disease in mice, leading not only to the accumulation of CD207+ histiocytes, but also to the recruitment of additional inflammatory cells and subsequent formation of granuloma with highly active cytokine expression. These data support the ability of MAPK activation in myeloid DC precursors to drive myeloid differentiation and granuloma formation in mice, similar to pathology observed in LCH. We further propose that LCH should be defined as an inflammatory myeloid neoplasia characterized by the formation of highly active, inflamma-tory granuloma induced by transformed DC clones.
LCH as consequence of misguided myeloid differentiation
Revisiting the cell of origin
As discussed above, it has been assumed that aberrant or transformed epidermal LCs embody the cellular origin of the histiocytes in LCH, which was mainly based on certain structural similarities of both cells types, including intracytoplasmatic Birbeck granules and especially the common expression of the antigen CD1A and the C-type lectin langerin (CD207) (Nezelof et al, 1973). Both features have traditionally been considered to be exclusive to LCs.
Langerhans cells represent a specific subpopulation of cells of the mononuclear phagocyte system residing in the outer layer of the skin, the epidermis. They are characterized by lower major histocompatibility complex (MHC)-II expression levels, intermediate CD11c levels as compared to other DCs and high expression of langerin (Merad et al, 2008). LCs arise from embryonic precursors – mostly fetal liver-derived monocytes (Hoeffel et al, 2012) that populate the skin prior to birth and maintain themselves locally throughout life in steady state conditions (Merad et al, 2002). Following severe inflammatory injury, such as ultraviolet (UV) light exposure, a transient population of LCs can be identified in the skin that derive from circulating Gr-1hi blood monocytes (Ginhoux et al, 2006; Sere et al, 2012). However, it remains unclear if these inflammation-associated, monocyte-derived LCs residing in the epidermis after UV light exposure are equivalent to steady-state LCs in terms of function and tissue homeostasis.
In contrast to a primary embryonic origin of LCs, most other classical DCs residing in lymphoid as well as the non-lymphoid tissue arise from common circulating precursors–the pre-DC (Naik et al, 2007; Onai et al, 2007; Liu et al, 2009). Pre-DC share a common progenitor with plasmocytoid DCs (pDC), which is thus termed common DC precursor or CDP. CDPs are localized in the bone marrow and originate from a multipotent bone marrow resident myeloid precursor named macrophage-DC progenitor (MDP), which gives rise to monocytes and DC but has lost granulocyte potential as compared to the granulocyte-monocyte progenitor (GMP) and thereby marks the stage of commitment to the mononuclear phagocyte systems during development (reviewed in Merad et al, 2013).
The concept that LCH cells represent transformed cells of LC origin has been recently challenged by the observation that the expression of langerin (CD207) and the formation of Birbeck granules is not exclusive to LC but can also be identified in other subpopulations of the mononuclear phagocyte system. These CD207+ DCs constitutively reside in all lymphoid and non-lymphoid tissues where LCH lesions can be found in patients (Chikwava & Jaffe, 2004; Ginhoux et al, 2007; Poulin et al, 2007; Segerer et al, 2008; Helft et al, 2010). This is in striking contrast to the restricted tropism of LCs to the epidermis and skin-draining lymph nodes. Gene expression profiling studies demonstrated that human CD207+ DCs within LCH lesions only display a minimal profile overlap with differentiated human LCs, while their profile is more consistent with those of immature myeloid DC precursors (Allen et al, 2010b). Additionally, the maturation status of LCH cells within the lesions is heterogenous with variable CD1A+/CD207− subpopulations (Chikwava & Jaffe, 2004; Coury et al, 2008; Peters et al, 2011) and extended immunohistological studies were able to identified expression of the common BRAF V600E mutation not only in CD207+ cells but also in CD207− cells as well as in CD14+ monocytes (Sahm et al, 2012).
These new findings open up the possibility that LCH might not, as tradionally speculated, result from the accumulation of dysregulated or transformed mature fetal liver-derived epidermal LCs, but may rather arise from dysregulated differentiation and/or recruitment of precursor cells from the bone marrow-derived myeloid lineage. A dys-regulated differentiation of early multipotent myeloid progenitors as origin of the disease is also consistent with reports of concurrent or serial lesions of more than one histiocytic phenotype in individual cases with overlapping features of LCH, juvenile xanthogranuloma (JXG) and Erdheim-Chester disease (ECD) (Hoeger et al, 2001; Patrizi et al, 2004; Tsai et al, 2010; Haroche et al, 2013; Berres et al, 2014; Chakraborty et al, 2014; Hervier et al, 2014).
Misguided myeloid differentiation translate into disease severity and dissemination
Langerhans cell histiocytosis is characterized by a broad spectrum of clinical presentations ranging from single lesions to multiple lesions within a single organ system to multisystemic disease. As discussed above, the involvement of liver, spleen and bone marrow is associated with higher risk of mortality. Histologically, lesions of high risk patients are indistinguishable from those with low risk disease. Additionally, with the exception of disease recurrence after therapy, all studies fail to correlate the presence of certain mutation with disease outcome or stage.
So, which mechanisms regulate disease severity and tropism in LCH? Assessing the BRAF V600E mutational status in circulating cells of patients with LCH, we recently observed that in patients with high risk multi-system disease and BRAF V600E mutation in lesional DCs, BRAF V600E was also identified in circulating cells and resident bone marrow cells. Contrarily, circulating cells with BRAF V600E mutation were absent in the blood and bone marrow of all patients with single-system low risk disease (Berres et al, 2014). Lineage analysis further localized the BRAF V600E mutation to CD11c+ DC as well as CD14+ monocytes in high-risk patients. These results point to a somatic mutation of a specific common DC and monocyte progenitor in these cases. Indeed, when bone marrow aspirates of patients with circulating BRAF V600E positive cells were analysed, cells carrying the mutation were identified within the CD34+ haematopoietic stem cell (HSC) compartment, and clonal potential for BRAF V600E positive cells was further confirmed by colony-forming-unit assays in vitro. Interestingly, in single cases where BRAF V600E was identified in CD34+ cells, it was also detectable in the CD19+ B cells as well as the CD11c+ and CD14+ fractions, but was absent from CD3+ T cells (Berres et al, 2014).
The association of disease severity with detection of BRAF V600E in HSC in the bone marrow is in line with the murine data presented above showing that expression of the mutation already at the stage of bone marrow resident DC progenitors resulted in an aggravated and accelerated phenotype with increased mortality as compared to langerin-driven expression, which is limited to the stage of differentiated DC (Berres et al, 2014).
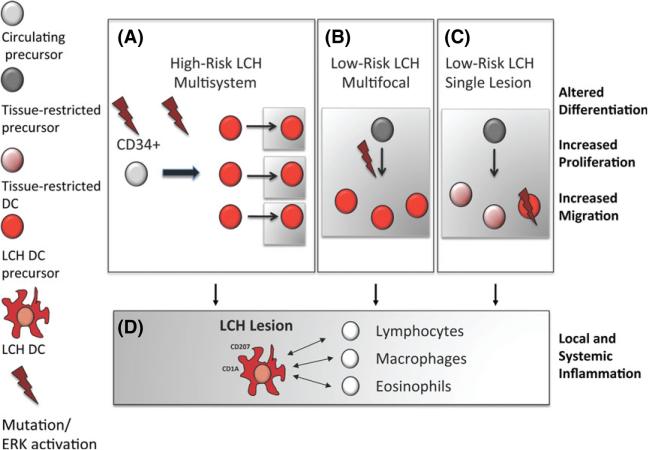
We propose that the misguided myeloid differentiation model of LCH pathogenesis would account for the range of clinical manifestations in LCH. In this model, the extent of disease severity and dissemination is not defined by genomic or functional alteration at the histiocyte level but by the stage at which pathological ERK activation occurs during myeloid differentiation (Fig 2). Based on the data discussed above, we hypothesize that if the mutation occurs at the stage of a tissue-restricted precursor or even tissue-resident precursor/DC, disease will be limited to specific organs (multisystem low-risk) or even a single location (single lesion low risk). On the contrary, mutations impacting early myeloid progenitors with high proliferative capacity and multipotent potential, the disease will be highly aggressive and disseminate to various organs resulting in high-risk multisystemic disease. In all cases, the pathological DC seed to the tissue and follow a common path of terminal differentiation in which they acquire the expression of CD207 and CD1A and recruit and activate additional inflammatory cells, e.g., macrophages, eosinophils, B cells and T cells, to the lesion, mediated by altered cytokine/chemokines expression. This will then result in the formation of complex LCH granuloma. However, the relative contributions of these recruited ‘innocent bystander’ cells to LCH pathogenesis are uncertain.
Fig 2.
Developmental stage of pathological DC precursor defines extent of disease. (A) Somatic mutation of BRAF or other inciting event in CD34+ haematopoietic stem cells or early DC progenitors induces proliferation, maturation and migration of pathological DCs in multiple tissues that results in high-risk multisystem LCH. (B) Somatic mutation of BRAF or other inciting event in a tissue-restricted DC precursor induces proliferation, maturation and tissue-limited migration of pathological DCs that results in low-risk multi-system LCH. (C) Somatic mutation of BRAF or other inciting event in mature DC results in proliferation and maturation of pathological DCs leading to low-risk single lesion LCH. (D) Regardless of cell of origin, the pathological DCs recruit ‘bystander’ immune cells in an inflammatory lesion characteristic of LCH. Cells in white areas indicate cells in circulation; cells in grey areas indicate cells that have migrated to tissue targets. LCH, Langerhans Cell Histiocytosis; DC, dendritic cell.
While ERK activiation appears to be universal in LCH, the mechanisms through which ERK activation drives pathogenesis in myeloid precursors in LCH remain to be defined. Interestingly, BRAF V600E has been identified in CD34+ haematopoeitic cells in patients with hairy cell leukaemia (Chung et al, 2014). Similarly, some patients display the BRAF V600E mutation in hybrid, synchronous or serial LCH/JXG or LCH/ECD lesions (Berres et al, 2014; Hervier et al, 2014). Furthermore, mutations in NRAS, upstream from RAF, have been described in ECD, but not LCH (Fig 1) (Diamond et al, 2013; Emile et al, 2014). The terminal phenotype of cells in which ERK is pathologically activated may depend on the state of differentiation of the original cell, the somatic mutation, or additional genetic or epigenetic factors that remain to be defined.
Clinical Implications of the misguided myeloid differentiation model
If future studies support the early observation that BRAF V600E confers increased risk of refractory/recurrent disease, these patients may benefit from prolonged or intensified chemotherapy. Furthermore, once the genetic lesion is established, the presence of circulating cells that carry the mutation may become a valuable diagnostic tool to define high versus low-risk disease. Finally, in patients in whom circulating cells bearing the somatic mutation are identified, persistence of circulating cells with the mutation may be a marker with which to follow residual disease (Berres et al, 2014).
While the Histiocyte Society and other cooperative group trials have contributed tremendously to the understanding and treatment of LCH, overall outcomes remain suboptimal. Due to the scientific progress on LCH pathology in the last years, we now have the opportunity to move beyond empiricism to rationale strategies. The relative efficacy of the second generation nucleoside analogue, clofarabine, in patients refractory to other therapies may be consistent with its known activity against immature myeloid cells in AML and myelodysplastic syndrome (Ghanem et al, 2013).
In addition to refining the therapeutic profile of chemotherapy strategies, recurrent somatic mutations in LCH offer an opportunity to develop targeted therapeutic strategies. Mutations in the MAPK pathway have been identified in approximately 75% of patients with LCH, and drugs that specifically inhibit kinase activity of RAF, MEK and ERK proteins have been developed and are in various stages of therapeutic evaluation for paediatric patients (Belden & Flaherty, 2012). Unlike melanoma or other aggressive malignancies associated with MAPK activation, the overall genomic landscape of LCH lesions is relatively quiet, which may make the efficiacy of these agents more durable in LCH than in tumours that can rapidly develop resistance through multiple mechanisms including acquisition of new mutations (Poulikakos & Rosen, 2011; van Allen et al, 2014). An early report of vemurafanib, which has activity against BRAFV 600E, in patients with mixed ECD and LCH, demonstrated promising early responses (Haroche et al, 2013). However, the side effect profile of BRAF inhibitors, which includes high rates of de novo squamous cell carcinoma among other toxicities, makes the the risk-benefit profile of these agents in LCH uncertain relative to rapidly fatal BRAF V600E-associated malignancies such as melanoma. Dabrafenib, another agent with activity against activated BRAF, is being evaluated in a paediatric phase I/II study that includes patients with LCH (clinicaltrials.gov NCT01677741). Efficacy, tolerability and optimal role for targeted therapies along with or instead of chemotherapeutic agents will be defined in future clinical trials.
Conclusions
Langerhans cell histiocytosis is a disease caused by somatic driver mutations at critical stages of myeloid differentiation that result in cellular transformation. An important implication of the new understanding of LCH biology is the re-definition of LCH as an inflammatory myeloid neoplasia. Like other neoplastic myeloid disorders, we believe LCH should be considered under the umbrella of cancer research. Patients with LCH will ultimately benefit from inclusion in portfolios of cooperative cancer networks to be considered for research funding and clinical trial support. The recent advances in the understanding of LCH pathogenesis also question if ‘Langerhans cell histiocytosis’ is the appropriate nomenclature, given that pathological lesional cells arise from variable points along the myeloid/monocytic lineage. ‘Histiocytosis X’ may be more accurate after all. While ERK activation appears to be universal in LCH, pathways of ERK activation and cellular context in which pathological ERK activation arises are variable. Increased understanding of LCH pathogenesis will provide opportunities to optimize and personalize therapy through improved risk-stratification, targeted therapy and assessment of therapy response based on specific molecular features and origin of the pathological cells.
Acknowledgement
MLB is supported in part by grants of the German Research Association (Deutsche Forschungsgemeinschaft, BE 4818/1-1, SFB TRR57 P07). CEA is supported in part by the National Institutes of Health (NIH), R01 grant CA154489. MM is supported by NIH grant CA154947A. CEA and MM are also supported by the North American Consortium for Histiocytosis Research (St. Baldrick's Foundation).
Footnotes
Author contributions
All author's wrote substantial parts of the manuscript and revised all final versions.
All authors have no financial conflicts to disclose.
References
- Abraham A, Alsultan A, Jeng M, Rodriguez-Galindo C, Campbell PK. Clofarabine salvage therapy for refractory high-risk langer-hans cell histiocytosis. Pediatric Blood & Cancer. 2013;60:E19–E22. doi: 10.1002/pbc.24436. [DOI] [PubMed] [Google Scholar]
- Aguilera-Montilla N, Chamorro S, Nieto C, Sanchez-Cabo F, Dopazo A, Fernandez-Salguero PM, Rodriguez-Fernandez JL, Pello OM, Andres V, Cuenda A, Alonso B, Dominguez-Soto A, Sanchez-Ramon S, Corbi AL. Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood. 2013;121:e108–e117. doi: 10.1182/blood-2012-07-445106. [DOI] [PubMed] [Google Scholar]
- Allen CE, McClain KL. Interleukin-17A is not expressed by CD207(+) cells in Langerhans cell histiocytosis lesions. Nature Medicine. 2009;15:483–484. doi: 10.1038/nm0509-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CE, Flores R, Rauch R, Dauser R, Murray JC, Puccetti D, Hsu DA, Sondel P, Hetherington M, Goldman S, McClain KL. Neurodegenerative central nervous system Langer-hans cell histiocytosis and coincident hydrocephalus treated with vincristine/cytosine arabinoside. Pediatric Blood & Cancer. 2010a;54:416–423. doi: 10.1002/pbc.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, Merad M, McClain KL. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. Journal of Immunology. 2010b;184:4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, Place CS, Taylor-Weiner A, Whittaker S, Kryukov GV, Hodis E, Rosenberg M, McKenna A, Cibulskis K, Farlow D, Zimmer L, Hillen U, Gutzmer R, Goldinger SM, Ugurel S, Gogas HJ, Egberts F, Berking C, Trefzer U, Loquai C, Weide B, Hassel JC, Gabriel SB, Carter SL, Getz G, Garraway LA, Schadendorf D, Dermatologic Cooperative Oncology Group of, G. The genetic landscape of clinical resistance to RAF inhibition in meta-static melanoma. Cancer Discovery. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatric Blood & Cancer. 2008;50:304–307. doi: 10.1002/pbc.21198. [DOI] [PubMed] [Google Scholar]
- Arceci RJ. The histiocytoses: the fall of the Tower of Babel. European Journal of Cancer. 1999;35:747–767. doi: 10.1016/s0959-8049(99)00039-8. [DOI] [PubMed] [Google Scholar]
- Arico M, Scappatici S, Danesino C. The genetics of Langerhans cell histiocytosis. In: Weitzman S, Egeler RM, editors. Histiocytic Disorders of Children and Adults: Basic Science, Clinical Features and Therapy. Cambridge Press; Cambridge: 2005. pp. 83–94. [Google Scholar]
- Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Medical and Pediatric Oncology. 1997;28:9–14. doi: 10.1002/(sici)1096-911x(199701)28:1<9::aid-mpo3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Belden S, Flaherty KT. MEK and RAF inhibitors for BRAF-mutated cancers. Expert Reviews in Molecular Medicine. 2012;14:e17. doi: 10.1017/erm.2012.11. [DOI] [PubMed] [Google Scholar]
- Bernard F, Thomas C, Bertrand Y, Munzer M, Landman Parker J, Ouache M, Colin VM, Perel Y, Chastagner P, Vermylen C, Donadieu J. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langer-hans cell histiocytosis with haematological dys-function. European Journal of Cancer. 2005;41:2682–2689. doi: 10.1016/j.ejca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Berres ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, Idoyaga J, Ruzo A, Lupo PJ, Hicks MJ, Shih A, Simko SJ, Abhyankar H, Chakraborty R, Leboeuf M, Beltrao M, Lira SA, Heym KM, Bigley V, Collin M, Manz MG, McClain K, Merad M, Allen CE. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. The Journal of Experimental Medicine. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NA, Furtado LV, Betz BL, Kiel MJ, Weigelin HC, Lim MS, Elenitoba-Johnson KS. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124:1655–1658. doi: 10.1182/blood-2014-05-577361. [DOI] [PubMed] [Google Scholar]
- Bubolz AM, Weissinger SE, Stenzinger A, Arndt A, Steinestel K, Bruderlein S, Cario H, Lubatschofski A, Welke C, Anagnostopoulos I, Barth TF, Beer AJ, Moller P, Gottstein M, Viardot A, Lennerz JK. Potential clinical implications of BRAF mutations in histiocytic proliferations. Oncotarget. 2014;5:4060–4070. doi: 10.18632/oncotarget.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcino-genesis and molecular therapy. Molecular Cancer Therapeutics. 2011;10:385–394. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Hampton OA, Shen X, Simko S, Shih A, Abhyankar H, Lim KP, Covington K, Trevino L, Dewal N, Muzny DM, Doddapaneni H, Hu J, Wang L, Lupo PJ, Hicks MJ, Bonilla DL, Dwyer KC, Berres ML, Poulikakos PI, Merad M, McClain KL, Wheeler DA, Allen CE, Parsons DW. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124:3007–3015. doi: 10.1182/blood-2014-05-577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikwava K, Jaffe R. Langerin (CD207) staining in normal pediatric tissues, reactive lymph nodes, and childhood histiocytic disorders. Pediatric and Developmental Pathology. 2004;7:607–614. doi: 10.1007/s10024-004-3027-z. [DOI] [PubMed] [Google Scholar]
- Chung SS, Kim E, Park JH, Chung YR, Lito P, Teruya-Feldstein J, Hu W, Beguelin W, Monette S, Duy C, Rampal R, Telis L, Patel M, Kim MK, Huberman K, Bouvier N, Berger MF, Melnick AM, Rosen N, Tallman MS, Park CY, Abdel-Wahab O. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Science Translational Medicine. 2014;6:238ra271. doi: 10.1126/scitranslmed.3008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CE, Annels NE, Faaij CM, Forsyth RG, Hogendoorn PC, Egeler RM. Presence of osteoclast-like multinucleated giant cells in the bone and nonostotic lesions of Langerhans cell histiocytosis. The Journal of Experimental Medicine. 2005;201:687–693. doi: 10.1084/jem.20041785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CE, Szuhai K, van Eijk R, Hooge-boom M, Sciot R, Mertens F, Bjorgvinsdottir H, Debiec-Rychter M, de Krijger RR, Hogendoorn PC, Egeler RM, Annels NE. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes, Chromosomes & Cancer. 2009;48:239–249. doi: 10.1002/gcc.20634. [DOI] [PubMed] [Google Scholar]
- Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, Azocar O, Flacher M, Djebali S, Tebib J, Brytting M, Egeler RM, Rabourdin-Combe C, Henter JI, Arico M, Delprat C. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nature Medicine. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dhall G, Finlay JL, Dunkel IJ, Ettinger LJ, Kellie SJ, Allen JC, Egeler RM, Arceci RJ. Analysis of outcome for patients with mass lesions of the central nervous system due to Langerhans cell histiocytosis treated with 2-chlorodeoxyadenosine. Pediatric Blood & Cancer. 2008;50:72–79. doi: 10.1002/pbc.21225. [DOI] [PubMed] [Google Scholar]
- Diamond EL, Abdel-Wahab O, Pentsova E, Borsu L, Chiu A, Teruya-Feldstein J, Hyman DM, Rosenblum M. Detection of an NRAS mutation in Erdheim-Chester disease. Blood. 2013;122:1089–1091. doi: 10.1182/blood-2013-02-482984. [DOI] [PubMed] [Google Scholar]
- Emile JF, Diamond EL, Helias-Rodzewicz Z, Cohen-Aubart F, Charlotte F, Hyman DM, Kim E, Rampal R, Patel M, Ganzel C, Au-mann S, Faucher G, Le Gall C, Leroy K, Colombat M, Kahn JE, Trad S, Nizard P, Donadieu J, Taly V, Amoura Z, Abdel-Wahab O, Haroche J. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014;124:3016–3019. doi: 10.1182/blood-2014-04-570937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H, Gresik M, Henter JI, Imashuku S, Janka-Schaub G, Jaffe R, Ladisch S, Nezelof C, Pritchard J. Con temporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Medical and Pediatric Oncology. 1997;29:157–166. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. The Journal of Experimental Medicine. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadner H, Minkov M, Grois N, Potschger U, Thiem E, Arico M, Astigarraga I, Braier J, Donadieu J, Henter JI, Janka-Schaub G, McC-lain KL, Weitzman S, Windebank K, Ladisch S, Histiocyte S. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–5014. doi: 10.1182/blood-2012-09-455774. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Lepelletier Y, Fraitag S, Valladeau J, Bodemer C, Debre M, Leborgne M, Saeland S, Brousse N. Differentiation of Langerhans cells in Langerhans cell histiocytosis. Blood. 2001;97:1241–1248. doi: 10.1182/blood.v97.5.1241. [DOI] [PubMed] [Google Scholar]
- Ghanem H, Kantarjian H, Ohanian M, Jabbour E. The role of clofarabine in acute myeloid leukemia. Leukemia & Lymphoma. 2013;54:688–698. doi: 10.3109/10428194.2012.726722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nature Immunology. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. The Journal of Experimental Medicine. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grois N, Potschger U, Prosch H, Minkov M, Arico M, Braier J, Henter JI, Janka-Schaub G, Ladisch S, Ritter J, Steiner M, Unger E, Gadner H, Dalhx, Lch, I. & Committee, I.I.S Risk factors for diabetes insipidus in langerhans cell histiocytosis. Pediatric Blood & Cancer. 2006;46:228–233. doi: 10.1002/pbc.20425. [DOI] [PubMed] [Google Scholar]
- Grois N, Fahrner B, Arceci RJ, Henter JI, McClain K, Lassmann H, Nanduri V, Prosch H, Prayer D. Central nervous system disease in Langerhans cell histiocytosis. Journal of Pediatrics. 2010;156:873–881. 881. doi: 10.1016/j.jpeds.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Guyot-Goubin A, Donadieu J, Barkaoui M, Bellec S, Thomas C, Clavel J. Descriptive epidemiology of childhood Langer-hans cell histiocytosis in France, 2000–2004. Pediatric Blood & Cancer. 2008;51:71–75. doi: 10.1002/pbc.21498. [DOI] [PubMed] [Google Scholar]
- Hamdorf M, Berger A, Schule S, Reinhardt J, Flory E. PKCdelta-induced PU.1 phosphorylation promotes hematopoietic stem cell differentiation to dendritic cells. Stem Cells. 2011;29:297–306. doi: 10.1002/stem.564. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haroche J, Charlotte F, Arnaud L, von Deimling A, Helias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay D, Lesot A, Mokhtari K, Canioni D, Galmiche L, Rose C, Schmalzing M, Croockewit S, Kambouchner M, Copin MC, Fraitag S, Sahm F, Brousse N, Amoura Z, Donadieu J, Emile JF. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- Haroche J, Cohen-Aubart F, Emile JF, Arnaud L, Maksud P, Charlotte F, Cluzel P, Drier A, Hervier B, Benameur N, Besnard S, Donadieu J, Amoura Z. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langer-hans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Pritzker MS. Electron microscopic study of reticulohistiocytoma. An unusual case of congenital, self-healing reticulohistiocytosis. Archives of Dermatology. 1973;107:263–270. [PubMed] [Google Scholar]
- Haupt R, Minkov M, Astigarraga I, Schafer E, Nanduri V, Jubran R, Egeler RM, Janka G, Micic D, Rodriguez-Galindo C, van Gool S, Visser J, Weitzman S, Donadieu J, Network Euro Histio Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatric Blood & Cancer. 2013;60:175–184. doi: 10.1002/pbc.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rush WL, Travis WD, Liotta LA, Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary Langerhans’ cell granulomatosis. Archives of Pathology & Laboratory Medicine. 1997;121:930–937. [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunological Reviews. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Hervier B, Haroche J, Arnaud L, Charlotte F, Donadieu J, Neel A, Lifermann F, Villabona C, Graffin B, Hermine O, Rigolet A, Roubille C, Hachulla E, Carmoi T, Bezier M, Meignin V, Conrad M, Marie L, Kostrzewa E, Michot JM, Barete S, Taly V, Cury K, Emile JF, Amoura Z, French Histiocytoses Study Group Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124:1119–1126. doi: 10.1182/blood-2013-12-543793. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. The Journal of Experimental Medicine. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger PH, Diaz C, Malone M, Pritchard J, Harper JI. Juvenile xanthogranuloma as a sequel to Langerhans cell histiocytosis: a report of three cases. Clinical and Experimental Dermatology. 2001;26:391–394. doi: 10.1046/j.1365-2230.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- Idbaih A, Donadieu J, Barthez MA, Geiss-mann F, Bertrand Y, Hermine O, Brugieres L, Genereau T, Thomas C, Hoang-Xuan K. Retinoic acid therapy in “degenerative-like” neuro-langerhans cell histiocytosis: a prospective pilot study. Pediatric Blood & Cancer. 2004;43:55–58. doi: 10.1002/pbc.20040. [DOI] [PubMed] [Google Scholar]
- Imashuku S, Okazaki N, Nakayama M, Fujita N, Fukuyama T, Koike K, Minato T, Kobayashi R, Morimoto A, Japan LCH Study Group Treatment of neurodegenerative CNS disease in Langerhans cell histiocytosis with a combination of intravenous immunoglobulin and chemotherapy. Pediatric Blood & Cancer. 2008;50:308–311. doi: 10.1002/pbc.21259. [DOI] [PubMed] [Google Scholar]
- Jeziorski E, Senechal B, Molina TJ, Devez F, Leruez-Ville M, Morand P, Glorion C, Mansuy L, Gaudelus J, Debre M, Jaubert F, Seigneurin JM, Thomas C, Joab I, Donadieu J, Geissmann F. Herpes-virus infection in patients with Langerhans cell histiocytosis: a case-controlled sero-epidemiological study, and in situ analysis. PLoS ONE. 2008;3:e3262. doi: 10.1371/journal.pone.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal R, Quintanilla-Martinez L, Datta V, Lopategui J, Garshfield G, Nathwani BN. Identification of the V600D mutation in Exon 15 of the BRAF oncogene in congenital, benign langerhans cell histiocytosis. Genes, Chromosomes & Cancer. 2013;52:99–106. doi: 10.1002/gcc.22010. [DOI] [PubMed] [Google Scholar]
- Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans-cell histiocytosis ‘insight into DC biology’. Trends in Immunology. 2003;24:190–196. doi: 10.1016/s1471-4906(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. Histiocytosis X; integration of eosinophilic granuloma of bone, Letterer-Siwe disease, and Schuller-Christian disease as related manifestations of a single nosologic entity. A.M.A. Archives of Pathology. 1953;56:84–102. [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makras P, Polyzos SA, Anastasilakis AD, Terpos E, Papatheodorou A, Kaltsas GA. Is serum IL-17A a useful systemic bio-marker in patients with Langerhans cell histiocytosis? Molecular Therapy. 2012;20:6–7. doi: 10.1038/mt.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature Immunology. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature Reviews. Immunology. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual Review of Immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkov M, Steiner M, Potschger U, Arico M, Braier J, Donadieu J, Grois N, Henter JI, Janka G, McClain K, Weitzman S, Winde-bank K, Ladisch S, Gadner H. Reactivations in multisystem Langerhans cell histiocytosis: data of the international LCH registry. Journal of Pediatrics. 2008;153:700–705. doi: 10.1016/j.jpeds.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Miranda MB, Xu H, Torchia JA, Johnson DE. Cytokine-induced myeloid differentiation is dependent on activation of the MEK/ ERK pathway. Leukemia Research. 2005;29:1293–1306. doi: 10.1016/j.leukres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Horie Y, Hayashi K, Imamura T, Morimoto A, Imashuku S, Gogusev J, Jaubert F, Takata K, Oka T, Yoshino T. Merkel cell polyomavirus DNA sequences in peripheral blood and tissues from patients with Langerhans cell histiocytosis. Human Pathology. 2014;45:119–126. doi: 10.1016/j.humpath.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nature Immunology. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nelson DS, Quispel W, Badalian-Very G, van Halteren AG, van den Bos C, Bovee JV, Tian SY, Van Hummelen P, Ducar M, MacConaill LE, Egeler RM, Rollins BJ. Somatic activating ARAF mutations in Langerhans cell histiocytosis. Blood. 2014;123:3152–3155. doi: 10.1182/blood-2013-06-511139. [DOI] [PubMed] [Google Scholar]
- Nezelof C, Basset F, Rousseau MF. Histiocytosis X histogenetic arguments for a Langer-hans cell origin. Biomedicine. 1973;18:365–371. [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nature Immunology. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Patrizi A, Neri I, Bianchi F, Guerrini V, Misciali C, Paone G, Burnelli R. Langerhans cell histiocytosis and juvenile xanthogranuloma. Two case reports. Dermatology. 2004;209:57–61. doi: 10.1159/000078589. [DOI] [PubMed] [Google Scholar]
- Peters TL, McClain KL, Allen CE. Neither IL-17A mRNA nor IL-17A protein are detectable in Langerhans cell histiocytosis lesions. Molecular Therapy. 2011;19:1433–1439. doi: 10.1038/mt.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Rosen N. Mutant BRAF melanomas–dependence and resistance. Cancer Cell. 2011;19:11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. The Journal of Experimental Medicine. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Xing F, Solit DB. Targeting oncogenic BRAF in human cancer. Current Topics in Microbiology and Immunology. 2012;355:83–98. doi: 10.1007/82_2011_162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–2182. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Galindo C, Jeng M, Khuu P, Mc-Carville MB, Jeha S. Clofarabine in refractory Langerhans cell histiocytosis. Pediatric Blood & Cancer. 2008;51:703–706. doi: 10.1002/pbc.21668. [DOI] [PubMed] [Google Scholar]
- Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M, Kulozik A, Anagnostopoulos I, Mullauer L, Mechtersheimer G, von Deimling A. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120:e28–e34. doi: 10.1182/blood-2012-06-429597. [DOI] [PubMed] [Google Scholar]
- Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Archives of Disease in Childhood. 2009;94:376–380. doi: 10.1136/adc.2008.144527. [DOI] [PubMed] [Google Scholar]
- Satoh T, Smith A, Sarde A, Lu HC, Mian S, Trouillet C, Mufti G, Emile JF, Fraternali F, Donadieu J, Geissmann F. B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS ONE. 2012;7:e33891. doi: 10.1371/journal.pone.0033891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten B, Egeler RM, Leenen PJ, Taminiau AH, van den Broek LJ, Hogendoorn PC. Expression of cell cycle-related gene products in Langerhans cell histiocytosis. Journal of Pediatric Hematology-Oncology. 2002;24:727–732. doi: 10.1097/00043426-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Segerer S, Heller F, Lindenmeyer MT, Schmid H, Cohen CD, Draganovici D, Mandelbaum J, Nelson PJ, Grone HJ, Grone EF, Figel AM, Nossner E, Schlondorff D. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney International. 2008;74:37–46. doi: 10.1038/ki.2008.99. [DOI] [PubMed] [Google Scholar]
- Senechal B, Elain G, Jeziorski E, Grondin V, Patey-Mariaud de Serre N, Jaubert F, Beldjord K, Lellouch A, Glorion C, Zerah M, Mary P, Barkaoui M, Emile JF, Boccon-Gibod L, Josset P, Debre M, Fischer A, Donadieu J, Geissmann F. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Medicine. 2007;4:e253. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sere K, Baek JH, Ober-Blobaum J, Muller-Newen G, Tacke F, Yokota Y, Zenke M, Hieronymus T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity. 2012;37:905–916. doi: 10.1016/j.immuni.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Simko SJ, Garmezy B, Abhyankar H, Lupo PJ, Chakraborty R, Shih A, Lim KPH, Wright T, Levy M, Hicks MJ, McClain KL, Allen CE. Differentiating skin-limited versus multisystem Langerhans cell histiocytosis. The Journal of Pediatrics. 2014a;165:990–996. doi: 10.1016/j.jpeds.2014.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko SJ, Tran HD, Jones J, Bilgi M, Beaupin LK, Coulter D, Garrington T, McCavit TL, Moore C, Rivera-Ortegon F, Shaffer L, Stork L, Turcotte L, Welsh EC, Hicks MJ, McC-lain KL, Allen CE. Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease. Pediatric Blood & Cancer. 2014b;61:479–487. doi: 10.1002/pbc.24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter JI. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatrics Blood & Cancer. 2008;51:76–81. doi: 10.1002/pbc.21504. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Tsou JH, Hung LY, Wu HB, Chang KC. Combined Erdheim-Chester disease and Langerhans cell histiocytosis of skin are both monoclonal: a rare case with human androgen-receptor gene analysis. Journal of the American Academy of Dermatology. 2010;63:284–291. doi: 10.1016/j.jaad.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nature Immunology. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S, Braier J, Donadieu J, Egeler RM, Grois N, Ladisch S, Potschger U, Webb D, Whitlock J, Arceci RJ. 2′ Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). results of the LCH-S-98 protocol of the Histiocyte Society. Pediatric Blood & Cancer. 2009;53:1271–1276. doi: 10.1002/pbc.22229. [DOI] [PubMed] [Google Scholar]
- Willis B, Ablin A, Weinberg V, Zoger S, Wara WM, Matthay KK. Disease course and late sequelae of Langerhans’ cell histiocytosis: 25-year experience at the University of California, San Francisco. Journal of Clinical Oncology. 1996;14:2073–2082. doi: 10.1200/JCO.1996.14.7.2073. [DOI] [PubMed] [Google Scholar]
- Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, Gilli-land DG. Langerhans’-cell histiocytosis (Histiocytosis X)–a clonal proliferative disease. The New England Journal of Medicine. 1994;331:154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343:767–768. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]