Abstract
Objective
To review our current understanding of immunotherapy, the immune mechanisms underlying food allergy, and the methodological advances that are furthering our understanding of the role of immune cells and other molecules in mediating food allergies.
Data Sources
Literature searches were performed using the following combination of terms: allergy, immunotherapy, food, and mechanisms. Data from randomized clinical studies using state-of-the-art mechanistic tools were prioritized.
Study Selections
Articles were selected based on their relevance to food allergy.
Results
Current standard of care for food allergies is avoidance of allergenic foods and the use of epinephrine in case of severe reaction during unintentional ingestion. During the last few decades, great strides have been made in understanding the cellular and molecular mechanisms underlying food allergy, and this information is spearheading the development of exciting new treatments.
Conclusion
Immunotherapy protocols are effective in desensitizing individuals to specific allergens; however, recurrence of allergic sensitization is common after discontinuation of therapy. Interestingly, in a subset of individuals, immunotherapy is protective against allergens even after discontinuation of immunotherapy. Whether this protection is permanent is currently unknown because of inadequate long-term follow-up data. Research on understanding the underlying mechanisms may assist in modifying protocols to improve outcome and enable sustained unresponsiveness, rather than a temporary relief against food allergies. The cellular changes brought about by immunotherapy are still a black box, but major strides in our understanding are being made at an exciting pace.
Introduction
Food allergies (FAs) are recurring immune-mediated adverse reactions to common foods and should not be confused with food intolerances, which are caused by nonimmune factors, such as enzyme deficiencies or reactions against certain substances.1 FAs are broadly classified into IgE-mediated, non–IgE mediated, or mixed (IgE and non-IgE) types. Because IgE-mediated FAs are the most common, best understood, and carry the risk of severe or fatal reactions, this review primarily focuses on IgE-mediated FAs. A diagnosis of IgE-mediated FA is suggested by clinical history and further evaluated by serum IgE levels and skin prick tests (SPTs), but these tests are not definitive because they lack specificity and result in a high number of false-positives. However, in conjunction with clinical history, these tests are invaluable for diagnosis.2 The gold standard for diagnosis of FA is the double-blind, placebo-controlled food challenge, but its use in clinical settings is limited because it is time consuming, expensive, and carries the risk of severe or even life-threatening anaphylactic reactions.
There has been a general consensus that FAs are increasing in prevalence; however, there is much inconsistency in estimating its prevalence because of differences among studies in methods and FA definitions used, populations studied, environmental and dietary exposures, and other factors.3 On the basis of a number of studies, approximately 5% of adults and 8% of children are estimated to have FAs.4 In recent years, immunotherapy has shown great promise as a treatment option for FAs and has renewed interest in FA research.5 This review highlights our current knowledge of the mechanism of IgE-mediated FAs, the differences in immune response between individuals with and without FAs, the potential mechanisms underlying immunotherapy, the limitations of current methods, and the unanswered questions in this field.
Defining Immune Responses to Food: FA, Desensitization, Sustained Unresponsiveness, and Immune Tolerance (Permanent Unresponsiveness)
A dynamic equilibrium between proallergic and tolerogenic immune cells and cytokines differentiate innocuous from pathogenic antigens, thereby maintaining a balanced response to environmental insults. Although all food antigens have the potential for inducing allergic response, immunologic unresponsiveness is the normal healthy response to common food antigens. This state of permanent unresponsiveness to common food antigens is usually termed immune tolerance. Perturbations to the immune system could contribute to inappropriate inflammatory responses to common foods, leading to FAs, which are defined as an allergenic reaction to a food challenge.1
Currently, there are no approved cures for FA. Standard care for FAs is a strict elimination diet with the use of antihistamines to control minor symptoms and the emergency use of epinephrine in life-threatening anaphylaxis on unintentional exposure.6 In recent years, however, immunotherapy has shown great promise in clinical trials by establishing either a state of sustained unresponsiveness or desensitization in individuals with FA (Table 1). Desensitization indicates a temporary state of unresponsiveness to causal food allergens, allowing individuals to ingest higher amounts of the offending foods (an increased threshold of reactivity) without adverse reactions. To maintain desensitization, individuals need to regularly consume allergenic foods. In a smaller subset of individuals, unresponsiveness is sustained even after discontinuation of regular consumption of allergenic foods after cessation of immunotherapy. Because long-term data on unresponsiveness after discontinuation of immunotherapy are limited, the term sustained unresponsiveness is used to differentiate this state from that of a permanent state of immunologic unresponsiveness as seen in immune tolerance. Whether the mechanisms underlying desensitization, sustained unresponsiveness, and immune tolerance are similar or whether they represent different immunologic mechanisms is under investigation.
Table 1.
Defining Immune Responses to Foods
| Term | Definition |
|---|---|
| Tolerance | This refers to a permanent and active state of healthy immunologic unresponsiveness to common food antigens. Maintenance of a tolerogenic state does not require regular, continued ingestion of the food allergen. |
| Food allergy | This refers to an inappropriate and unhealthy immunologic state that leads to local or systemic inflammatory responses to commonly ingested foods. Symptoms may be mild, severe, or life threatening. |
| Desensitization | This refers to a temporary state of immunologic unresponsiveness caused by immunotherapy. To maintain desensitization, regularly consumption of the causative allergen is necessary. The mechanisms underlying desensitization are not well understood and likely differ from that of tolerance. |
| Sustained unresponsiveness | This refers to a state of immunologic unresponsiveness caused by immunotherapy. Unlike desensitization, unresponsiveness is sustained even after discontinuation of allergen consumption. Because long-term data on sustained unresponsiveness are limited, it is currently unclear whether this reflects a temporary or a permanent state or whether this reflects an immunologic state distinct from tolerance. |
Immune Cells in FA: Antigen-Presenting Cells, T Cells, B Cells, Type 2 Innate Lymphoid Cells, Natural Killer T Cells, and Granulocytes
The main immune cells involved in FA are the antigen-presenting cells (APCs), T cells, B cells, and granulocytes (mast cells, eosinophils, and basophils). APCs are the first to encounter antigens in the lamina propria and are responsible for differentiating harmful antigens from innocuous ones and initiating appropriate polarization of T cells. The most common APCs are the dendritic cells (DCs) and macrophages. CD103+ DCs are migratory cells that sample antigens that pass through the epithelial barrier by transcytosis or endocytosis (via microfold cells or M cells found interspersed between the epithelial cells) and migrate to the mesenteric lymph nodes. These cells are indispensable for oral tolerance, express indoleamine 2,3-dioxygenease (IDO), and induce naive T-cell differentiation into regulatory T cells (Tregs) via mechanisms that involve transforming growth factor β (TGF-β) and retinoic acid.7 Tissue resident CX3R1+ macrophages are nonmigratory cells that are abundantly localized to the gut, extend dendrites between epithelial cells, and sample antigens present in the lumen. It is likely that these cells transfer antigen to CD103+ migratory DCs; however, the exact mechanism and role of CX3R1+ macrophages in oral tolerance is unclear.8
CD4+ T cells play a pivotal role in allergic response and tolerance. CD4+ T cells have been classically divided into TH cells that protect from pathogens and cancerous cells and Tregs that suppress excessive inflammatory reactions. In general, T-cell subsets are distinguished by function and unique cytokine profiles. Proallergic TH2 and TH9 cells secrete type 2 cytokines interleukin (IL) 4, IL-5, IL-9, and IL-13, which promote hallmark features of allergy, such as mucous production, alterations in epithelial and stromal architecture, recruitment of mast cells, basophils, and eosinophils, and increases in serum IgE antibody levels.9 Treg populations dampen responses through a variety of mechanisms, including cell-cell contact and anti-inflammatory cytokine production, such as TGF-β and IL-10.10
B cells also play a major role in allergic responses by coordinating humoral responses as directed by T cells. Specifically, B cells undergo isotype class switching from IgM to IgE through paracrine secretions of IL-4 and IL-13 by TH2 cells and CD40:CD40 ligand ligation between B and T cells, respectively.11 B-cell induction of allergen specific IgE and subsequent binding of IgE through FcεRI on effector basophils and mast cells lead to IgE-mediated inflammation on allergen exposure. Recently, IgG4 and IgA antibody subclasses have emerged as key immunoglobulins in regulation of FAs based on data from immunotherapy trials. High levels of IgG4 correlated with sustained unresponsiveness in patients undergoing immunotherapy.12 It has been postulated that IgG4 confers a protective effect through inhibitory receptors on effectors cells that counterbalance IgE-mediated activation.13,14 Recent data from Wright et al indicate that egg specific IgA is associated with clinical responsiveness to egg oral immunotherapy (OIT).15 Finally, a sub-population of regulatory B cells that produce anti-inflammatory cytokine IL-10 have been identified. However, their role in modulating immune responses to food allergens is unclear and is currently being explored.16
Mast cells, eosinophils, and basophils are cellular contributors that modulate IgE-mediated release of proinflammatory factors. Allergic inflammation consists of 2 phases: an early phase and a late phase. In sensitized individuals with high IgE titers, food allergens attach to IgE prebound to FcεR1 receptors on mast cells, eosinophils, and basophils. Cross-linking by food allergens leads to the release of preformed proinflammatory mediators, such as histamine, tryptase, and tumor necrosis factor α (TNF-α), leading to the onset of immediate allergic reactions. Newly synthesized proinflammatory molecules, such as leukotrienes, platelet-activating factor, and cytokines IL-4, IL-5, and IL-13 are later produced, which recruit and activate a variety of immune cells to maintain allergic inflammation.17
In recent years, type 2 innate lymphoid cells (ILC2s) and natural killer T (NKT) cells have also been implicated in allergic response. ILC2s promote inflammatory response by producing IL-4 and IL-13. NKT cells have characteristics of both T cells and natural killer cells and when stimulated via toll-like receptors regulate airway inflammation. However, our understanding of the roles of ILC2s and NKT cells is still in its infancy.18,19
Overview of Mechanism of Allergic Response in the Gastrointestinal Tract
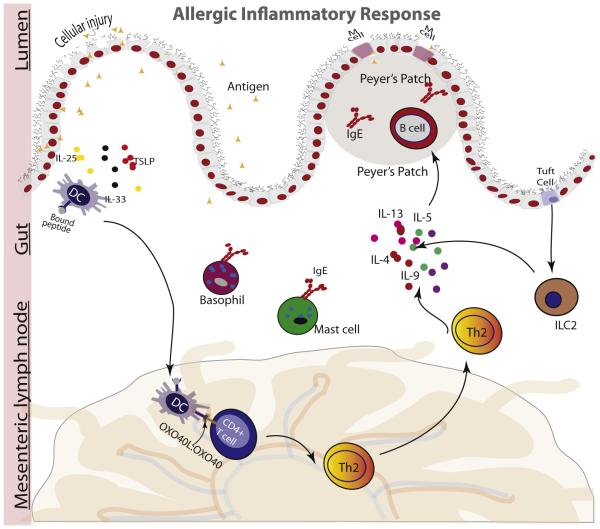
IgE-mediated FAs are initiated by a sensitization phase that occurs on initial exposure to the causal allergen. This is followed by an effector phase on recurring allergen exposure with amplification of IgE-mediated allergic inflammation.1 Antigens are initially encountered at barrier surfaces, such as skin, gastrointestinal tract, or respiratory tract, and sensitization is thought to potentially occur through one or more of these routes. In the gastrointestinal tract, food proteins undergo denaturation and degradation and pass through the epithelial barrier, where they encounter and are processed by APCs, such as DCs and macrophages (Fig 1). DCs in the lamina propria sample the antigens, process them into small peptide fragments (T-cell epitopes), migrate to the lymphoid tissue, and present the epitopes to naive T lymphocytes.
Figure 1.
Breakdown of the epidermal barrier increases secretion of epithelium-derived cytokines (interleukin [IL] 25, IL-33, and thymic stromal lymphopoietin [TSLP]), promoting increases in TH2 cells, IgE class switching by B cells, and accumulation of proinflammatory mediators. DC indicates dendritic cell.
CD4+ naive T cells recognize allergen-specific T-cell epitopes on DCs and bind them through their T-cell receptors. Upregulation of OX40L on exposure of DCs to proinflammatory cytokines and to food antigens is associated with differentiation of naive T cells to TH2 cells. On differentiation, TH2 cells proliferate and produce IL-4, IL-5, and IL-13, which mediate a number of cellular responses, including recruitment and activation of effector cells and B cell isotope switching from IgM to IgE.7 IgE then binds to high-affinity FcεRI receptors on the surface of mast cells and basophils and mediates inflammatory responses.
Disruption of the epithelial barrier enabling increased penetration by intact food allergen is believed to play a key role in allergic sensitization. This is supported by a number of disparate observations. A population-based study of more than 4,000 infants found that those who developed eczema in the first 3 months after birth were 6 and 11 times more likely to develop oral food challenge–proven egg and peanut allergy, respectively, by 12 months of age than infants without eczema.20 Increased risk of eczema and FA is associated with filaggrin mutation, which is a loss-of-function mutation that leads to dysfunctions in the skin barrier.21 Sensitization through the skin is thought to increase likelihood of peanut allergy in individuals with filaggrin loss-of-function mutation exposed to environmental dust containing peanuts. It has also been hypothesized that the introduction of fruits and vegetables containing fermentable fiber in early infancy could increase microbial diversity and short-chain fatty acids, which are known to promote epithelial integrity and reduce penetration of intact food allergens. Loss of epithelial integrity in the skin or gut increases antigen uptake and promotes secretion of epithelial-derived cytokines IL-33, thymic stromal lymphopoietin, and IL-25.22 These cytokines promote TH2-type allergic response by activation of ILC2s, mast cells, basophils, and DCs.7 Activation of ILC2s stimulates production of IL-4–, IL-5–, and IL-13–promoting TH2-type allergic responses.23 Overall, the state of the epithelial barrier and the extent of degradation of the food proteins are thought to be important for sensitization to food antigens.
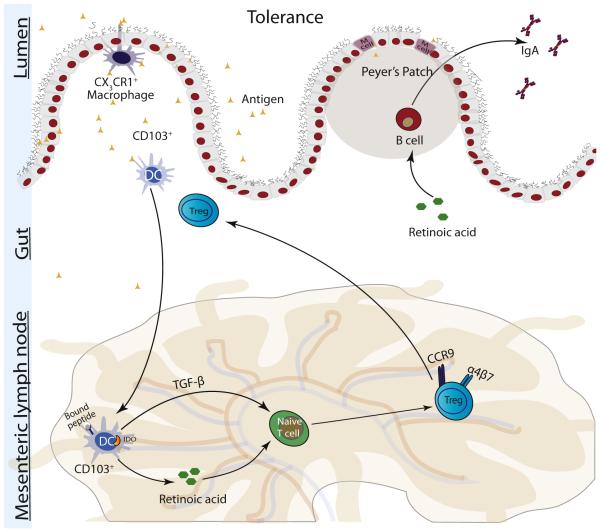
Overview of Mechanisms of Immune Tolerance (Permanent Unresponsiveness)
Healthy immune unresponsiveness to commonly ingested foods is an active process that occurs within the gut-associated lymphoid tissue and is termed oral tolerance. As in allergy, in immune tolerance, food antigens are processed by DCs and presented to naive T cells (Fig 2). How the uptake and presentation of antigens differ in allergy and tolerance is not well understood.7 In tolerance, DCs secrete costimulatory molecules, such as TGF-β, IL-10, retinoic acid, IDO, and retinal aldehyde dehydrogenase. These molecules promote the differentiation of naive T cells into CD4+ Tregs, which are essential for the suppression of TH2-mediated inflammation, induction of oral tolerance, and oral desensitizations. DCs also induce expression of the CCR9 and α4β7 receptors, which are some of the factors that induce T cell migration to the gut.24 Although not inclusive of all Tregs, an important cellular marker used for identifying Tregs is the transcription factor forkhead box 3 (Foxp3). Mutations of Foxp3+ in humans result in a severe autoimmune disease called IPEX, and low levels of Foxp3 in the placenta are associated with atopy in infants. In adult mice, ablation of Foxp3+ Tregs leads to onset of autoimmune diseases showing the continued importance of the Foxp3 transcription factor in auto-immunity throughout life.25 There are multiple subtypes of Tregs, but 3 main subtypes of CD4+ Tregs are now thought to be involved in tolerance: Foxp3+ natural Tregs, Foxp3-Tr1 cells, and Foxp3+ TH3 cells. In humans, there are no unique cellular markers that distinguish Tregs from TH cells, and identification of Tregs requires functional assays in addition to cellular markers and cytokine secretion profiles (Table 2).10 Tr1 regulatory cells predominantly secrete IL-10, TH3 cells predominantly secrete TGF-β, and natural Tregs secrete moderate amounts of both cytokines. The central role of Tregs in tolerance is well established; however, the importance of the individual Treg subtypes in oral tolerance is less clear because there is now evidence that Tregs exhibit phenotypic plasticity. Overall, tolerance is thought to be brought about by one or more of the following mechanisms: suppression of TH2 cells, decreased production of IgE by B cells, increased IgA and IgG4 production by B cells, suppression of effector T-cell migration to tissues, induction of IL-10–producing DCs, and suppression of basophil, eosinophil, and mast cell activation.26
Figure 2.
Tolerance is an active immune process. Dendritic cells (DCs) present food antigens to naive T cells, promoting their differentiation into T-regulatory cells (Tregs). These cells secrete interleukin 10 (IL-10) and transforming growth factor β (TGF-β), which suppresses allergic response. IDO indicates indoleamine 2,3-dioxygenease.
Table 2.
Characteristics of Regulatory T-Cell Subtypesa
| nTregs | Tr1 | TH3 | |
|---|---|---|---|
| Development | Thymus | Periphery | Periphery |
| CD4 | + | + | + |
| CD25 | + | + | + |
| Foxp3 | + | − | + |
| IL-10 | + | +++ | + |
| TGF-β | + | + | +++ |
Abbreviations: IL-10, interleukin 10; nTregs, natural T-regulatory cells; TGF-β, transforming growth factor β.
Plus sign indicates positive; minus sign, negative.
Immunotherapy: Cellular and Molecular Responses and Potential Mechanisms of Desensitization and Sustained Unresponsiveness
Current evidence indicates that, as with sensitization, tolerance and desensitization can occur by many routes. Immunotherapy treatments for FA have mainly included sublingual immunotherapy (SLIT), epicutaneous immunotherapy (EPIT), and OIT.27 Although subcutaneous immunotherapy is effective in desensitization, it has been associated with high rates of anaphylactic reactions and is no longer used for FA immunotherapy.28
During SLIT, small amounts of food allergen extracts are placed under the tongue. The food allergens are taken up by APCs, which are present in the oral mucosa. It is hypothesized that by bypassing denaturation and degradation in the gastrointestinal tract during SLIT, a larger number of epitopes of the protein are presented to these cells than in OIT. However, there are practical limitations because only low volumes of allergens can be delivered sub-lingually. The safety profile of SLIT is superior to OIT; however, efficacy appears to be lower than that achieved with OIT.27,29
EPIT is novel patch delivery system developed by Viaskin (DBV Technologies SA, Paris, France) that can be used on intact skin and has a safety profile similar to SLIT. It delivers microgram amounts of allergens through the skin. EPIT has shown promise in phase 1 and phase 2 studies. Phase 3 studies of EPIT are under way.5 The review by Sindher et al27 discusses relevant clinical studies with SLIT and EPIT. A study in mice suggests that EPIT enables sustained protection against food-induced anaphylaxis by selectively increasing gut-homing latency-associated peptide Tregs. Interestingly, these Tregs did not suppress IgE but directly suppressed mast cell activation.30 Epigenetic modifications with increased methylation of the GATA-3 promoter region has also been observed.31
Although OIT is associated with higher rates of adverse effects than SLIT and EPIT, it is the predominant form of immunotherapy for FAs in clinical trials. OIT is typically administered in 3 steps or stages: an initial escalation phase during which allergens are administered in increasing amounts within a single day to determine threshold of reactivity, a buildup phase in which allergen dose is increased periodically (in most instances every 1–2 weeks) to milligram or gram quantities, and a final maintenance phase in which allergens are ingested daily at a regular constant dose.5 Treatment with omalizumab during OIT buildup to prevent IgE-mediated reactions has also shown great success in treating individuals at high risk of severe reactions and simultaneously treating individuals for multiple allergens.32,33 In a study of OIT in children with egg allergy, 75% of children were desensitized after 22 months of OIT.12 However, long-term studies of successful OIT with rechallenge after a period of allergen avoidance (2 weeks to 6 months) indicates sustained unresponsiveness in only a subset of patients.34 A correlation between low baseline IgE and sustained unresponsiveness has been observed.29 Other biomarkers that can differentiate those who are likely to achieve sustained unresponsiveness, such as Foxp3 methylation status, Tregs, and IgG, are being explored.34,35 Reported rates of sustained unresponsiveness are 50% (1 month of peanut avoidance),36 28% (4–6 weeks of egg avoidance),12 30% (3 months of peanut avoidance),35 and 13% (6 months of peanut avoidance).35 OIT induces desensitization in individuals with FA; however, whether OIT results in true tolerance is uncertain.37 There are many unanswered questions and whether optimization of allergen dose, route, or treatment period can result in an eventual cure rather than just a period of desensitization is unclear.
The mechanisms underlying sustained unresponsiveness or desensitization with immunotherapy are still mostly a black box and an area of intense investigation. Tremendous progress has been made, but current understanding of the immune pathways involved in FA is as yet incomplete. It is not known whether sustained unresponsiveness differs from desensitization or whether these are steps in the pathway toward tolerance. It has been proposed that, as in tolerance, the main mechanism underlying OIT is induction of Tregs with concomitant increases in IL-10 and TGF-β.38 However, there is no conclusive evidence of this in humans, and it is as yet unclear whether a waning of allergic sensitization, an induction of Tregs, or other factors cause tolerance.39,40
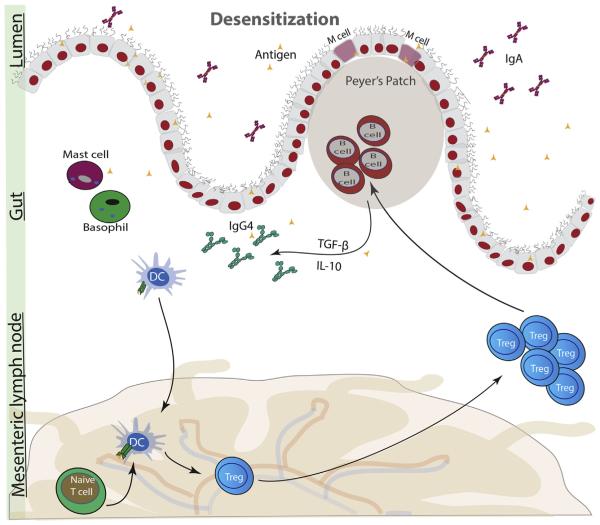
A number of changes have been observed in OIT (Fig 3). Decreases in activation of mast cells and basophils are observed as determined by SPTs and basophil activation tests.41,42 Increases in IgG4 after immunotherapy have been consistently observed in studies.43 Furthermore, a study by Santos et al13 revealed that removal of IgG4 in peanut sensitive patients mimicked peanut allergic serum. Increases in IgA have also been reported, but not all studies have observed these changes.44,45 IgA is the most abundant antibody and by binding antigen it either prevents attachment of the antigen to the epithelium or promotes antigen agglutination. Increased risk of FA in children has been associated with IgA deficiency.46 A number of studies reported decreases in IgE, although some have reported no changes in IgE. Current data support an initial increase in IgE followed by an eventual decrease in IgE with OIT.36,47,48 The mechanism behind a shift from IgE to IgG4 is unclear and may likely involve anergy or deletion of IgE-producing B cells or increases in IgG4-producing B cells.
Figure 3.
During immunotherapy, current evidence indicates increases in T-regulatory cells (Tregs) and IgG4 class switching by B cells. Mast cells and basophil activation is decreased. IgG4 may compete with IgE to dampen allergic response. IL-10 indicates interleukin 10; TGF-β, transforming growth factor β.
Decreases in TH2 cells,49 increases in anergic or apoptotic antigen specific TH2 cells,50 increases in Foxp3+Tregs,42 and increases in TGF-β and IL-10 have been reported,48 but these results, based on immunotherapy studies to food and aeroallergens, have not been consistent across studies. The subtypes of Tregs involved in tolerance or desensitization have been hard to conclusively determine because of the lack of definitive markers, their short lifespan, and their low frequencies.51 It was initially hypothesized that the observed increases in IL-10 after immunotherapy are attributable to its secretion by Foxp3−Tr1 cells, but it is now thought that this increase is brought about by B-regulatory cells rather than T cells.52 A study in ovalbumin-sensitized mice found that administration of low levels of antigens resulted in the generation of antigen-specific Tregs. However, the same study found that administration of high doses of allergens induced deletion or anergy of antigen-specific effector T cells,53 suggesting that a waning of allergic sensitization may also cause tolerance. Foxp3+Tregs are known to be important in immune regulation and in tolerance. In a study by Syed et al,35 hypomethylation of CpG sites on Foxp3+ Tregs was seen in individuals who achieved sustained unresponsiveness 6 months after treatment withdrawal, suggesting that epigenetic changes may also play a role in desensitization and tolerance. Epigenetic analysis of samples obtained from children in a Dutch birth cohort study found that hypermethylation was observed in children with cow’s milk allergy compared with children in the tolerant and control groups.54 Although much progress has been made in understanding key cells and molecules associated with FA and tolerance, there is still much we do not understand. Research needs to focus on unraveling the immune pathways associated with immunotherapy.
Mechanistic Studies: Common Methods and Experimental Models in Immunotherapy
There is a large repertoire of experimental models and methods that are being used to study FA and OIT mechanisms.55 CyTOF is a novel mass cytometry technique, which combines flow cytometry and mass spectrometry and allows for more than 40 targets to be detected in a single sampling of cells by labeling with metal isotypes rather than flurochromes. This method provides lesser signal overlap and offers a platform for a more comprehensive investigation of immune cell phenotypes compared with the traditional flow cytometry method. It also provides the option to use phospho-specific antibodies, which enables investigators to look at signaling pathways, such as B- and T-cell signaling.
Other methods target smaller immune cell subpopulations in cultures to look at more specific phenotypes. Syed et al55 looked at CD4+CD25hi-Foxp3+ Tregs, previously proliferated in the presence of peanut, to assess immune tolerance during an OIT trial. The same study also used epigenetic investigations to track hypomethylation of Foxp3 CpG sites of allergen-induced Tregs during OIT, which may also help to indicate clinical tolerance. The role of epigenetics in FA is increasingly recognized, and genome-wide DNA methylation studies have shown promise in predicting clinical outcome during food challenge.56 Other studies include the assay for transposase-accessible chromatin with high-throughput sequencing, or ATAC-seq, which probes DNA accessibility with hyperactive Tn5 transposase A to quickly map genome-wide chromatin accessibility57 and the flow cytometricebased basophil activation test, which measures markers of basophil activation as an indicator of allergy after stimulation of the individual with specific antigens.58
For questions that are directed at functions and behavior of single cells, which cannot be answered at the population level, methods such as single-cell sorting are invaluable. Cell sorting is another flow cytometry–based method that physically sorts cells into separate entities based on fluorescent labels on specific cell population targets. This method was used by a research group to isolate specific B cells that bound to only peanut antigens. The researchers then applied a combination of genomic and biochemical assays to evaluate the behavior of the isolated peanut-specific B cells with respect to isotype switching during peanut OIT.59
Investigators now often incorporate “omics” studies into their research. In one study looking at CD4+ T cells and their role in clinical tolerance, investigators single-cell sorted allergen-specific T cells and sequenced T-cell receptors of these single cells to assess changes to their functionality and phenotypes in individuals undergoing OIT.60 By using a combination of methods, studies are now able to track changes in behavior of individual immune cells during immunotherapy.
Conclusion
Currently, there are still many gaps in our understanding of the mechanisms underlying immunotherapy. Although immunotherapy is promising for desensitizing individuals to FA, the dose, delivery method, and maintenance period need to be optimized to increase safety and bring about lasting tolerance. Recent methodologic advances, including the ability to analyze single cells, have now provided us with powerful techniques that can aid in the improvement of existing therapies and the development of potential cures of FA. They can also provide valuable guidance for assessing safety during immunotherapy, choosing the best mode of therapy, and establishing optimal dosing regimens during immunotherapy trials.
Acknowledgments
Funding Sources: This study was supported by the Sean N. Parker Center for Allergy and Asthma Research at Stanford University.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- [1].Valenta R, Hochwallner H, Linhart B, Pahr S. Food allergies: the basics. Gastroenterology. 2015;148:1120–1131. doi: 10.1053/j.gastro.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chokshi NY, Sicherer SH. Interpreting IgE sensitization tests in food allergy. Expert Rev Clin Immunol. 2016;12:389–403. doi: 10.1586/1744666X.2016.1124761. [DOI] [PubMed] [Google Scholar]
- [3].Shu SA, Chang C, Leung PS. Common methodologies in the evaluation of food allergy: pitfalls and prospects of food allergy prevalence studies. Clin Rev Allergy Immunol. 2014;46:198–210. doi: 10.1007/s12016-012-8337-8. [DOI] [PubMed] [Google Scholar]
- [4].Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- [5].Wood RA. Food allergen immunotherapy: current status and prospects for the future. J Allergy Clin Immunol. 2016;137:973–982. doi: 10.1016/j.jaci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- [6].Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31:61–75. doi: 10.1016/j.nutres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137:984–997. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- [9].Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- [10].Conrad ML, Renz H, Blaser K. Immunological approaches for tolerance induction in allergy. Curr Top Microbiol Immunol. 2011;352:1–26. doi: 10.1007/82_2011_128. [DOI] [PubMed] [Google Scholar]
- [11].Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39:440–456. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]
- [12].Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134:1310–1317. doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wright BL, Kulis M, Orgel KA, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71:1552–1560. doi: 10.1111/all.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011;3:168–177. doi: 10.4168/aair.2011.3.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van Rijt L, von Richthofen H, van Ree R. Type 2 innate lymphoid cells: at the cross-roads in allergic asthma. Semin Immunopathol. 2016;38:483–496. doi: 10.1007/s00281-016-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zakeri A, Yazdi FG. Toll-like receptor-mediated involvement of innate immune cells in asthma disease [published online August 17, 2016] Biochim Biophys Acta. doi: 10.1016/j.bbagen.2016.08.009. http://dx.doi.org/10.1016/j.bbagen.2016.08.009. [DOI] [PubMed]
- [20].Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? results from a population-based cohort. Clin Exp Allergy. 2015;45:255–264. doi: 10.1111/cea.12406. [DOI] [PubMed] [Google Scholar]
- [21].Wawrzyniak P, Akdis CA, Finkelman FD, Rothenberg ME. Advances and highlights in mechanisms of allergic disease in 2015. J Allergy Clin Immunol. 2016;137:1681–1696. doi: 10.1016/j.jaci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- [22].Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [23].Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim KS, Surh CD. Induction of immune tolerance to dietary antigens. Adv Exp Med Biol. 2015;850:93–118. doi: 10.1007/978-3-319-15774-0_8. [DOI] [PubMed] [Google Scholar]
- [25].Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- [26].Soyer OU, Akdis M, Ring J, et al. Mechanisms of peripheral tolerance to allergens. Allergy. 2013;68:161–170. doi: 10.1111/all.12085. [DOI] [PubMed] [Google Scholar]
- [27].Sindher S, Fleischer DM, Spergel JM. Advances in the treatment of food allergy: sublingual and epicutaneous immunotherapy. Immunol Allergy Clin North Am. 2016;36:39–54. doi: 10.1016/j.iac.2015.08.008. [DOI] [PubMed] [Google Scholar]
- [28].Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- [29].Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–1282. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC. Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis [published online June 11, 2016] J Allergy Clin Immunol. doi: 10.1016/j.jaci.2016.03.057. http://dx.doi.org/10.1016/j.jaci. 2016.03.057. [DOI] [PMC free article] [PubMed]
- [31].Mondoulet L, Dioszeghy V, Puteaux E, et al. specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol. 2015;135:1546–1557. doi: 10.1016/j.jaci.2014.11.028. [DOI] [PubMed] [Google Scholar]
- [32].Umetsu DT, Rachid R, Schneider LC. Oral immunotherapy and anti-IgE antibody treatment for food allergy. World Allergy Organ J. 2015;8:20. doi: 10.1186/s40413-015-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Begin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yee CS, Rachid R. The heterogeneity of oral immunotherapy clinical trials: implications and future directions. Curr Allergy Asthma Rep. 2016;16:25. doi: 10.1007/s11882-016-0602-0. [DOI] [PubMed] [Google Scholar]
- [35].Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cabrera CM, Urra JM. Food allergy and the oral immunotherapy approach. Arch Immunol Ther Exp (Warsz) 2015;63:31–39. doi: 10.1007/s00005-014-0304-z. [DOI] [PubMed] [Google Scholar]
- [39].Wambre E. Effect of allergen-specific immunotherapy on CD4+ T cells. Curr Opin Allergy Clin Immunol. 2015;15:581–587. doi: 10.1097/ACI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kwok WW. Modulation of Peanut-specific humoral and cellular responses pre- and post-oral immunotherapy. Clin Exp Allergy. 2015;45:1146–1149. doi: 10.1111/cea.12552. [DOI] [PubMed] [Google Scholar]
- [41].Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- [42].Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tang ML, Martino DJ. Oral immunotherapy and tolerance induction in childhood. Pediatr Allergy Immunol. 2013;24:512–520. doi: 10.1111/pai.12100. [DOI] [PubMed] [Google Scholar]
- [44].Vazquez-Ortiz M, Pascal M, Juan M, Alsina L, Martin-Mateos MA, Plaza AM. Serum allergen-specific IgA is not associated with natural or induced tolerance to egg in children. Allergy. 2013;68:1327–1332. doi: 10.1111/all.12217. [DOI] [PubMed] [Google Scholar]
- [45].Savilahti EM, Saarinen KM, Savilahti E. Duration of clinical reactivity in cow’s milk allergy is associated with levels of specific immunoglobulin G4 and immunoglobulin A antibodies to beta-lactoglobulin. Clin Exp Allergy. 2010;40:251–256. doi: 10.1111/j.1365-2222.2009.03409.x. [DOI] [PubMed] [Google Scholar]
- [46].Janzi M, Kull I, Sjoberg R, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133:78–85. doi: 10.1016/j.clim.2009.05.014. [DOI] [PubMed] [Google Scholar]
- [47].Sugimoto M, Kamemura N, Nagao M, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27:276–282. doi: 10.1111/pai.12535. [DOI] [PubMed] [Google Scholar]
- [48].Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Int. 2010;59:43–51. doi: 10.2332/allergolint.09-OA-0107. [DOI] [PubMed] [Google Scholar]
- [49].Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- [50].Ryan JF, Hovde R, Glanville J, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016;113:E1286–E1295. doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Palomares O. The role of regulatory T cells in IgE-mediated food allergy. J Investig Allergol Clin Immunol. 2013;23:371–382. [PubMed] [Google Scholar]
- [52].Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013;62:425–433. doi: 10.2332/allergolint.13-RAI-0608. [DOI] [PubMed] [Google Scholar]
- [53].Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- [54].Petrus NC, Henneman P, Venema A, et al. Cow’s milk allergy in Dutch children: an epigenetic pilot survey. Clin Transl Allergy. 2016;6:16. doi: 10.1186/s13601-016-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sathe SK, Liu C, Zaffran VD. Food allergy. Annu Rev Food Sci Technol. 2016;7:191–220. doi: 10.1146/annurev-food-041715-033308. [DOI] [PubMed] [Google Scholar]
- [56].Martino D, Dang T, Sexton-Oates A, et al. Blood DNA methylation biomarkers predict clinical reactivity in food-sensitized infants. J Allergy Clin Immunol. 2015;135:1319–1328. doi: 10.1016/j.jaci.2014.12.1933. [DOI] [PubMed] [Google Scholar]
- [57].Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol. 2015;109:21–29. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Santos AF, Lack G. Basophil activation test: food challenge in a test tube or specialist research tool? Clin Transl Allergy. 2016;6:10. doi: 10.1186/s13601-016-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hoh RA, Joshi SA, Liu Y, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J Allergy Clin Immunol. 2016;137:157–167. doi: 10.1016/j.jaci.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]