Abstract
The two primary factors leading to poor clinical results after intrasynovial tendon repair are adhesion formation within the digital sheath and repair-site elongation and rupture. As the outcomes following modern tendon multi-strand repair and controlled rehabilitation techniques are often unsatisfactory, alternative approaches, such as the application of growth factors and mesenchymal stem cells (MSCs), have become increasingly attractive treatment options. Successful biological therapies require carefully controlled spatiotemporal delivery of cells, growth factors, and biocompatible scaffold matrices in order to simultaneously (1) promote matrix synthesis at the tendon repair site leading to increased biomechanical strength and stiffness and (2) suppress matrix synthesis along the tendon surface and synovial sheath preventing adhesion formation. This review summarizes recent cell and biologic-based experimental treatments for flexor tendon injury, with an emphasis on large animal translational studies.
Keywords: flexor tendon repair, cell, growth factor, tissue engineering
Introduction
Among the most common and challenging hand injuries, intrasynovial flexor tendon transections have motivated over five decades of research designed to improve primary operative and rehabilitation techniques.1–10 Finger lacerations are the most common upper extremity injury encountered in the emergency room, with an incidence of 221 per 100,000 person-years or 1 in 452 people per year,11 mostly caused by glass or knives.12 Even small lacerations < 2 cm presenting to the emergency room often cause deep tendon injuries (~60% of cases).12 Major repair technique advances by Kessler,9 then Pennington,10 and then Winters and Gelberman4 have changed Zone II intrasynovial flexor digitorum profundus (FDP) tendon treatment from an inoperable “no man’s land”8 to a common surgical procedure. Following several decades of repair3,4,9,10,13–22 and rehabilitation23–25 improvements, we have reached a plateau in Zone II flexor tendon repair outcomes with current methods. Clincial outcomes remain highly variable, necessitating alternative approaches.3,26,27
The two primary factors leading to poor results are adhesion formation within the digital sheath and repair-site elongation and rupture. Adhesions severe enough to limit range of motion occur in up to 40% of flexor tendon repairs.28 While adhesions can be decreased with passive motion rehabilitation,6,29 they still occur frequently, even with closely controlled techniques.25,30 Experimental studies report repair-site elongation and gap formation preventing satisfactory healing in up to 48% of canine FDP tendons undergoing state-of-the-art operative repairs. In a clinically relevant, controlled canine repair model, repair site gap formation during the first six postoperative weeks did not correlate with formation of intrasynovial adhesions or loss of digital motion.31 In clinical settings, surgeons pursue a balance between repair and rehabilitation approaches promoting tendon strength and digital excursion.32 Flexor tendon repair complications are attributed to a slow accrual of repair-site strength and stiffness and to an increase in gliding resistance within the digital sheath during the first few weeks following tendon suture.31–39 The healing of paucicellular, hypovascular intrasynovial tendon appears to be limited by the relatively low levels of collagen synthesis and remodeling during the early stages of healing.40,41
Recent approaches in the canine model seek to increase time-zero strength, enabling better coaptation of tendon stumps, by increasing interaction between the suture and tendon tissue. Adhesive-coatings on sutures increase the interaction and distribute load transfer over a longer length of suture. Mechanically optimized adhesive coatings have potential to improve repair strength by several fold.42 Experimental crosslinking agents coating sutures, including 1-ethyl-3-(3-dimethylaminopropyl) carbo-diimide hydrochloride (EDC) and cyanoacrylate, also increase suture-tendon interactions and crosslink the tendon tissue immediately adjacent to the suture.43,44 These mechanical approaches offer an opportunity to improve repair strength, but do not inherently decrease adhesions or enhance the healing process.
Therefore, we look to biological approaches, such as the application of growth factors and mesenchymal stem cells (MSCs), for the next generation of approaches to improve tendon and ligament repair.37,39,45–48 The goal of recent studies has been to: (1) promote matrix synthesis at the tendon repair site leading to increased biomechanical strength and stiffness and (2) suppress matrix synthesis along the tendon surface and synovial sheath preventing adhesion formation.31,33,35,36 Biological approaches to augment repair have the potential to advance both of these goals. This review summarizes recent cell and biologic-based experimental treatments for flexor tendon injury, with an emphasis on large animal translational studies.
Flexor tendon natural healing response
Similar to healing paradigms in other tissues, intrasynovial flexor tendons follow three successive, overlapping stages of healing: acute inflammation (days 0–7 post injury), proliferation (days 3–14), and remodeling (days 10+).32,35,49 The commonly injured region of the flexor tendon is intrasynovial, defined as Zone II by Kleinert and Verdan.50 The tendon lies within a synovium-lined fibro-osseous sheath that extends from the distal aspect of the palm to the distal aspect of the A4 pulley. Intrasynovial flexor tendons are paucicellular51 and hypovascular,52,53 with limited blood supply delivered by long and short vinculae originating from the digital arteries and supplying the tendon segmentally.41 In addition, the tendon receives nutrients and lubrication from the synovial fluid produced by the tendon sheath.3,32 As healing intrasynovial tendon has few intrinsic cells and has limited vascularization, there is little intrinsic healing from tendon fibroblasts until delayed time points. At early time points, cell proliferation and matrix synthesis are dominated by cells that migrate to the injury site (Figure 1).32,33,35 As a result, zone II flexor tendon injuries have substantially poorer healing outcomes following operative repair than do tendon injuries to extrasynovial flexor tendons.3,8,15
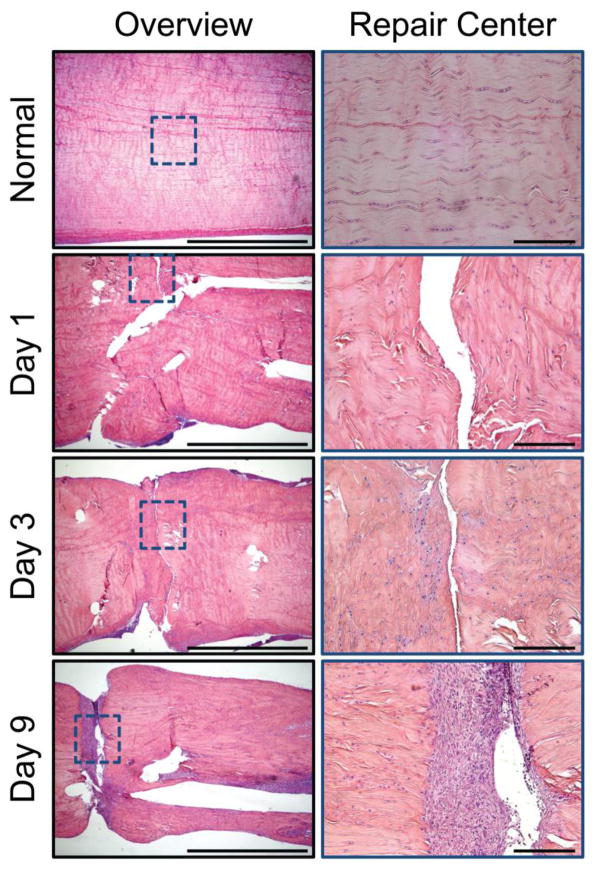
Figure 1.
Representative histologic sections of healthy and repaired canine flexor tendons 1, 3, and 9 days post-operatively. The sections were stained with H&E and viewed under bright field for cell identification. An overview of a representative section from each time point is shown to the left (4x objective, 2mm scale bar). High magnification images (20x objective, 200mm scale bar) of the section outlined in blue are shown to the right. Inflammatory cells are seen infiltrating the repair site via the tendon surface.
Acute inflammation in the first several days after tendon injury attracts circulating inflammatory cells to the injured tendon.35,54,55 This inflammatory infiltration is dominated by polymorphonuclear cells during the first day, especially in the fibrin clot that forms at the repair site, followed by a transition to monocytes and macrophages by the third day.49 Activated macrophages exhibit two major phenotypes: M1 and M2. The M1 macrophages, prevalent during acute inflammation,56,57 promote extracellular matrix deposition (scar) and inflammation,55,58 bridging the transected tendon ends but also leading to adhesions. Following acute inflammation, the proliferative phase of healing ensues. In addition to M1 macrophages,55 there is an increase in the number of fibroblast-like cells synthesizing extracellular matrix at the proliferative phase.49 Most of the fibroblast-like cells are likely derived from epitenon cells49 and resident tendon fibroblasts.59 Morphologic studies of repaired canine tendons at 7 days after tendon transection and repair show that regions with well coapted collagen fibers had a stronger endotendon response compared to those where the gap only had a few fibrinous strands serving as a scaffold for epitenon cell migration.35 New blood vessels emerge at the surface of canine tendons 9 days following suture.49 By 14 days, repaired canine tendon stumps show spontaneous neo-vascularization.35 The final phase, remodeling, lasts many weeks to months, during which M1 macrophages subside and M2 macrophages appear. M2 macrophages suppress inflammation, promote matrix deposition, and facilitate tissue remodeling.55,56,60 Reorganization of the granulation tissue at the repair site leads to improved tendon strength.
Animal models
The most commonly used animal models for studying flexor tendon repair and tendon rehabilitation18,61 are the canine, mouse, horse,62–64 rabbit,65 and chicken.66–69 The canine model for Zone II FDP tendon laceration and repair has been extensively used since 1962.1,70 Canine flexor tendons are similar to human flexor tendons in both anatomy and function,61,71 as well as in response to tendon injury, repair, and rehabilitation.3,24 The canine FDP tendon size is approximately one half the size of a human FDP tendon. Approximate size match enables surgeons both to perform surgical repairs identical to those performed clinically and to achieve similar time-zero mechanical strength to that seen in humans.72,73 The canine Zone II FDP tendon repair surgical model allows direct testing of surgical modifications and biological approaches before performing clinical trials in humans.24,25,43,48,74–76
Several groups are currently investigating murine models for flexor tendon repair.46,54,59,77–80 These models offer high genetic versatility and low cost, enabling in vivo studies of the healing response, biology of adhesion formation,54,59,79 and effects of biological interventions.46 However, the models and hypotheses tested need to be considered carefully due to anatomic and technical challenges that limit clinical relevance. Specifically, the small size of the tendon requires a simpler surgical technique using 8-0 caliber or smaller suture. Furthermore, to prevent repair rupture, all murine models to date require either partial laceration, which modifies the healing process, or proximal unloading. Wong and colleagues perform a partial laceration in Zone II in the murine digit.54 Other groups opted to fully or partially lacerate the extrasynovial Zone III tendon and perform proximal transection to protect the repair,59,78,80 leading to large scar formation between tendon ends.79 Finally, rehabilitation postoperatively cannot be controlled due to the small size of the animal. Despite these limitations, the availability of transgenic mouse models opens up possibilities for mechanistic basic science experiments, including cell lineage tracing, gene deletion, and cell ablation.
Biologic treatments
A number of recent reports have indicated that biological approaches, such as the application of growth factors and mesenchymal stem cells (MSCs), have the potential to improve tendon and ligament repair.37,39,45,47,48,81 By introducing cells into the paucicellular intrasynovial flexor tendon milieu and inducing a developmental paradigm between the repaired tendon ends, biological approaches attempt to accelerate healing and regenerate normal tissue. Multipotent MSCs from a variety of adult tissues have an excellent capacity to differentiate into the relevant tissue-specific phenotype and to provide potent immunosuppressive and anti-inflammatory effects.82,83 However, MSC delivery has been ineffective in improving the strength and stiffness following the repair of intrasynovial tendons in vivo.48 Similarly, likely due to the paucity of tendon fibroblasts in the region of repair, growth factor application in isolation has been unsuccessful in stimulating enhanced tensile properties following tendon suture, although some improvements have been achieved in digital range of motion.37,39,84 This has led to more recent focus on combinations of growth factors, cells, and specialized delivery approaches to improve flexor tendon repair.
Delivery of biofactors
Several biofactor delivery approaches have been investigated to improve healing after flexor tendon suture. The simplest delivery method, systemic drug delivery, has not been widely adopted clinically due to low bioavailability at the tendon and concern of side effects. Oral nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., ibuprofen) have been used, with varying results, to limit adhesions experimentally and clinically.85–88 Local bolus delivery of cells89 or growth factors90,91 by simple injection has yielded limited results, since few cells graft to host tissue without a supporting scaffold and the delivered growth factor is rapidly cleared from the repair site92. Recent studies have shown that biological interventions require controlled spatiotemporal delivery to the repair site to improve tendon healing.39,93–96
In order to effectively deliver cells and growth factors to the repair site, two major tissue engineering paradigms have been investigated using a variety of scaffold biomaterials. Approaches typically either interpose cell- and/or growth factor-seeded scaffolds between the repaired tendon stumps97–99 or deliver scaffolds on the surface of the repaired tendon.48,100 Interposition delivers factors directly to the injury site where they are needed for repair, but scaffolds may form a barrier between the tendon stumps that is detrimental for healing.101 Our group has explored scaffold delivery in a longitudinal slit made within the canine flexor tendon, enabling factor delivery to the injured site while retaining tendon stump coaptation. However, the slit was found to have injurious mechanical effects that must be overcome before improvement in healing can be achieved.81,84,102 Alternatively, scaffolds placed on the surface of tendon adjacent to the repair site deliver factors to the general vicinity but rely on biofactor diffusion or migration to impact the repair itself. Furthermore, scaffolds wrapped around the tendon may induce adhesions or cause excessive bulking that limits tendon gliding within the fibro-osseous sheath. To minimize adhesion formation, lubricating biomaterials such as lubricin and hyaluronic acid46,48,103–107 and anti-mitotic drugs such as 5-fluorouracil33,108 have been successfully utilized in animal models. While these materials improve tendon gliding, some studies have shown deleterious effects on repair strength.48,103,109 Other materials including silicone, polyethylene, and cellophane have been used clinically as an artificial sheath to reduce adhesions, but have not gained widespread acceptance in the United States.2,3,110,111
Biomaterial selection is crucial to the function of tissue engineered scaffolds. Fibrin delivery systems with heparin-bound growth factors have enabled sustained drug delivery during healing,39,76,81,93,102,112,113 as have some microsphere-based approaches.114–116 The scaffold backbone is also essential for promoting stem cell integration and differentiation. Scaffold mechanical properties and fiber diameter influence cell activity and differentiation.117 Synthetic polymer approaches using electrospun polylactic co-glycolic acid (PLGA) nanofiber scaffolds have provided a strong, fibrous backbone and delivered viable cells and growth factors to the repair site (Figure 2). However, these scaffolds release acidic byproducts that increase the proinflammatory cytokine IL-1β and negatively impact healing.81,102 Naturally occurring polymers, including collagen- and fibrin/heparin-based delivery systems, have been explored for their enhanced biocompatibility. Future delivery approaches should be biocompatible, appropriate for cell seeding, able to provide sustained growth factor delivery, and have appropriate surgical handling characteristics for implantation into the relatively dense tendon tissue.
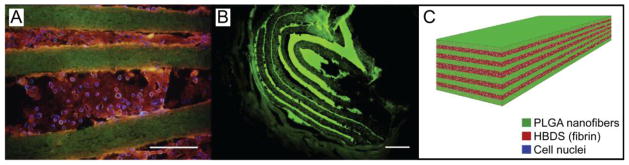
Figure 2.
A representative PLGA-fibrin scaffold with 11 alternating layers of aligned electrospun PLGA nanofiber mats separated by fibrin containing adipose-derived MSCs. (A) Micrograph showing the scaffold in vitro; the PLGA was labeled with FITC (green), the fibrin was labeled with Alexa Fluor 546 (red) and the adipose-derived MSC nuclei were labeled with Hoechst 33258 (blue) (scale bar = 200 μm). (B) Micrograph showing the scaffold in vivo 9 days after implantation in a canine flexor tendon repair (scale bar = 100 μm). (C) A schematic of the layered scaffold is shown.
Growth factor treatments
The growth factors bone morphogenic protein (BMP) 12, BMP13, and BMP14, (a.k.a., GDF7, GDF6, and GDF5, respectively) which are expressed in developing tendons and ligaments, have been shown to have the greatest potential for improving tendon healing.45,118–122 These BMPs act by inducing tenogenesis in stem cells in vitro via Smad 1/5/8 phosphorylation.45,118,121,123 BMP12 effectively increased the expression of the tendon markers scleraxis and tenomodulin in canine adipose-derived mesenchymal stromal cells (ASCs) in vitro at both mRNA and protein levels.45 Consistent with these results, BMP12 induced scleraxis promoter driven-GFP and tenomodulin expression in mouse ASCs. BMP12 administration concurrently reduced expression of the bone marker osteocalcin, but not the osteogenic transcription factor runx-2. There was a mild increase in the expression of the cartilage matrix gene aggrecan, though still to considerably lower levels than those detected in tendon fibroblasts. BMP14 had similar but less potent effects.45 However, these factors alone, without concurrent cell delivery, have not been sufficient to improve repair strength. Hayashi et al. interposed collagen gels with BMP14 without cells between cut ends of canine FDP tendon under in vitro tissue culture conditions, but this did not significantly change ultimate healing strength or stiffness compared to repaired controls.47 Similarly, adenoviral-mediated gene transfer of human BMP13 did not improve healing in a rat rotator cuff repair model.124
Several other growth factor approaches have attempted to promote cell proliferation and matrix synthesis in order to improve flexor tendon healing. Exogenous basic fibroblast growth factor (bFGF) was shown to accelerate wound closure and promote fibroblast proliferation and matrix synthesis in vitro in rat patellar tendon fibroblasts and in canine tendon fibroblasts.76,125,126 In an in vivo chicken flexor tendon injury and repair model, adeno-associated virus-2 carrying bFGF was directly injected into injured tendons. The bFGF treated chicken flexor tendons had significantly higher ultimate strength at 2, 4, and 8 weeks, fewer ruptures, and similar adhesion scores.69 These promising in vitro and chicken in vivo studies motivated testing the effects of sustained bFGF delivery to injured flexor tendons in the clinically relevant canine large animal model. Sustained delivery of biologically active bFGF was achieved by incorporating bFGF into a fibrin delivery system. The bFGF release profile was tuned by bFGF dosage and heparin concentration, where increasing concentrations of heparin significantly slowed release. At a 1:1000 growth factor to heparin ratio, 37% of the loaded bFGF was released within the first 2 days and 71% was released within the first 10 days by passive release in vitro (i.e., mediated by diffusion and degradation of the fibrin carrier). Active bFGF release (i.e., mediated by canine tendon fibroblast cells) also achieved a sustained delivery profile over at least 10 days. The released bFGF stimulated increased cell number, increased gene expression of the extracellular matrix lubricating proteins lubricin and hyaluronic acid synthase 2, increased expression of the degradation proteins matrix metalloproteinase 1 and 13, and decreased expression of collagen I and III.76 However, at 21 days in vivo in the canine flexor tendon injury and repair model, sustained bFGF delivery not only accelerated the cell-proliferation phase of tendon healing, but also promoted neovascularization and inflammation in the earliest stages following the suturing of the tendon. Despite a substantial biologic response, the administration of basic fibroblast growth factor failed to produce improvements in either the mechanical or functional properties of the repair. Rather, increased cellular activity resulted in peritendinous scar formation and diminished range of motion (Figure 3).84
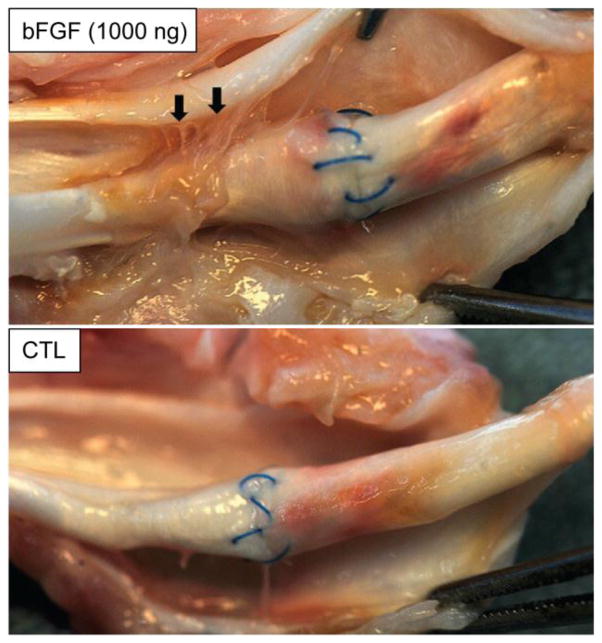
Figure 3.
There were more adhesions (arrows) between the flexor tendon surface and its surrounding sheath in the bFGF-treated tendons (top panel) than there were in the non-bFGF-treated control (CTL) tendons (bottom panel). Arrows indicate adhesions proximal to the repair site in a tendon treated with 1000 ng bFGF. The paired control tendon did not have any apparent adhesions.
Similarly, exogenous platelet derived growth factor-BB (PDGF-BB) was shown to stimulate canine flexor tendon fibroblast proliferation, decrease collagen I and III gene expression, and increase lubricin, hyaluronic acid synthase-2, and matrix metalloproteinase 1 and 13 gene expression in vitro.76,93,126 Sustained delivery of PDGF-BB using a fibrin/heparin based delivery system in canine flexor tendon repairs in vivo consistently improved range of motion, collagen remodeling, and cell proliferation at 14, 21, and 42 days following suture. However, PDGF-BB delivery did not increase repair strength at any timepoint.37,39,40,81
Transforming growth factor β (TGFβ) has also produced mixed effects on flexor tendon healing: while TGFβ isoforms are important during flexor tendon healing to stimulate collagen production, they also lead to fibrosis and scar formation.38,127–131 While some studies used TGFβ with the goal of improving strength,118 many have attempted to inhibit TGFβ in order to reduce adhesions, albeit at the cost of reducing repair strength.132–136 This decrease in repair strength precludes the use of TGFβ inhibitors as a standalone therapy. Successful TGFβ-modulating treatments would require controlled spatiotemporal activity to enhance collagen production in the healing tendon itself, but decrease scar and adhesion formation at the tendon surface.
A promising recent growth factor approach is based on connective tissue growth factor (CTGF), which has been shown to induce MSC differentiation into tendon fibroblasts and/or chondrocytes.137–139 Similar to BMP12 and positively promoting BMP12 effects, in vitro CTGF effectively increased the expression of the tenocyte lineage markers scleraxis and tenomodulin, as well as the fibroblast proteins collagen I and tenascin-C.138 During rat rotator cuff healing, CTGF is highly expressed in the tendon midsubstance and at the tendon-to-bone insertion for several weeks following injury.140 In chicken flexor tendons, CTGF is relatively highly expressed in normal tendons and throughout healing.141 CTGF and cell combination studies are described below.
Cell treatments
As noted above, early enthusiasm for cell therapy, based on patellar tendon89,142 and Achilles tendon143 results, has been largely unsuccessful in rotator cuff144 and flexor tendon animal models. In two studies using a canine in vitro tissue culture model, interposition of a multilayered collagen patch seeded with bone marrow-derived MSCs into the repair site did not improve flexor tendon healing mechanics compared with control repairs without interposed patches.47,145 MSC implantation in vivo in rabbits decreased adhesions but did not improve biomechanical properties 3 or 8 weeks after surgery.146 Racehorses that received direct injection of bone marrow-derived MSCs during superficial digital flexor tendon repair had reduced re-injury rates compared with historical controls,147–149 however, the equine superficial digital flexor tendon has substantially different functional, structural, and material properties from human FDP tendon.63,150
Cell-growth factor combination treatments
Though cells and growth factors in isolation have not markedly improved flexor tendon healing, combination therapies offer greater potential to improve outcomes. While interposition of bone marrow-derived MSCs only or BMP14 only did not improve repair mechanics in an in vitro canine flexor tendon tissue culture model, the combination of MSCs with BMP14 or platelet-rich plasma on collagen patches improved strength and stiffness.47,145 This approach, combined with surface lubricin for in vivo canine flexor tendon repairs to decrease adhesions, unfortunately resulted in substantially worse repair strength 42 days after repair.48 Similarly, application of adipose-derived MSCs in combination with BMP12 in an in vivo canine Zone II flexor tendon repair using PLGA and fibrin scaffolds led to increased total collagen compared to repairs with acellular scaffolds, but did not improve tensile properties at 28 days after surgery compared to the acellular group. The delivery method used in these studies was a critical component driving the outcomes: the PLGA-fibrin scaffolds had a deleterious effect that may have counteracted any beneficial effects from the MSCs and/or BMP12.102 A previous study delivering the same PLGA-fibrin scaffolds containing MSCs and PDGF-BB demonstrated retained cell viability after 10 days, but also mild inflammatory reactions, possibly due to the PLGA scaffold (Figure 2).81
Connective tissue growth factor and cell combination approaches have not been thoroughly evaluated in flexor tendon in vivo, but CTGF-based approaches show promise in other tendon repair scenarios. Tendon-derived CD146+ stem cells cultured with CTGF promoted tenogenic differentiation in vitro.151 Tendon-derived stem cell sheets stimulated with CTGF promoted improved anterior cruciate ligament graft healing and biomechanics in vivo in rats, including improved osteointegration.152 Similarly, cell sheets with CTGF and ascorbic acid enhanced biomechanical and histology-based outcomes at 8 weeks in an in vivo rat patellar tendon repair model. Further studies introducing CTGF and/or BMP growth factors with cells and biocompatible matrices will be important for defining the next generation of therapies for flexor tendon repair.
Conclusions
Intrasynovial flexor tendons are notoriously challenging to repair, with highly variable clinical outcomes due to the competing requirements for repair strength to avoid rupture and minimal adhesion formation to maintain adequate range of motion. Advances in rehabilitation protocols have led to significant improvements in tendon healing and gliding outcomes, but post-operative complications remain. Biological approaches have potential to simultaneously tackle both of these problems. Successful biological therapies will require carefully controlled spatiotemporal delivery of cells, growth factors, and biocompatible scaffold matrices in order to simultaneously promote matrix synthesis at the tendon repair site to improve strength and suppress matrix synthesis along the tendon surface to prevent adhesion formation.
Acknowledgments
Supported by National Institutes of Health grants R01-AR062947 (to RHG and ST) and T32-AR060719 (to SWL).
Footnotes
Conflict of Interest Statement: No conflict.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Jt Surg Am. 1962;44-A(1):49–64. [PubMed] [Google Scholar]
- 2.Kessler I, Nissim F. Primary repair without immobilization of flexor tendon division within the digital sheath. An experimental and clinical study. Acta Orthop Scand. 1969;40:587–601. doi: 10.3109/17453676908989524. [DOI] [PubMed] [Google Scholar]
- 3.Boyer MI, Strickland JW, Engles DR, Sachar K, Leversedge FJ. Flexor Tendon Repair and Rehabilitation: state of the art in 2002. Instr Course Lect. 2003;52:137–61. [PubMed] [Google Scholar]
- 4.Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., III The Effects of Multiple-Strand Suture Methods on the Strength and Excursion of Repaired Intrasynovial Flexor Tendons : A Biomechanical Study in Dogs. J Hand Surg Am. 1998;23(1):97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 5.Woo SL-Y, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. Acta Orthop Scand. 1981;52(6):615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 6.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982;7(2):170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 7.Verdan CE. Primary repair of flexor tendons. J Bone Jt Surg. 1960;42(4):647–657. [PubMed] [Google Scholar]
- 8.Bunnell S. In: Surgery of the Hand. 3. Lippincott J, editor. Philadelphia: JB Lippincott; 1956. [Google Scholar]
- 9.Kessler I. The “grasping” technique for tendon repair. Hand. 1973;5(3):253–255. doi: 10.1016/0072-968x(73)90038-7. [DOI] [PubMed] [Google Scholar]
- 10.Pennington DG. The locking loop tendon suture. Plast Reconstr Surg. 1979;63(5):648–52. doi: 10.1097/00006534-197905000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ootes D, Lambers KT, Ring DC. The epidemiology of upper extremity injuries presenting to the emergency department in the United States. Hand. 2012;7(1):18–22. doi: 10.1007/s11552-011-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55(2):146–148. doi: 10.1097/01.sap.0000168884.88016.e1. [DOI] [PubMed] [Google Scholar]
- 13.Fufa DT, Osei DA, Calfee RP, Silva MJ, Thomopoulos S, Gelberman RH. The effect of core and epitendinous suture modifications on repair of intrasynovial flexor tendons in an in vivo canine model. J Hand Surg Am. 2012;37(12):2526–31. doi: 10.1016/j.jhsa.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao E, Hariharan S, Soejima O, Lotz JC, Francisco S. Effect of Peripheral Suture Depth on Strength of Tendon Repairs. J Hand Surg Am. 1996;21(2):234–239. doi: 10.1016/S0363-5023(96)80106-7. [DOI] [PubMed] [Google Scholar]
- 15.Nelson GN, Potter R, Ntouvali E, et al. Intrasynovial flexor tendon repair: a biomechanical study of variations in suture application in human cadavera. J Orthop Res. 2012;30(10):1652–9. doi: 10.1002/jor.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade PJF, Wetherell RG, Amis AA. Flexor tendon repair: Significant gain in strength from the halsted peripheral suture technique. J Hand Surg J Br. 1989;14(2):232–235. doi: 10.1016/0266-7681_89_90135-6. [DOI] [PubMed] [Google Scholar]
- 17.Hatanaka H, Manske PR. Effect of suture size on locking and grasping flexor tendon repair techniques. Clin Orthop Relat Res. 2000;(375):267–274. doi: 10.1097/00003086-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Strickland JW. Development of flexor tendon surgery: Twenty-five years of progress. J Hand Surg Am. 2000;25(2):214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 19.Savage R, Risitano G. Flexor tendon repair using a “six strand” method of repair and early active mobilisation. J Hand Surg Br. 1989;14(4):396–9. doi: 10.1016/0266-7681_89_90154-x. [DOI] [PubMed] [Google Scholar]
- 20.Becker H, Davidoff M. Eliminating the Gap in Flexor Tendon Surgery: A New Method of Suture. Hand. 1977;9(3):306–311. doi: 10.1016/s0072-968x(77)80122-8. [DOI] [PubMed] [Google Scholar]
- 21.Wagner WF, Carroll C, Strickland JW, Heck DA, Toombs JP. A biomechanical comparison of techniques of flexor tendon repair. J Hand Surg Am. 1994;19(6):979–83. doi: 10.1016/0363-5023(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 22.McLarney E, Hoffman H, Wolfe SW. Biomechanical analysis of the cruciate four-strand flexor tendon repair. J Hand Surg Am. 1999;24(2):295–301. doi: 10.1053/jhsu.1999.0295. [DOI] [PubMed] [Google Scholar]
- 23.Silva MJ, Boyer MI, Ditsios K, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and structure repair. J Orthop Res. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 24.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Jt Surg. 2001;86(6):891–899. [PubMed] [Google Scholar]
- 25.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased In vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 26.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing: The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–86. doi: 10.1197/j.jht.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: What is the evidence? Br Med Bull. 2009;90(1):85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 28.Aydin A, Topalan M, Mezdeği A, et al. Single-stage flexor tendoplasty in the treatment of flexor tendon injuries. Acta Orthop Traumatol Turc. 2004;38(1):54–9. [PubMed] [Google Scholar]
- 29.Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg Am. 1977;2(6):441–451. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 30.Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech. 1999;32(2):175–181. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 31.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The Effect of Gap Formation at the Repair Site on the Strength and Excursion of Intrasynovial Flexor Tendons. J Bone Jt Surg Am. 1999;81-A(7):975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Jt Surg Am. 2003;85-A(3):539–550. doi: 10.2106/00004623-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Khan U, Kakar S, Akali A, Bentley G, McGrouther DA. Modulation of the formation of adhesions during the healing of injured tendons. J Bone Jt Surg Br. 2000;82-B(7):1054–1058. doi: 10.1302/0301-620x.82b7.9892. [DOI] [PubMed] [Google Scholar]
- 34.Matthews P, Richards H. Factors in the adherence of flexor tendon after repair: an experimental study in the rabbit. J Bone Jt Surg Br. 1976;58(2):230–6. doi: 10.1302/0301-620X.58B2.777010. [DOI] [PubMed] [Google Scholar]
- 35.Gelberman RH, Vande Berg JS, Manske PR, Akeson WH. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10(6 Pt 1):776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Jt Surg Am. 2004;86-A(2):320–7. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27(9):1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 39.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The Early Effects of Sustained Platelet-Derived Functional and Structural Properties of Repaired Intrasynovial Flexor Tendons: An In Vivo Biomechanic Study at 3 Weeks in Canines. J Hand Surg Am. 2007;32A(3):373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Thomopoulos S, Zaegel MA, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358–68. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 41.Gelberman RH, Amiel D, Gonsalves M, Woo SL, Akeson WH. The Influence of Protected Passive Mobilization on the Healing of Flexor Tendons: A Biochemical and Microangiographic Study. Hand. 1981;13(2):120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 42.Linderman SW, Kormpakis I, Gelberman RH, et al. Shear lag sutures: Improved suture repair through the use of adhesives. Acta Biomater. 2015;23:229–39. doi: 10.1016/j.actbio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C, Sun Y, Zobitz ME, An K, Amadio PC. Enhancing the Strength of the Tendon–Suture Interface Using 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Hydrochloride and Cyanoacrylate. J Hand Surg Am. 2007;32A(5):606–611. doi: 10.1016/j.jhsa.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Thoreson AR, Hiwatari R, An K-N, Amadio PC, Zhao C. The Effect of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Suture Coating on Tendon Repair Strength and Cell Viability in a Canine Model. J Hand Surg Am. 2015;40(10):1986–1991. doi: 10.1016/j.jhsa.2015.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen H, Gelberman RH, Silva MJ, Sakiyama-Elbert SE, Thomopoulos S. BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS One. 2013;8(10):e77613. doi: 10.1371/journal.pone.0077613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi M, Zhao C, Thoreson AR, et al. The effect of lubricin on the gliding resistance of mouse intrasynovial tendon. PLoS One. 2013;8(12):e83836. doi: 10.1371/journal.pone.0083836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi M, Zhao C, An K-N, Amadio PC. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Eur. 2011;36(4):271–9. doi: 10.1177/1753193410394521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao C, Ozasa Y, Reisdorf RL, et al. Engineering Flexor Tendon Repair With Lubricant, Cells, and Cytokines in a Canine Model. Clin Orthop Relat Res. 2014;472(9):2569–78. doi: 10.1007/s11999-014-3690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res. 2014;32(5):645–52. doi: 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleinert HE, Verdan C. Report of the Committee on Tendon Injuries. J Hand Surg Am. 1983;8(5):794–798. doi: 10.1016/s0363-5023(83)80275-5. [DOI] [PubMed] [Google Scholar]
- 51.Gelberman RH, Vandeberg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Jt Surg Am. 1983;65(1):70–80. [PubMed] [Google Scholar]
- 52.Lundborg G, Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg Am. 1978;3(1):21–31. doi: 10.1016/s0363-5023(78)80114-2. [DOI] [PubMed] [Google Scholar]
- 53.Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4(4):252–60. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong JKF, Lui YH, Kapacee Z, Kadler KE, Ferguson MWJ, McGrouther DA. The Cellular Biology of Flexor Tendon Adhesion Formation. Am J Pathol. 2009;175(5):1938–1951. doi: 10.2353/ajpath.2009.090380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dakin SG, Werling D, Hibbert A, et al. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7(2):e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32(7):944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsolais D, Côté CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001;19(6):1203–1209. doi: 10.1016/S0736-0266(01)00031-6. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 59.Hasslund S, Jacobson JA, Dadali T, et al. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res. 2008;26(6):824–33. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loiselle AE, Kelly M, Hammert WC. Biological Augmentation of Flexor Tendon Repair. J Hand Surg Am. 2016;41(1):144–149. doi: 10.1016/j.jhsa.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Gelberman RH, Manske PR, Vande Berg JS, Lesker PA, Akeson WH. Flexor tendon repair in vitro: a comparative histologic study of the rabbit, chicken, dog, and monkey. J Orthop Res. 1984;2(9):39–48. doi: 10.1002/jor.1100020107. [DOI] [PubMed] [Google Scholar]
- 62.Thorpe CT, Birch HL, Clegg PD, Screen HRC. The role of the non-collagenous matrix in tendon function. Int J Exp Pathol. 2013;94(4):248–59. doi: 10.1111/iep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorpe CT, Klemt C, Riley GP, Birch HL, Clegg PD, Screen HRC. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater. 2013;9(8):7948–56. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Cadby JA, Buehler E, Godbout C, van Weeren PR, Snedeker JG. Differences between the Cell Populations from the Peritenon and the Tendon Core with Regard to Their Potential Implication in Tendon Repair. PLoS One. 2014;9(3):e92474. doi: 10.1371/journal.pone.0092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J Hand Surg Am. 1998;23(6):1052–8. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 66.Halikis MN, Manske PR, Kubota H, Aoki M. Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. J Hand Surg. 1997;22(3):464–472. doi: 10.1016/S0363-5023(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 67.Wray RC, Jr, Holtman B, Weeks PM. Clinical treatment of partial tendon lacerations without suturing and with early motion. Plast Reconstr Surg. 1977;59(2):231–234. [PubMed] [Google Scholar]
- 68.Chow SP, Yu OD. An experimental study on incompletely cut chicken tendons--a comparison of two methods of management. J Hand Surg Br. 1984;9(2):121–125. [PubMed] [Google Scholar]
- 69.Tang JB, Cao Y, Zhu B, Xin K-Q, Wang XT, Liu PY. Adeno-Associated Virus-2-Mediated bFGF Gene Transfer to Digital Flexor Tendons Significantly Increases Healing Strength. J Bone Jt Surg Am. 2008;90(5):1078. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- 70.Potenza AD. Detailed evaluation of healing processes in canine flexor digital tendons. Mil med. 1962;127:34–47. [PubMed] [Google Scholar]
- 71.Horibe S, Woo SL, Spiegelman JJ, Marcin JP, Gelberman RH. Excursion of the flexor digitorum profundus tendon: a kinematic study of the human and canine digits. J Orthop Res. 1990;8(2):167–74. doi: 10.1002/jor.1100080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19(4):580–6. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 73.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51(5):917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Zhao C, Moran SL, Cha SS, Kai-Nan-An, Amadio PC. An analysis of factors associated with failure of tendon repair in the canine model. J Hand Surg Am. 2007;32(4):518–25. doi: 10.1016/j.jhsa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi M, Seiler JG, Gelberman RH, Sofranko RA, Woo SL. In vitro biomechanical analysis of suture methods for flexor tendon repair. J Orthop Res. 1993;11(4):603–11. doi: 10.1002/jor.1100110415. [DOI] [PubMed] [Google Scholar]
- 76.Thomopoulos S, Das R, Sakiyama-Elbert SE, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: Controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38(2):225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong J, Bennett W, Ferguson MWJ, McGrouther DA. Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D reconstruction. J Anat. 2006;209(4):533–45. doi: 10.1111/j.1469-7580.2006.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beason DP, Kuntz AF, Hsu JE, Miller KS, Soslowsky LJ. Development and evaluation of multiple tendon injury models in the mouse. J Biomech. 2012;45(8):1550–1553. doi: 10.1016/j.jbiomech.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loiselle AE, Bragdon GA, Jacobson JA, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27(6):833–840. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasslund S, O’Keefe RJ, Awad HA. A mouse model of flexor tendon repair. Methods Mol Biol. 2014;1130:73–88. doi: 10.1007/978-1-62703-989-5_6. [DOI] [PubMed] [Google Scholar]
- 81.Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater. 2013;9:6905–6914. doi: 10.1016/j.actbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose Tissue-Derived Mesenchymal Stem Cells Have In Vivo Immunosuppressive Properties Applicable for the Control of the Graft-Versus-Host Disease. Stem Cells. 2006;24(11):2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 83.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13(6):1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 84.Thomopoulos S, Kim HM, Das R, et al. The Effects of Exogenous Basic Fibroblast Growth Factor on Intrasynovial Flexor Tendon Healing in a Canine Model. J Bone Jt Surg Am. 2010;92A(13):2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rouhani A, Tabrizi A, Ghavidel E. Effects of Non-Steroidal Anti-Inflammatory Drugs on Flexor Tendon Rehabilitation After Repair. Arch Bone Jt Surg. 2013;1(1):28–30. [PMC free article] [PubMed] [Google Scholar]
- 86.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29(4):551–563. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 87.Kulick MI, Smith S, Hadler K. Oral ibuprofen: evaluation of its effect on peritendinous adhesions and the breaking strength of a tenorrhaphy. J Hand Surg Am. 1986;11(1):110–20. doi: 10.1016/s0363-5023(86)80116-2. [DOI] [PubMed] [Google Scholar]
- 88.Tan V, Nourbakhsh A, Capo J, Cottrell JA, Meyenhofer M, O’Connor JP. Effects of nonsteroidal anti-inflammatory drugs on flexor tendon adhesion. J Hand Surg Am. 2010;35(6):941–7. doi: 10.1016/j.jhsa.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 89.Awad HA, Butler DL, Malaviya P, Huibregtse B, Caplan AI. Autologous Mesenchymal Stem Cell-Mediated Repair of Tendon. Tissue Eng. 1999;5(3):267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 90.Woo SLY, Smith DW, Hildebrand KA, Zeminski JA, Johnson LA. Engineering the healing of the rabbit medial collateral ligament. Med Biol Eng Comput. 1998;36(3):359–364. doi: 10.1007/BF02522484. [DOI] [PubMed] [Google Scholar]
- 91.Hildebrand KA, Woo SL, Smith DW, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26(4):549–54. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 92.Robinson SN, Talmadge JE. Sustained release of growth factors. In Vivo. 2002;16(6):535–40. [PubMed] [Google Scholar]
- 93.Sakiyama-Elbert SE, Das R, Gelberman RH, Harwood F, Amiel D, Thomopoulos S. Controlled-Release Kinetics and Biologic Activity of Platelet-Derived Growth Factor-BB for Use in Flexor Tendon Repair. J Hand Surg Am. 2008;33A(9):1548–1557. doi: 10.1016/j.jhsa.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122(6):570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 95.Butler DL, Juncosa-Melvin N, Boivin GP, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26(1):1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 96.Breidenbach AP, Gilday SD, Lalley AL, et al. Functional tissue engineering of tendon: Establishing biological success criteria for improving tendon repair. J Biomech. 2013:1–8. doi: 10.1016/j.jbiomech.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12(12):3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 98.Lu HH, Subramony SD, Boushell MK, Zhang X. Tissue engineering strategies for the regeneration of orthopaedic interfaces. Ann Biomed Eng. 2010;38(6):2142–2154. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uehara K, Zhao C, Gingery A, Thoreson AR, An K-N, Amadio PC. Effect of Fibrin Formulation on Initial Strength of Tendon Repair and Migration of Bone Marrow. J Bone Jt Surg Am. 2015;97(21):1792–1798. doi: 10.2106/JBJS.O.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao C, Chieh H-F, Bakri K, et al. The effects of bone marrow stromal cell transplants on tendon healing in vitro. Med Eng Phys. 2009;31(10):1271–1275. doi: 10.1016/j.medengphy.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qu F, Lin J-MG, Esterhai JL, Fisher MB, Mauck RL. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013;9(5):6393–402. doi: 10.1016/j.actbio.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gelberman RH, Shen H, Kormpakis I, et al. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J Orthop Res. 2015:1–11. doi: 10.1002/jor.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao C, Sun Y-L, Kirk RL, et al. Effects of a Lubricin-Containing Compound on the Results of Flexor Tendon Repair in a Canine Model in Vivo. J Bone Jt Surg Am. 2010;92(6):1453. doi: 10.2106/JBJS.I.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao C, Sun Y-L, Jay GD, Moran SL, An K-N, Amadio PC. Surface modification counteracts adverse effects associated with immobilization after flexor tendon repair. J Orthop Res. 2012;30(12):1940–4. doi: 10.1002/jor.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amiel D, Ishizue K, Billings E, et al. Hyaluronan in flexor tendon repair. J Hand Surg Am. 1989;14(5):837–43. doi: 10.1016/s0363-5023(89)80085-1. [DOI] [PubMed] [Google Scholar]
- 106.Wiig M, Abrahamsson SO, Lundborg G. Tendon repair-cellular activities in rabbit deep flexor tendons and surrounding synovial sheaths and the effects of hyaluronan: An experimental study in vivo and in vitro. J Hand Surg Am. 1997;22(5):818–825. doi: 10.1016/S0363-5023(97)80075-5. [DOI] [PubMed] [Google Scholar]
- 107.Miller JA, Ferguson RL, Powers DL, Burns JW, Shalaby SW. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J Biomed Mater Res. 1997;38(1):25–33. doi: 10.1002/(sici)1097-4636(199721)38:1<25::aid-jbm4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 108.Khan U, Occleston NL, Khaw PT, McGrouther DA. Single exposures to 5-Fluorouracil: A possible mode of targeted therapy to reduce contractile scarring in the injured tendon. Plast Reconstr Surg. 1997;99:465–71. doi: 10.1097/00006534-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 109.Cerovac S, Afoke A, Akali A, McGrouther DA. Early breaking strength of repaired flexor tendon treated with 5-fluorouracil. J Hand Surg Br. 2001;26:220–223. doi: 10.1054/jhsb.2000.0537. [DOI] [PubMed] [Google Scholar]
- 110.Stark HH, Boyes JH, Johnson L, Ashworth CR. The use of paratenon, polyethylene film, or silastic sheeting to prevent restricting adhesions to tendons in the hand. J Bone Jt Surg Am. 1977;59(7):908–913. [PubMed] [Google Scholar]
- 111.Eskeland G, Eskeland T, Hovig T, Teigland J. The ultrastructure of normal digital flexor tendon sheath and of the tissue formed around silicone and polyethylene implants in man. J Bone Jt Surg Br. 1977;59(2):206–212. doi: 10.1302/0301-620X.59B2.873981. [DOI] [PubMed] [Google Scholar]
- 112.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing brin-based cell ingrowth matrix. J Control Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 113.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 114.Solorio LD, Phillips LM, McMillan A, et al. Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. Adv Healthc Mater. 2015;4(15):2306–13. doi: 10.1002/adhm.201500598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solorio LD, Dhami CD, Dang PN, Vieregge EL, Alsberg E. Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-β1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Transl Med. 2012;1(8):632–639. doi: 10.5966/sctm.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeon O, Wolfson DW, Alsberg E. In-Situ Formation of Growth-Factor-Loaded Coacervate Microparticle-Embedded Hydrogels for Directing Encapsulated Stem Cell Fate. Adv Mater. 2015;27(13):2216–2223. doi: 10.1002/adma.201405337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erisken C, Zhang X, Moffat KL, Levine WN, Lu HH. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A. 2013;19(3–4):519–28. doi: 10.1089/ten.tea.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100(2):321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tashiro T, Hiraoka H, Ikeda Y, et al. Effect of GDF-5 on ligament healing. J Orthop Res. 2006;24(1):71–79. doi: 10.1002/jor.20002. [DOI] [PubMed] [Google Scholar]
- 120.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19(6):1199–202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 121.Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116(4):940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-Derived Mesenchymal Stem Cells Treated with Growth Differentiation Factor-5 Express Tendon-Specific Markers. Tissue Eng Part A. 2010;16(9):2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JY, Zhou Z, Taub PJ, et al. BMP-12 treatment of adult mesenchymal stem cells In Vitro augments tendon-like tissue formation and defect repair In Vivo. PLoS One. 2011;6(3):1–7. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sport Med. 2011;39(1):180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- 125.Chan BP, Chan KM, Maffulli N, Webb S, Lee KKH. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin Orthop Relat Res. 1997;342:239–247. [PubMed] [Google Scholar]
- 126.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30(3):441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 127.Juneja SC, Schwarz EM, O’Keefe RJ, Awad HA. Cellular and molecular factors in flexor tendon repair and adhesions: A histological and gene expression analysis. Connect Tissue Res. 2013;54(2):218–226. doi: 10.3109/03008207.2013.787418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farhat YM, Al-Maliki AA, Chen T, et al. Gene Expression Analysis of the Pleiotropic Effects of TGF-β1 in an In Vitro Model of Flexor Tendon Healing. PLoS One. 2012;7(12):e51411. doi: 10.1371/journal.pone.0051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klein MB, Pham H, Yalamanchi N, Chang J. Flexor tendon wound healing in vitro: The effect of lactate on tendon cell proliferation and collagen production. J Hand Surg Am. 2001;26(5):847–854. doi: 10.1053/jhsu.2001.26185. [DOI] [PubMed] [Google Scholar]
- 130.Katzel EB, Wolenski M, Loiselle AE, et al. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011;29(May):684–693. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farhat YM, Al-Maliki AA, Easa A, O’Keefe RJ, Schwarz EM, Awad HA. TGF-beta1 Suppresses Plasmin and MMP Activity in Flexor Tendon Cells via PAI-1: Implications for Scarless Flexor Tendon Repair. J Cell Physiol. 2014;(June):318–326. doi: 10.1002/jcp.24707. [DOI] [PMC free article] [PubMed]
- 132.Zhou Y, Zhang L, Zhao W, Wu Y, Zhu C, Yang Y. Nanoparticle-mediated delivery of TGF-β1 miRNA plasmid for preventing flexor tendon adhesion formation. Biomaterials. 2013;34(33):8269–8278. doi: 10.1016/j.biomaterials.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 133.Wong JKF, Metcalfe AD, Wong R, et al. Reduction of Tendon Adhesions following Administration of Adaprev, a Hypertonic Solution of Mannose-6-Phosphate: Mechanism of Action Studies. PLoS One. 2014;9(11):e112672. doi: 10.1371/journal.pone.0112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-B1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 135.Loiselle AE, Yukata K, Geary MB, et al. Development of antisense oligonucleotide (ASO) technology against Tgf-β signaling to prevent scarring during flexor tendon repair. J Orthop Res. 2015;33(6):859–66. doi: 10.1002/jor.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu YF, Mao WF, Zhou YL, Wang XT, Liu PY, Tang JB. Adeno-associated virus-2-mediated TGF-β1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury. Gene Ther. 2016;23(2):167–175. doi: 10.1038/gt.2015.97. [DOI] [PubMed] [Google Scholar]
- 137.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu J, Tao X, Chen L, Han W, Zhou Y, Tang K. CTGF Positively Regulates BMP12 Induced Tenogenic Differentiation of Tendon Stem Cells and Signaling. Cell Physiol Biochem. 2015;35(5):1831–1845. doi: 10.1159/000373994. [DOI] [PubMed] [Google Scholar]
- 139.Fukunaga T, Yamashiro T, Oya S, Takeshita N, Takigawa M, Takano-Yamamoto T. Connective tissue growth factor mRNA expression pattern in cartilages is associated with their type I collagen expression. Bone. 2003;33(6):911–918. doi: 10.1016/j.bone.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 140.Würgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elb Surg. 2007;16(5 Suppl):S198–203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am. 2008;33(10):1834–1842. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Juncosa-Melvin N, Boivin GP, Gooch C, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12(2):369–79. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 143.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16(4):406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 144.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–33. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 145.Morizaki Y, Zhao C, An K-N, Amadio PC. The effects of platelet-rich plasma on bone marrow stromal cell transplants for tendon healing in vitro. J Hand Surg Am. 2010;35(11):1833–41. doi: 10.1016/j.jhsa.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He M, Gan AWT, Lim AYT, Goh JCH, Hui JHP, Chong AKS. Bone Marrow–Derived Mesenchymal Stem Cell Augmentation of Rabbit Flexor Tendon Healing. J Hand Surg Am. 2015;20(3):421–429. doi: 10.1142/S0218810415500343. [DOI] [PubMed] [Google Scholar]
- 147.Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 148.Smith RKW. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30(20–22):1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 149.Pacini S, Spinabella S, Trombi L, et al. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007;13(12):2949–55. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 150.Thorpe CT, Clegg PD, Birch HL. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet J. 2010;42(2):174–80. doi: 10.2746/042516409X480395. [DOI] [PubMed] [Google Scholar]
- 151.Lee CH, Lee FY, Tarafder S, et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015;125(7):2690–2701. doi: 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lui PPY, Wong OT, Lee YW. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):681–9. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]