SUMMARY
Case reports of Zika virus (ZIKV) sexual transmission and genital persistence are mounting. Venereal transmission and genital persistence threaten public health within and beyond the range of ZIKVs’ mosquito vectors. In this study, we administered ZIKV into vaginas of AG129 mice and LysMCre+IFNARfl/fl C57BL/6 mice after hormonal treatments. Mice infected during estrus-like phase were resistant to vaginal infection. In contrast when infected during diestrus-like phase, AG129 mice succumbed to infection, whereas LysMCre+IFNARfl/fl mice experienced transient illness. Patency of transgenital transmission (TGT) in diestrus-like mice was demonstrated by detection of viremia and ZIKV replication in spleen and brain, and viral RNA persisted in vaginal washes as late as 10 days post infection. In these lethal and sublethal mouse models, this study indicates that intravaginal deposition of ZIKV can cause TGT, hormonal changes in the female reproductive tract (FRT) influence transmission, and ZIKV replication persists in the FRT for several days.
Keywords: Zika virus, semen, sperm, sexual, venereal, sexual, genital, vagina, mouse model, diestrus
INTRODUCTION
Relatively little is currently known about Zika virus (ZIKV) which, like other members of family Flaviviridae, is enveloped with a positive-sense, single-stranded RNA genome (Lazear and Diamond, 2016). ZIKV is transmitted by mosquitoes of the Aedes genus, including Aedes aegypti. From the 1950s through 2014, small outbreaks of ZIKV were detected from the virus’ African origin to Southeast Asia and Oceania, with recognized symptomology ranging from asymptomatic infection to self-limiting, febrile illness. Beginning in French Polynesia in 2013, Guillain Barre Syndrome was recognized in large numbers of ZIKV-infected patients (Watrin et al., 2016). ZIKV then emerged on a larger scale in Brazil in 2015 with infections associated with new clinical problems including congenital microcephaly (Rasmussen et al., 2016). Since then, ZIKV has spread to mosquito populations to produce endemic human infections from Latin America to Florida (McCarthy, 2016), and imported infections throughout the world (World Health Organization, 2016a). The United States Centers for Disease Control and Prevention has confirmed that ZIKV causes congenital microcephaly (Rasmussen et al., 2016), data are being collected to further characterize congenital Zika syndrome (van der Linden et al., 2016), and the World Health Organization (WHO) has declared the current ZIKV outbreak to be a global health emergency (World Health Organization, 2016b).
Although ZIKV has rightfully gained notoriety as an arbovirus, case reports of heterosexual and homosexual transmission (Brooks et al., 2016; D’Ortenzio et al., 2016; Davidson et al., 2016; Deckard et al., 2016; Freour et al., 2016; Hills et al., 2016; Venturi et al., 2016), and reports of viral persistence in semen (Atkinson et al., 2016; Mansuy et al., 2016; Matheron et al., 2016) are mounting. The public health implications of sexual transmission are far reaching, as this mode allows the virus to travel beyond its vector’s geographic ranges to unsuspecting human populations. Implications of ZIKV infection, whether acquired sexually or by other routes, extend to whether infection might impact fertility, and to the safety of banked sperm and eggs.
Multiple routes may be implicated with sexual transmission (vaginal intercourse vs. kissing, fellatio, etc.), and the current study models the deposition of virus into the vagina as would occur with atraumatic intravaginal ejaculation. Upon deposition of ejaculate into the vagina, a portion of the semen ascends across the cervix into the lumen of the uterus and upper female reproductive tract (FRT). Since transgenital ZIKV infection of the female could occur anywhere from the vagina to the fallopian tubes (Wira et al., 2015), in the present report we use the term transgenital transmission (TGT) to signify an infection which originates with virus deposited into the lumen of the FRT and results in viremia.
A mouse model has been reported for TGT of West Nile virus (Burke et al., 2004), Japanese encephalitis virus has been transmitted to sows by artificial insemination (Habu et al., 1977), and naturally occurring TGT is seen with pestiviral flaviviruses including border disease virus in sheep (Braun et al., 2015), bovine viral diarrhea virus (Bielanski et al., 2013), and classical swine fever virus (Floegel et al., 2000). A recent study demonstrated vaginal ZIKV replication in C57BL/6 mice and TGT in mice with interferon (IFN) pathway mutations (Yockey et al., 2016). In the current study, we administered ZIKV into the vaginas of AG129 mice, which globally lack type I (IFNAR) and type II (IFNGR) IFN receptors, and LysMCre+IFNARfl/fl C57BL/6 mice, which lack IFNAR in myeloid cells, in estrus-like and diestrus-like phases after respective hormonal treatments. We observed that diestrus-like AG129 mice developed lethal disease and that diestrus-like LysMCre+IFNARfl/fl mice recovered from disease, whereas estrus-like mice were resistant to ZIKV TGT. We verified patency of TGT by qRT-PCR detection of viremia and immunohistochemical detection of ZIKV NS2B, a marker of viral replication, in spleen and cerebrum. Immunohistochemistry (IHC) also detected NS2B in the vaginal tissues and cells within draining lymph nodes at an early time point after infection. Vaginal swab viral RNA persisted until the last day of measurement at 10 days post-infection (dpi). These results demonstrate TGT of ZIKV, imply that infected semen can cause ZIKV TGT, and suggest a protective influence of estrus-like phase and a permissive influence of progesterone on genital anti-ZIKV immunity. Further, these lethal and sublethal mouse models of female ZIKV TGT establish platforms on which the pathogenesis of and preventative strategies against ZIKV sexual transmission can be investigated.
RESULTS
Diestrus-like mice show morbidity and mortality following atraumatic intravaginal ZIKV administration
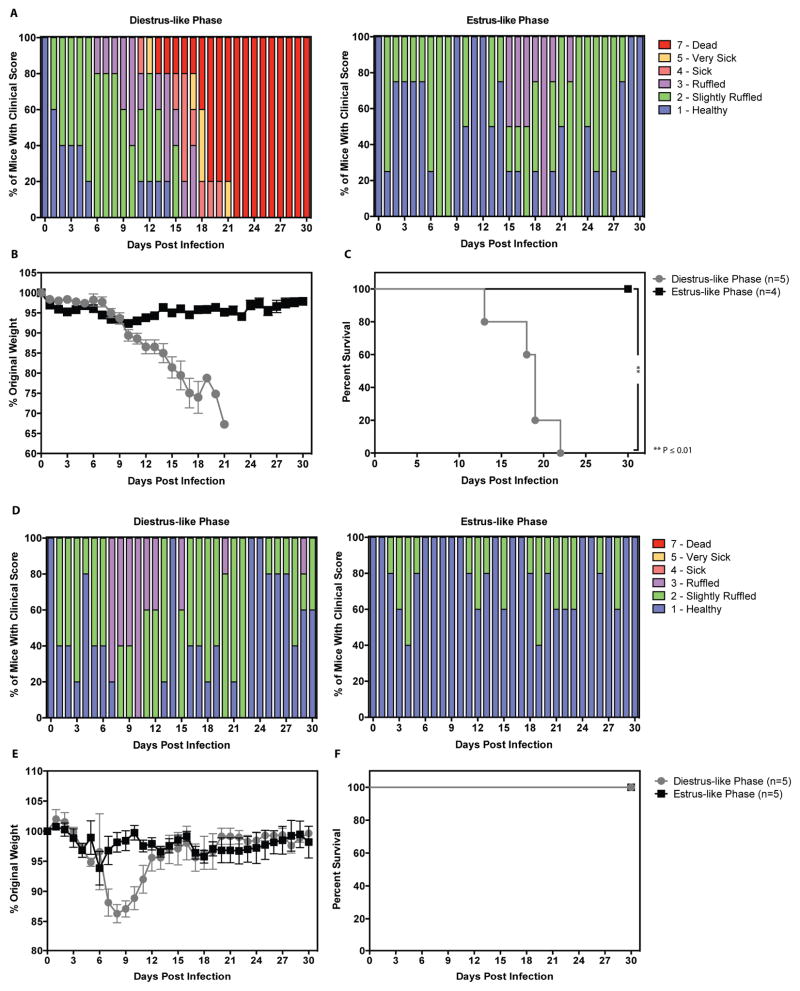
To begin evaluating whether ZIKV TGT was feasible in sexually mature mice, 8–12 week old female AG129 and LysMCre+IFNARfl/fl mice were induced into diestrus-like and estrus-like phases by injection of progesterone and pregnant mare serum gonadotropin (PMSG), respectively. Following atraumatic administration of 1 × 105 focus-forming units (FFU) ZIKV (strain FSS13025; 2010 clinical isolate from Cambodia) into the vagina of AG129 mice and 1 × 106 FFU ZIKV FSS13025 into the vagina of LysMCre+IFNARfl/fl mice, mice were weighed and observed for clinical score daily. Diestrus-like AG129 mice exhibited progressive increase in clinical score (Figure 1A) and weight loss (Figure 1B) beginning at 9dpi and extending to the times of their death or severe disease necessitating euthanasia. Time to severe disease/mortality ranged from 13 to 22dpi (Figure 1C). Diestrus-like LysMCre+IFNARfl/fl mice showed increased clinical scores (Figure 1D) and weight loss (Figure 1E) beginning at 6dpi and peaking at 9dpi. These mice recovered from ZIKV disease with no mortality (Figure 1F) and returned to 100% of original weight by 16dpi. Both estrus-like AG129 and LysMCre+IFNARfl/fl mice did not show any weight loss, morbidity, or mortality. Collectively, these results demonstrate that AG129 and LysMCre+IFNARfl/fl mice in diestrus-like but not estrus-like phase manifest clinical signs upon infection via atraumatic intravaginal route. Consistent with their immunodeficiencies, AG129 mice succumb to infection, while LysMCre+IFNARfl/fl mice recover.
Figure 1. Mice in diestrus-like phase are susceptible to intravaginal ZIKV infection.
Eight- to Twelve-week old AG129 (Figure 1A–C) and LysMCre+IFNARfl/fl C57BL/6 (Figure 1D–E) mice were intravaginally inoculated with 1 × 105 FFU and 1 × 106 FFU of ZIKV strain FSS13025, respectively. N = 4–5 mice per group. P-values were obtained using the Gehan-Breslow-Wilcoxon test (A–F), ** P≤0.01.
Kinetics of viremia
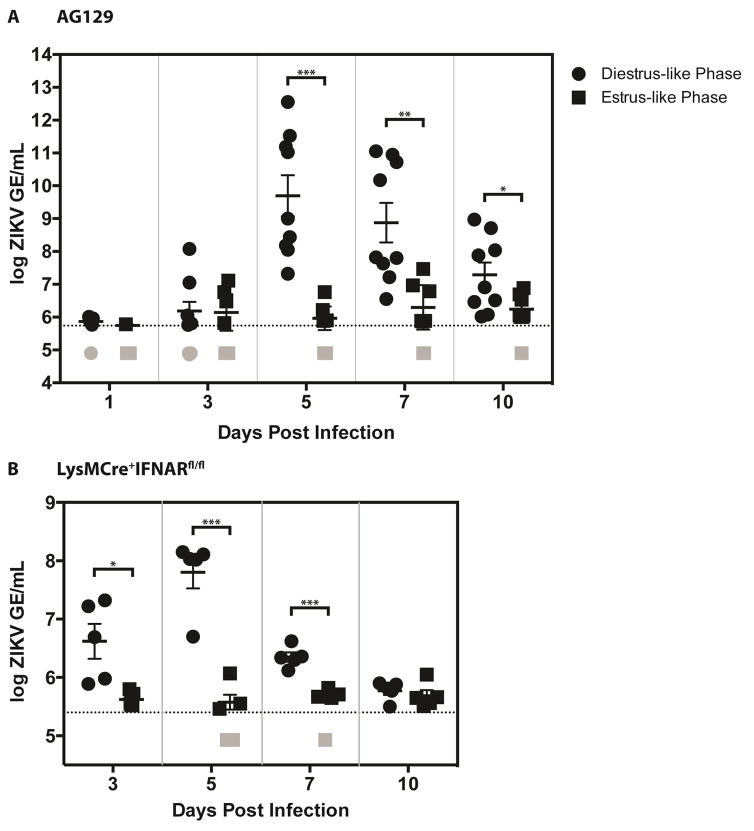
To determine whether attempted ZIKV TGT resulted in viremia, qRT-PCR was performed on serum samples following intravaginal infection (as described above) taken at 1, 3, 5, 7, and 10 dpi of diestrus-like and estrus-like AG129 mice and at 3, 5, 7, and 10 dpi of diestrus-like and estrus-like LysMCre+IFNARfl/fl mice. For diestrus-like AG129 mice, serum ZIKV RNA levels were not elevated at 3dpi (Figure 2A) whereas they were significantly higher for 3dpi diestrus-like LysMCre+IFNARfl/fl mice (Figure 2B). For both mouse strains in diestrus-like phase, viremia peaked at 5dpi and viral RNA levels were close to the lower limit of detection by 10dpi. Serum ZIKV RNA levels did not rise significantly for either of the mouse strains infected in estrus-like phase. These results, in combination with those shown in Figure 1, demonstrate TGT of ZIKV in diestrus-like mice.
Figure 2. Viremia in diestrus-like mice with intravaginal ZIKV infection.
Eight- to Twelve-week old AG129 (Figure 2A) and LysMCre+IFNARfl/fl C57BL/6 (Figure 2B) mice were intravaginally inoculated with 1 × 105 FFU and 1 × 106 FFU of ZIKV strain FSS13025, respectively. N = 4–5 mice per group. For figure 2A, two independent experiments were performed and data were pooled and expressed as mean ± SEM. Viral RNA levels were measured from serum collected on days 1, 3, 5, 7, and 10 for AG129 mice and on days 3, 5, 7, and 10 for LysMCre+IFNARfl/fl C57BL/6 mice. P-values were obtained using the parametric two-tailed unpaired t-test with Welch’s correction, * P≤0.05, ** P≤0.01, *** P≤0.001.
Histopathologic detection of ZIKV replication in tissues
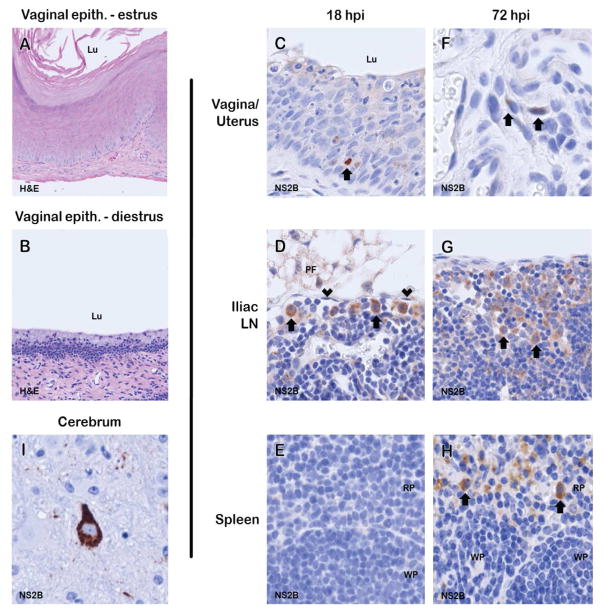
To determine whether ZIKV TGT resulted in local viral replication and systemic infection, formalin-fixed paraffin embedded tissues of estrus-like (Figure 3A) and diestrus-like (Figure 3B) AG129 mice were stained for expression of ZIKV NS2B, a viral nonstructural protein that is not carried into the virion and is thus a marker of viral replication (Lei et al., 2016). In diestrus-like mice at 18hpi, strong expression of NS2B was detected in rare cells within the vaginal epithelium (Figure 3C) and round cells (interpreted as macrophages based on morphology by a board-certified veterinary pathologist) in the subcapsular sinus of iliac lymph nodes (Figure 3D). In addition, there was weak NS2B expression in rare uterine stromal spindle cells in both diestrus-like (Figure 3F) and estrus-like (data not shown) mice. At 72hpi in diestrus-like mice, increased numbers of NS2B-positive cells (interpreted as macrophages) were seen in iliac lymph node sinuses (Figure 3G) and NS2B-positive cells (interpreted as macrophages) were also seen in the splenic red pulp (Figure 3H). In diestrus-like mice at 10dpi, strong NS2B expression was detected in neurons of the cerebrum (Figure 3I). These results demonstrate that ZIKV replicates locally in the vaginal tissue, followed by presence within cells in the draining lymph nodes and then eventual replication in the spleen and cerebrum. Taken together, these results indicate patency of ZIKV TGT.
Figure 3. Estrus- and diestrus-like vaginal histopathology and immunohistochemical detection of ZIKV protein NS2B in diestrus-like AG129 mice with intravaginal ZIKV infection.
At 18hpi, the vaginal mucosa was approximately 10 cell layers thick and covered by a thick layer of keratin in estrus-like mice (A), whereas the mucosa of diestrus-like mice (B) was approximately 5 cell layers thick and unkeratinized. At 18hpi NS2B immunoreactivity was detected in (C) rare cells in the vaginal epithelium and (D) round cells interpreted as macrophages in the subcapsular sinus of the iliac lymph nodes. Morphologically the subcapsular sinus is identified as a channel subjacent to the lymph node capsule that is lined by endothelium and usually does not contain erythrocytes. Little to no NS2B expression was seen in (E) spleen at 18hpi. At 72hpi, increased numbers of NS2B-positive cells were detected in (F) the uterine stroma, (G) iliac lymph node sinuses, and (J) cells within the splenic red pulp interpreted as macrophages. At 10dpi, there was strong NS2B expression in rare (I) neurons. No chromogenic reaction was present in control slides of each tissue incubated with non-specific IgG substituted for NS2B antibody [Lu, lumen; PF, perinodal fat; arrowheads, lymphatic endothelial cells; RP, red pulp; WP, white pulp; H&E, hematoxylin & eosin stain; arrows, NS2B-expressing cells; NovaRED chromogen; hematoxylin counterstain].
Viral burdens in the vaginal canal
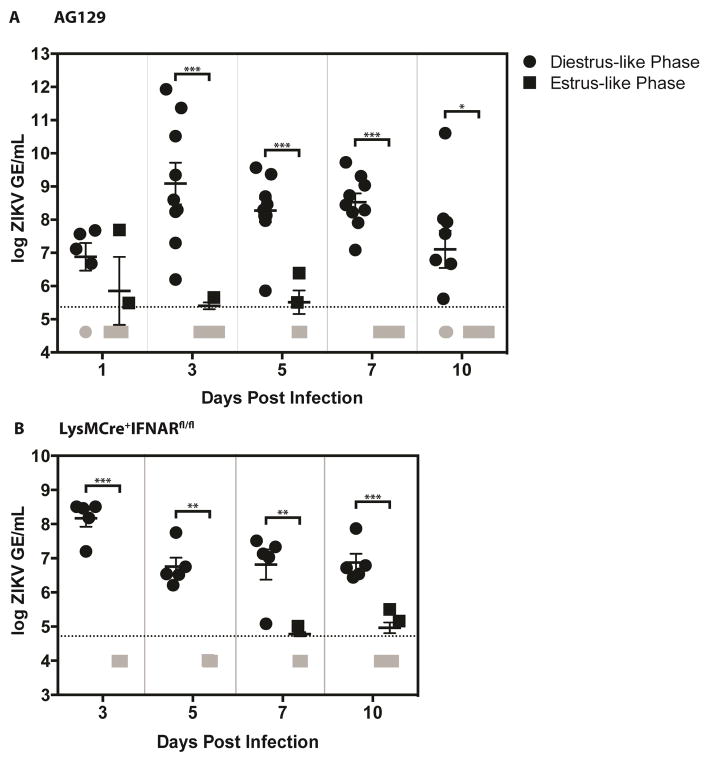
To determine the duration of ZIKV replication in the vaginal canal following intravaginal infection, qRT-PCR was performed on AG129 (Figure 4A) and LysMCre+IFNARfl/fl mice (Figure 4B) douche samples collected at 1, 3, 5, 7, and 10 dpi. In some diestrus-like AG129 and all diestrus-like LysMCre+IFNARfl/fl mice, viral RNA persisted in the vaginal canal up to the last day of measurement at 10dpi. Virus was not detected in estrus-like mice beginning from 3dpi. These results reveal that ZIKV replication can persist in the vaginal canal for a longer time in diestrus-like mice than in estrus-like mice following intravaginal administration of the virus. Viral RNA persistence may thus be a key factor in driving ZIKV TGT in diestrus-like but not estrus-like mice.
Figure 4. ZIKV vaginal RNA persists for up to 10 days post intravaginal infection.
Eight- to Twelve-week old AG129 (Figure 4A) and LysMCre+IFNARfl/fl C57BL/6 (Figure 4B) mice were intravaginally inoculated with 1 × 105 FFU and 1 × 106 FFU of ZIKV strain FSS13025, respectively. N = 4–5 mice per group. For figure 4A, two independent experiments were performed and data were pooled and expressed as mean ± SEM. Viral RNA titers were measured from vaginal washes collected in PBS on days 1, 3, 5, 7, and 10 for AG129 mice and on days 3, 5, 7, and 10 for LysMCre+IFNARfl/fl C57BL/6 mice. P-values were obtained using the parametric two-tailed unpaired t-test with Welch’s correction, * P≤0.05, ** P≤0.01, *** P≤0.001.
DISCUSSION
Research on ZIKV is in its infancy, and currently minimal information is available regarding the epidemiology and pathogenesis of ZIKV sexual transmission. Sexual behaviors involve the intended and unintended exchange of multiple body fluids at numerous anatomical sites. Case reports have indicated which sexual partner was first infected, isolated virus from semen, and reasonably postulated that transmission has been by deposition of infected semen into the vagina or rectum. However, the exact role of semen in sexual transmission might only be determined experimentally since natural sexual contact can include the intended and unintended exchange of multiple body fluids (semen, urethral and vaginal secretions, urine, blood, saliva, nasal secretions, exogenous lubricants and fluids) and contact of multiple mucous membranes. Detection of ZIKV in saliva (Bonaldo et al., 2016; Fourcade et al., 2016), vaginal mucus (Prisant et al., 2016), and urine may indicate that sexual transmission is not only mediated by semen. Further suspicion is raised by a recent case report of viral transmission between a ZIKV patient and caregiver without sexual contact (Tavernise, 2016). Additionally, the closely related Japanese encephalitis virus can be transmitted without the arthropod vector by oronasal secretions and through the oronasal route in pigs (Ricklin et al., 2016). To begin addressing the question of which types of sex-associated behaviors and contact can result in ZIKV transmission, the current study isolates infection through the vaginal route after administration of exogenous hormones.
Women with reproductive tracts under the influence of endogenous and exogenous hormones engage in sex at various stages of the menstrual cycle and after menopause. It is noteworthy that the most common female oral contraceptives contain progesterone (Morrison et al., 2015), and that a 60-year-old woman has contracted ZIKV by sexual transmission (Turmel et al., 2016). Based on the results of this study we conclude that in these mouse models, deposition of ZIKV into the vagina can cause TGT, and that hormones can influence permissive and persistence natures of ZIKV infection in the FRT.
Taking the United States as an example, approximately one-third of women between 15–44 years of age use progesterone-based contraception in the form of pills, intrauterine devices, rings or injections (Daniels et al., 2015). If ZIKV TGT in women is progesterone-dependent as demonstrated in the current experimental mice, progesterone-based contraceptive strategies in women may alter infectivity of ZIKV. Any extrapolation of the results seen in these mouse models to human ZIKV infection must be made with extreme caution, as only thorough epidemiologic studies can determine whether progesterone influences anti-ZIKV immunity in the FRT. Whether hormonal status may alter infectivity following other modes of transmission, such as mosquito bite, remains to be seen.
The results of this study are based on transgenic mice with compromised antiviral immunity. AG129 mice globally lack IFNAR and IFNGR, whereas LysMCre+IFNARfl/fl mice only lack IFNAR in macrophages, neutrophils, and few dendritic cells (Clausen et al., 1999). A recent study has demonstrated that the FRT of wildtype C57BL/6 mice is susceptible to ZIKV infection (Yockey et al., 2016), but these mice did not become viremic. The lethal phenotype in diestrus-like AG129 mice provides a highly stringent system for testing of both preventative and therapeutic strategies, whereas diestrus-like LysMCre+IFNARfl/fl mice provide a model of self-limiting infection. Both models are easily manipulable, with many genetic and immunologic tools available for further study.
The 10uL volume of viral inoculum used in this study exceeds the normal mouse ejaculate volume of 1–5ul. However, during the time of administration it was noted that most volume that did not fit in the vaginal canal overflowed out of the vulva. Established mouse models of herpes simplex virus (HSV) transmission use similar or even larger volumes (Li et al., 2011). At no point in the present study was the viral inoculum administered under pressure that might have traumatically disrupted the epithelium of the FRT. It cannot be ruled out that, through grooming or other means, the mice in this study made oronasal contact with the viral inoculum following administration. However, IHC detection of viral replication in the vaginal tissues and draining lymph nodes at 18hpi would indicate that infection was transgenital. NS2B-expressing cells in the draining lymph nodes were rare and occurred in the subcapsular sinus, suggesting capture of the lymph-borne virus by these cells. In addition, the detection of NS2B protein in uterine spindle cells is consistent with a recent in vitro study demonstrating ZIKV infection of human uterine fibroblasts (Chen et al., 2016), which are essential components of FRT immunity. The absence of TGT in estrus-like mice also supports the FRT of diestrus-like mice as the original site of systemic infection.
Finally, detection of ZIKV in diestrus-like AG129 mouse vaginal washes up to 10 dpi parallels persistence of ZIKV that has been demonstrated in human vaginal mucosa 11 days after diagnosis of ZIKV (Prisant et al., 2016). Viral replication may persist to later time points, as 10 dpi was the last measurement in this study. Whether contact of a woman’s ZIKV-infected mucus with the mucosa of a sexual partner’s mouth, nose, vagina, or eyes results in ZIKV transmission remains to be seen. Regardless, the present study provides two different in vivo models of ZIKV TGT and persistent replication in the vaginal canal, and points a new avenue for dissecting mechanisms of protective immunity and pathogenesis of ZIKV TGT by comparing diestrus-like vs. estrus-like mice. Normal (i.e. IFNAR-competent) T, B, and dendritic cell responses in LysMCre+IFNARfl/fl mice will make the ZIKV TGT model in this mouse strain invaluable for assessing protective vs. potentially pathogenic effects of not only prior flaviviral exposure but also mucosal vs. nonmucosal vaccination. The lethal disease feature of ZIKV TGT in AG129 mice will allow the AG129 ZIKV TGT model to serve as a highly stringent challenge system for evaluating protective efficacy of both vaccine and antiviral candidates.
EXPERIMENTAL PROCEDURES
Mice and induction and determination of the estrus-like vs. diestrus-like phase
129/Sv mice deficient in IFNAR and IFNGR (AG129), and LysMCre+IFNARfl/fl C57BL/6 were housed under specific pathogen-free conditions. All experiments were approved by the Animal Care Committee at the La Jolla Institute for Allergy and Immunology. Mice at 8–12 weeks of age were injected with 5 IU of pregnant mare serum gonadotropin in 100uL PBS via intraperitoneal injection or 2mg of progesterone suspended in a solution containing 5% EtOH, 5% Kolliphor, and 90% H2O via subcutaneous injection to induce estrus-like and diestrus-like phases, respectively. Starting at 24h after injection, cytologies of vaginal washes were checked daily, as previously described (Byers et al., 2012; Caligioni, 2009), until all mice in a treatment group were synchronized into the desired phase.
Infections
Mice were infected in groups after all mice in a group had been synchronized to their respective cycle stage. Approximately 1 hour following vaginal cytology, for each AG129 mouse, 1 × 105 FFU of ZIKV strain FSS13025 in 10ul of PBS containing 10% of fetal bovine serum was drawn into 20ul pipette tips, and tips were externally lubricated with a solution of 0.5% carboxymethylcellulose and 0.9% glycerin. Mice were allowed to stand on a wire cage and the tail base was gently lifted to place the mouse in a prone position at a 45-degree angle, allowing clear visualization of the vulva. The lubricated pipette tip was gently inserted no more than 2mm into the vulva, and the viral dose was delivered. Mock-infected control mice were administered buffer without virus.
Cell cultures and viral stocks
ZIKV strain FSS13025 was obtained from the World Reference Center for Emerging Viruses and Arboviruses. Next generation sequencing was performed to confirm the sequence of FSS13025 and the absence of adventitious pathogens. Virus was cultured using the C6/36 Aedes albopictus mosquito cells. Briefly, C6/36 cells were maintained in culture using Leitbovitz’s L-15 medium with 10% Fetal Bovine Serum (FBS), 1% HEPES, and 1% Penicillin Streptomycin at 28°C in absence of CO2. At 80% of confluent monolayers, mosquito cells were infected with FSS13025 for one hour with gentle rocking every 15 minutes to facilitate infection of the cells by the virus. At the end of the hour, more L-15 media was added and cells were incubated for 7–10 days at 28°C in the absence of CO2. The supernatant was harvested, followed by clarification via centrifugation and concentration via ultracentrifugation as previously described for DENV (Prestwood et al., 2012). Virus was titrated using Baby Hamster Kidney (BHK)-21 cell-based Focus Forming Assay (FFA).
Clinical scoring
Following infection, mice were weighed and observed for clinical score daily. Clinical scores were based on a 7-point scale, as shown in the table in the Supplemental Experimental Procedures.
Collection of douche samples
Mice were positioned in 45-degree prone position as described in the infection section. 30ul of PBS was pipetted & withdrawn a total of 3–5cycles for each douche. Douche samples were frozen for RNA isolation.
qRT-PCR analysis of viral burdens
Total RNA from serum or vaginal washes of infected animals was isolated using the Qiagen viral RNA isolation kit. Real-time qRT-PCR was performed using the qScript™ 1-Step Qrt-PCR Kit (Quanta, BioSciences, Inc), CFX96 C1000 Touch™ real-time PCR detection system (Bio-Rad CFX Manager 3.1), and primers previously described (Lanciotti et al., 2008). Full primer sequences and cycling conditions are described in the Supplemental Experimental Procedures.
Histopathology
The FRT, iliac lymph nodes (LNs), and inguinal LNs were collected at 18 and 72 hpi, divided along the median plane, through the cervix, and fixed in zinc formalin for 24 hours at room temperature. Tissues for paraffinized sections were routinely processed and cut at 4um thickness. Sections were deparaffinized for routine hematoxylin & eosin (H&E) staining or for enzyme IHC as described in the Supplemental Experimental Procedures. A board-certified veterinary pathologist, who was blinded to each slide’s experimental conditions, read and scored each slide histopathologically.
Statistical analyses
All data were analyzed with Prism software version 6.0 (GraphPad Software, Inc., San Diego, CA) and expressed as mean ± SEM. Statistical significance was determined using the parametric two-tailed unpaired t-test with Welsh’s correction to compare two groups. Kaplan-Meier survival curves were analyzed by the Gehan-Breslow-Wilcoxon test.
Supplementary Material
Acknowledgments
We thank AnhThy Huynh for assistance with immunohistochemistry, Dr. Jinsheng Wen and Karla Viramontes for assistance with harvesting of samples, and Chris Lena for assistance with reagents. We also thank Genetex for antibody samples.
FUNDING
This research was funded by NIAID/NIH grant 1R01 AI116813 and the La Jolla Institute for Allergy & Immunology institutional support.
Footnotes
AUTHOR CONTRIBUTIONS
WWT, KK, and SS designed the study. WWT and KK performed experiments and data analysis. MPY, AM, and JARN performed experiments. WWT, KK, and SS interpreted the data and wrote the manuscript.
COMPETING INTERESTS
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. Detection of Zika Virus in Semen. Emerg Infect Dis. 2016;22:940. doi: 10.3201/eid2205.160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielanski A, Algire J, Lalonde A, Garceac A. Embryos produced from fertilization with bovine viral diarrhea virus (BVDV)-infected semen and the risk of disease transmission to embryo transfer (ET) recipients and offspring. Theriogenology. 2013;80:451–455. doi: 10.1016/j.theriogenology.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Bonaldo MC, Ribeiro IP, Lima NS, Dos Santos AA, Menezes LS, da Cruz SO, de Mello IS, Furtado ND, de Moura EE, Damasceno L, et al. Isolation of Infective Zika Virus from Urine and Saliva of Patients in Brazil. PLoS Negl Trop Dis. 2016;10:e0004816. doi: 10.1371/journal.pntd.0004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Frei S, Schweizer M, Zanoni R, Janett F. Short communication: Transmission of border disease virus to seronegative cows inseminated with infected semen. Res Vet Sci. 2015;100:297–298. doi: 10.1016/j.rvsc.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus - United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65:745–747. doi: 10.15585/mmwr.mm6529e2. [DOI] [PubMed] [Google Scholar]
- Burke SA, Wen L, King NJ. Routes of inoculation and the immune response to a resolving genital flavivirus infection in a novel murine model. Immunol Cell Biol. 2004;82:174–183. doi: 10.1046/j.0818-9641.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Wang Z, Huang H, Weitz SH, Wang A, Qiu X, Baumeister MA, Uzgiris A. Infection of human uterine fibroblasts by Zika virus in vitro: implications for viral transmission in women. Int J Infect Dis. 2016;51:139–140. doi: 10.1016/j.ijid.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Yazdanpanah Y, Leparc-Goffart I. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- Daniels K, Daugherty J, Jones J, Mosher W. Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15–44: United States, 2011–2013. Natl Health Stat Report. 2015:1–14. [PubMed] [Google Scholar]
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. Male-to-Male Sexual Transmission of Zika Virus - Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- Floegel G, Wehrend A, Depner KR, Fritzemeier J, Waberski D, Moennig V. Detection of classical swine fever virus in semen of infected boars. Vet Microbiol. 2000;77:109–116. doi: 10.1016/s0378-1135(00)00267-4. [DOI] [PubMed] [Google Scholar]
- Fourcade C, Mansuy JM, Dutertre M, Delpech M, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Viral load kinetics of Zika virus in plasma, urine and saliva in a couple returning from Martinique, French West Indies. J Clin Virol. 2016;82:1–4. doi: 10.1016/j.jcv.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Freour T, Mirallie S, Hubert B, Splingart C, Barriere P, Maquart M, Leparc-Goffart I. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.23.30254. [DOI] [PubMed] [Google Scholar]
- Habu A, Murakami Y, Ogasa A, Fujisaki Y. Disorder of spermatogenesis and viral discharge into semen in boars infected with Japanese encephalitis virus (author’s transl) Uirusu. 1977;27:21–26. doi: 10.2222/jsv.27.21. [DOI] [PubMed] [Google Scholar]
- Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and its Emergence in the Western Hemisphere. J Virol. 2016;90:4864–75. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Hansen G, Nitsche C, Klein CD, Zhang L, Hilgenfeld R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science. 2016;353:503–505. doi: 10.1126/science.aag2419. [DOI] [PubMed] [Google Scholar]
- Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- Matheron S, D’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y. Long Lasting Persistence of Zika Virus in Semen. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 doi: 10.1093/cid/ciw509. Published Online July 28, 2016. [DOI] [PubMed] [Google Scholar]
- McCarthy M. US officials issue travel alert for Miami area as Zika cases rise to 15. BMJ. 2016;354:i4298. doi: 10.1136/bmj.i4298. [DOI] [PubMed] [Google Scholar]
- Morrison CS, Chen PL, Kwok C, Baeten JM, Brown J, Crook AM, Van Damme L, Delany-Moretlwe S, Francis SC, Friedland BA, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Situation Report, Zika Virus (August 11 2016 report) 2016a. [Google Scholar]
- World Health Organization (2016) WHO statement on the first meeting of the International Health Regulations (2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations (first meeting of Emergency Committee) (teleconference:WHO).
- Prestwood TR, May MM, Plummer EM, Morar MM, Yauch LE, Shresta S. Trafficking and replication patterns reveal splenic macrophages as major targets of dengue virus in mice. J Virol. 2012;86:12138–12147. doi: 10.1128/JVI.00375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G. Zika virus in the female genital tract. The Lancet Infectious diseases. 2016;9:1000–1001. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects — Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Ricklin ME, Garcia-Nicolas O, Brechbuhl D, Python S, Zumkehr B, Nougairede A, Charrel RN, Posthaus H, Oevermann A, Summerfield A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nature communications. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernise S. Zika Virus Case in Utah Baffles Health Officials. A17 New York Times; 2016. [Google Scholar]
- Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, Leparc-Goffart I. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- van der Linden V, Filho EL, Lins OG, van der Linden A, de Aragao MF, Brainer-Lima AM, Cruz DD, Rocha MA, Sobral da Silva PF, Carvalho MD, et al. Congenital Zika syndrome with arthrogryposis: retrospective case series study. BMJ. 2016;354:i3899. doi: 10.1136/bmj.i3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- Watrin L, Ghawche F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre Syndrome (42 Cases) Occurring During a Zika Virus Outbreak in French Polynesia. Medicine (Baltimore) 2016;95:e3257. doi: 10.1097/MD.0000000000003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256. e1244. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.