Abstract
The aim of this study was to explore the role of body image, posttraumatic growth, and emotional state in recovery after transplantation. A total of 53 kidney transplant patients were assessed using our Self-Test and Organ Drawing Test, the Spielberger Anxiety Inventory, the Beck Depression Inventory, and the Posttraumatic Growth Inventory in a 3-year follow-up. Logistic regression analysis showed that lower levels of integrity of the body image and posttraumatic growth, and higher pre-discharge serum creatinine levels were significant predictors of graft rejection. Our results suggest that the integrity of the body image and posttraumatic growth might contribute to better health outcomes in organ transplantation.
Keywords: body image, drawing test, kidney transplant, posttraumatic growth, transplant outcomes
Introduction
There is an increasing body of evidence proving that transplanted patients’ perceptions of their disease and their body may have an influence on their physical recovery (Calia et al., 2011; Consoli, 2012; De Pasquale et al., 2010; Látos et al., 2012; Pérez-San-Gregorio et al., 2009). During transplantation, parallel with the restoration of anatomic and physiologic functions, a so-called psychic transplantation takes place, which means the cognitive and emotional integration of the new organ (De Pasquale et al., 2010). Patients after transplantation experience a range of positive and negative emotions like guilt, gratefulness, and fear (Schipper et al., 2013). A wide range of interdisciplinary investigations suggest that transplantation may also create self-representation problems and vulnerability of the body image (Castelnuovo-Tedesco, 1983; De Pasquale et al., 2010; Ilić and Avramović, 2002).
According to research evidence and clinical experience, the transplanted kidney as a “foreign body” may call forth archaic beliefs and reactions, which in turn would cause intrapsychological conflicts about the new organ and often obstruct the psychological acceptance of the graft (Basch, 1973; Consoli, 2012; Fukunishi et al., 2002; Ilić and Avramović, 2002; Scanner, 2005; Shimazono, 2013). Psychological conflicts about the new kidney may lead to depression and treatment non-compliance, and thus suggested as possible predictors of graft failure (Achille et al., 2006; Basch, 1973; Dickenmann et al., 2002). The interactions of psycho-immunological mechanisms in these processes are extremely complex. According to recent studies, the analysis of patients’ drawings of their diseased organs or their bodies seems to be a useful tool to assess underlying psychodynamic processes, cognitive representations, and psychological state (Daleboudt et al., 2011; De Pasquale et al., 2010; Hoogerwerf et al., 2012; Petrie and Weinman, 2012). Besides questionnaire and interview measures, organ drawings may be an alternative way to assess patients’ illness representations and can illustrate these representations more specifically than words (Broadbent et al., 2006). Following transplantation, the patient’s body undergoes an immediate change, which provokes a “psychosomatic crisis.” The solution to this crisis is a complex and painful task of rebuilding the self and body image to restructure the Self and integrate the new organ.
While the negative consequences of kidney disease and the stressful nature of transplantation have been well characterized in the literature, the idea that posttraumatic growth can occur in transplant patients has emerged as a novel target of empirical investigation. Posttraumatic growth is defined as “a positive cognitive process that is initiated to cope with traumatic events that extract an extreme cognitive and emotional toll” (Tedeschi and Calhoun, 1996: 5). Despite the growing knowledge of posttraumatic growth, only a minimal amount of research has been conducted on the relationship between posttraumatic growth and physical well-being. For example, heart attack victims who reported psychological growth from traumatic experiences were found to have lower rates of mortality than those who did not perceive any derived benefit (Epel et al., 1998). Women who reported deriving benefit from traumatic experiences in their lives had quicker cortisol habituation to stressors than those who did not report psychological growth (Bower et al., 1998). Qualitative analysis revealed that posttraumatic growth might provide additional perspectives for rehabilitation among stroke survivors (Kuenemund et al., 2014). Researchers also suggested that health-care providers might help the recovery of patients by facilitating posttraumatic growth (Schmidt et al., 2014; Wang et al., 2012). In summary, these findings suggest that posttraumatic growth may serve as a protective factor in relation to consequent health outcomes, but only a few studies have examined personal growth in the context of transplantation (Segatto et al., 2013; Yorulmaz et al., 2010).
The primary aim of our study was to explore post-operative personal growth and body image characteristics of post-transplant kidney patients in a prospective longitudinal research design. As a development of the Machover (1949) Draw-a-Person Test, which has already been used for the measurement of kidney transplant patients’ body image (Basch, 1973), our test was administered to patients with instructions to first draw themselves in the figure-drawing assignment and thereafter draw the newly received kidney. Our secondary goal was correlating test results with measures of anxiety, depression, and medical parameters. We hypothesized that the integrity of the body image (Witkin, 1962) and the size of the kidney in the drawings might reflect the patients’ difficulties in organ acceptance, related emotional state, and transplantation outcomes. We further assumed circular causal connections between these variables and kidney functions. We based these assumptions on our previous study, where we found that patients with a higher actual and dispositional anxiety drew the implanted kidney significantly larger (Látos et al., 2012). Further results of this study showed that patients who drew the kidney larger in the self-figure and kidney drawing had higher serum creatinine levels on their 10th day blood test, and this was a sign of poorer post-transplant outcome. The aim of this study was to determine whether kidney transplantation was associated with characteristics of body image as represented in self-figure and kidney drawings, and whether these features of body image and posttraumatic growth would predict poor renal outcomes (i.e. graft rejection) in both cross-sectional and prospective analyses.
Methods and materials
Data were collected at the Department of Surgery where a psychologist is a member of the renal team. Psychological examination of patients took place between the post-operative 5th and 10th days and during a 3-year period after transplantation (Table 1). During these individual examination sessions, we tested each patient with psychological tools. Medical parameters were collected from the routine clinical blood tests and biopsy results throughout the 3-year period after transplantation. The research protocol was approved by relevant institutional research ethics committees.
Table 1.
Study design.
| Date | Measurements | |
|---|---|---|
| Psychological tests | Between 5th and 10th post-operative days | Spielberger Anxiety Inventory |
| Beck’s Depression Inventory | ||
| Combined Self-Test and Organ Drawing Test | ||
| 3 years after transplantation | Spielberger Anxiety Inventory | |
| Beck’s Depression Inventory | ||
| Combined Self-Test and Organ Drawing Test | ||
| Posttraumatic Growth Inventory | ||
| Medical parameters | Post-operative days and quarterly | Blood test |
| Biopsy result |
The study sample comprised 53 patients who received a cadaver renal transplant. All patients were followed for 3 years after transplantation. In total, 28 recipients were males, with a mean age of 44.14 years (range = 23–75 years, standard deviation (SD) = 12.29 years), and 25 were females, with a mean age of 53.96 years (range = 28–69 years, SD = 11.79 years). Each patient was provided with comprehensive information regarding the study and informed consent was taken. All patients in the sample received psychological support after transplantation in the form of a standard 30-minute-long counseling session, based on a general post-operative protocol which includes psychological care to all post-operative patients at the Department of Surgery.
Psychological tests and measurements
The Spielberger State–Trait Anxiety Inventory (STAI-S; STAI-T) was administered to measure the level of anxiety after transplantation (Spielberger et al., 1970). Beck’s Depression Inventory (BDI) was used to assess the severity of depressive symptoms (Beck et al., 1961). We administered Posttraumatic Growth Inventory for assessing positive outcomes following a struggle with highly challenging life circumstances (Tedeschi and Calhoun, 1996). The questionnaire is composed of five subscales (Relating to Others, New Possibilities, Personal Strength, Spiritual Change, Appreciation of Life) and a total score of posttraumatic growth can be calculated.
The fourth tool, the Combined Self-Test and Organ Drawing Test which had been developed in our earlier study (Látos et al., 2012) comprised the self-drawing of the patient and subsequently the new organ. Patients were given a set of 12 colored pencils and received instructions to first draw their own body on an A4-size blank sheet and thereafter the newly received kidney. No further instructions were given about the position, size, color, or other details of the figures. Drawings were used to explore patients’ integrity of their body image (Gouda, 1989; Witkin, 1962). Self-drawings were evaluated by Witkin’s (1962) Sophistication-of-Body-Concept Scale which reflects the degree of differentiation in the body concept. Specific graphic features of the drawings were identified (form level, identity and sex differentiation, level of detailing) which comprised the degree of integrity. The final step was to evaluate the drawing’s level of integrity on a scale from 1 to 5. The following variables were assessed: (a) the size (diagonal, cm) of the kidney in organ drawings and (b) the size (height, cm) of the body. Drawings were blindly coded by two independent judges to verify the categories, with a third researcher as a tie-breaker to resolve any disagreement between the coders. The interrater reliability for the raters was found to be Kappa = 0.91 (p < 0.001), 95 percent confidence interval (CI) [0.00, 0.03]. As a rule of thumb values of Kappa, this measure means almost perfect agreements.
Medical parameters
Medical parameters (serum creatinine level) of patients were collected from the pre-discharge routine clinical blood test between the 14th and 20th post-operative days, and also 3 years after the transplantation, at the required control follow-up medical examination to assess allograft outcomes. Furthermore, we recorded acute rejection episodes throughout the 3-year period after transplantation. Rejection is one of the most common complications and a statistically significant indicator of poor outcome following a renal transplant (Dickenmann et al., 2002; Nankivell and Alexander, 2010). Graft rejection was diagnosed according to clinical and histopathological criteria. Human leukocyte antigen (HLA) donor mismatches were also registered, since these may correlate with an increased risk of death due to requiring more antirejection therapy (Opelz and Döhler, 2012). Cold ischemia time, donor age, duration of chronic kidney disease (in months), the recipient’s cardiovascular disease and/or diabetes mellitus were also considered in the research.
Statistical analyses
Data were analyzed using IBM SPSS 20.0 for Windows. Descriptive statistics were calculated for all variables. The Shapiro–Wilk tests were used to analyze for a normal or abnormal distribution of the data. To reveal the pattern of relations among the variables, Spearman’s and Pearson’s correlations were used. Paired t-test was applied to compare mean values on post-operative days and 3 years later. Group comparisons were performed with independent t-test and Mann–Whitney test. An interrater reliability analysis using the Kappa statistic was performed to determine consistency among raters. Binary logistic regression analyses (forward method) of psychological and somatic variables were performed to detect possible predictors for graft rejection. Results were considered statistically significant when the p value was less than 0.05.
Results
Assessing data normality
The Shapiro–Wilk test was chosen to assess if the data were normally distributed. Table 2 shows that most of the conditions were not normally distributed (p < 0.05).
Table 2.
Shapiro–Wilk test results.
| Shapiro–Wilk |
|||
|---|---|---|---|
| Statistic | df | Significance | |
| Depression | 0.839 | 53 | <0.001 |
| State anxiety | 0.968 | 53 | 0.172 |
| Trait anxiety | 0.978 | 53 | 0.431 |
| Duration of chronic kidney disease | 0.730 | 52 | <0.001 |
| Pre-discharge serum creatinine (µm/L) | 0.798 | 53 | <0.001 |
| Days spent in hospital | 0.783 | 53 | <0.001 |
| Serum creatinine (µm/L) 1 year after transplantation | 0.697 | 48 | <0.001 |
| Serum creatinine (µm/L) 2 years after transplantation | 0.564 | 47 | <0.001 |
| Serum creatinine (µm/L) 3 years after transplantation | 0.619 | 47 | <0.001 |
| Posttraumatic Growth Total Score | 0.963 | 49 | 0.124 |
| Posttraumatic Growth New Possibilities | 0.934 | 49 | 0.009 |
| Posttraumatic Growth Relating to Others | 0.973 | 49 | 0.313 |
| Posttraumatic Growth Personal Strength | 0.919 | 49 | 0.002 |
| Posttraumatic Growth Spiritual Change | 0.809 | 49 | <0.001 |
| Posttraumatic Growth Appreciation of Life | 0.865 | 49 | <0.001 |
| Integrity of the body drawing on post-operative day | 0.834 | 53 | <0.001 |
| Integrity of the body drawing 3 years after transplant | 0.866 | 49 | <0.001 |
Depression and anxiety after transplantation
The average level of state anxiety and depression measured with the BDI and the Spielberger Anxiety Inventory (STAI) fell into normal ranges (Table 3). No clinical depression (above 19 points) was found in the sample. On the dispositional measure of anxiety (STAI-T), 11.3 percent of the patients showed clinically high levels of trait anxiety (above 52 points) on post-operative days and 6 percent 3 years after surgery. None of the variables showed significant differences between the sexes.
Table 3.
Variables and mean values on the post-operative day and 3 years later (N = 53).
| Post-operative day, Mean (SD) | 3 years after transplantation, Mean (SD) | t | df | Significance | |
|---|---|---|---|---|---|
| Depression | 3.50 (2.67) | 4.27 (4.85) | −0.88 | 48 | 0.38 |
| State anxiety | 36.64 (8.67) | 31.90 (10.43) | 3.30 | 48 | <0.001 |
| Trait anxiety | 37.71 (9.27) | 35.16 (11.16) | 1.62 | 48 | 0.11 |
| Integrity of the body drawing | 2.53 (1.48) | 2.77 (1.47) | −1.60 | 48 | 0.11 |
| Size of the kidney drawing (cm) | 3.51 (2.38) | 2.48 (2.54) | 2.74 | 42 | <0.001 |
| Size of the body drawing (cm) | 10.65 (5.47) | 11.18 (5.70) | −0.75 | 48 | 0.45 |
SD: standard deviation.
Negative mood state as reflected in the drawing test
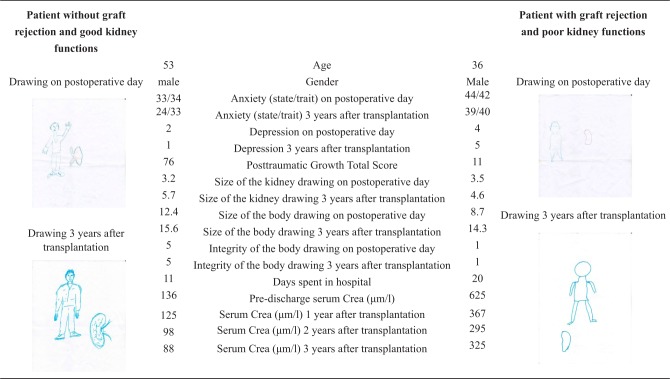
We found a relationship between distress and kidney size in the projective drawing test. Patients with higher depression scores drew significantly larger kidneys on post-operative days (BDI; p < 0.001, r = 0.35; n = 53). The size of the kidney drawing in the post-operative days (Mean: 3.51, SD = 2.38) was significantly larger than 3 years later (Mean = 2.48, SD = 2.54; p < 0.001, df = 42, t = 2.74). Examples for drawings of patients are depicted in Figure 1.
Figure 1.
Examples for test results of patients with and without graft rejection.
Predictors of graft rejection
To analyze the influence of medical and psychological factors on graft functioning, patients were separated into a “rejection group” (N = 22) and a “non-rejection group” (N = 31). In the “rejection group,” patients’ biopsy-proved rejection developed within the first 3 years after transplantation, and their average serum creatinine level was below 180 µmol/L. In the “non-rejection group,” patients did not show apparent signs of rejection during the 3-year period, and their average serum creatinine level was above 180 µmol/L. We compared all measured variables between the two groups: serum creatinine levels (pre-discharge, 1, 2, and 3 years after surgery), integrity of the body image (on post-operative day and 3 years after transplantation), and Posttraumatic Growth Total Score and two subscales (New Possibilities and Appreciation of Life) which showed differences (Table 4).
Table 4.
Comparison of medical and psychological parameters between non-rejection and rejection groups (N = 53).
| Non-rejection group (N = 31), Mean (SD)/Mean rank | Rejection group (N = 22), Mean (SD)/Mean rank | t/Z value | p value | |
|---|---|---|---|---|
| Pre-discharge serum creatinine (µm/L) | 156.32 (SD = 65.38) | 219.86 (SD = 124.65) | t = −2.41 | 0.01 |
| Serum creatinine (µm/L) 1 year after transplantation | 138.54 (SD = 56.87) | 298.05 (SD = 211.23) | Z = −3.11 | <0.001 |
| Serum creatinine (µm/L) 2 years after transplantation | 135.45 (SD = 49.90) | 338.43 (SD = 311.95) | Z = −2.51 | 0.01 |
| Serum creatinine (µm/L) 3 years after transplantation | 132.22 (SD = 57.71) | 391.75 (SD = 326.91) | Z = −3.32 | <0.001 |
| Posttraumatic Growth Total Score | 57.23 (SD = 24.23) | 43.94 (SD = 24.70) | t = 1.83 | 0.07 |
| Posttraumatic Growth New Possibilities | 57.23 (SD = 24.23) | 43.94 (SD = 24.70) | t = 2.40 | 0.02 |
| Posttraumatic Growth Appreciation of Life | 57.23 (SD = 24.23) | 43.94 (SD = 24.70) | Z = −2.14 | 0.03 |
| Integrity of the body drawing on post-operative day | 3.00 (SD = 1.48) | 1.77 (SD = 1.06) | Z = 3.09 | <0.001 |
| Integrity of the body drawing 3 years after transplantation | 3.22 (SD = 1.30) | 2.00 (SD = 1.45) | Z = 2.86 | <0.001 |
SD: standard deviation.
For further investigation, a binary logistic regression analysis was used to identify somatic and psychological factors contributing to an increased risk of graft rejection (dependent variable). The logistic regression with forward method among all variables identified three main predictors of graft rejection in our sample. The resulting model was statistically significant (χ2 = 23.27, df = 3, p < 0.001). The integrity of the body image (odds ratio (OR) = 0.411, 95% CI = 0.215–0.786, p < 0.01), the Posttraumatic Growth Total Score (OR = 0.963, 95% CI = 0.929–0.999, p < 0.05), and pre-discharge serum creatinine (OR = 1.017, 95% CI = 1.005–1.028, p < 0.01) were significant predictors of graft rejection episodes 3 years after transplantation. This model explained between 37.8 (Cox and Snell’s R2) and 51.7 percent (Nagelkerke’s R2) of the variance and correctly classified 63.3 percent of cases. There were no other significant interactions with other somatic and psychological variables. Examples for drawings of patients with and without graft rejection, together with medical and psychological test results, are depicted in Figure 1.
Discussion
The aim of this prospective study was to identify psychological risk factors which may help to predict graft rejection in kidney transplant patients using a test of self-figure and kidney drawings, together with the quantitative assessment of anxiety, depression, posttraumatic growth, and different medical parameters. We found relationships between negative mood state and kidney size in the drawings. This result is in accordance with conclusion of drawing studies of other organs, for example, of the heart, where higher levels of heart-specific anxiety were associated with significantly larger drawings of patients with heart failure (Reynolds et al., 2007). Furthermore, state anxiety and the size of the kidney drawing in the post-operative days were significantly higher than 3 years after transplantation. These results suggest that kidney size in the drawings might reflect the related emotional state.
Post-transplant patients must cope with several types of negative emotions which, in some cases, remain persistent (Achille et al., 2004; Consoli, 2012; Kaba et al., 2005; Kemph, 1967; Pérez-San-Gregorio et al., 2006). Consequently, the transplanted organ is not inert at a psychological level; the process of “psychic transplantation” (De Pasquale et al., 2010) is not able to run its course properly. In our study, this problem was indicated by the anxiety results related to the size of the new kidney whose mental representation was enlarged by anxiety. Simultaneously, we noted that the psychological process of the new organ’s integration has different phases, where the above-described “foreign-body phase” is just the first step, and related anxiety is a temporary response in the majority of cases (Castelnuovo-Tedesco, 1983; Joralemon, 1995). The next two phases—“partial incorporation” and “complete integration”—take longer time and may be aggravated by other psychological difficulties which, in unfavorable cases, may even lead to graft rejection.
The omission of some body parts (head, feet, hands) was also observed in the body drawings of kidney transplant patients in earlier research and was explained as a sign of problems in the redefinition process of the body image after transplantation (Nesci et al., 2001). Another qualitative study of kidney transplant patients showed that patients reacted to their altered body image by increasing their “barrier defenses”—a sign of psychological resistance—which was reflected in the stiff contours of their body drawings (Nesci et al., 2001).Withdrawal and feeling of emptiness were also found to be the cause of incomplete body-figure drawings (Kahill, 1984). The size of the human figure in other drawing studies was also related to lower self-esteem and energy levels (Kahill, 1984; Leibowitz, 1999; Lev-Wiesel and Drori, 2000; Machover, 1951). It was also shown that under-detailing in the drawing test was an indicator of anxiety, and the lower number of details referred to slower physical and psychological improvement (Handler, 1967; Hjorth and Harway, 1981; Horwitz et al., 2006; Kahill, 1984). In our sample, the integrity of the body image was related to rejection episodes and graft functions. Patients in the “rejection group” had less integrated body image as suggested by the drawings than those in the “non-rejection” group. This result suggests that transplantation may create vulnerability of the body image.
We found relationships between posttraumatic growth and physical well-being. Clients who reported growing psychologically from the stressful transplantation experiences were found to have lower rates of graft rejection than those who did not perceive any derived benefit. Transplanted patients who reported deriving benefit from hurtful traumatic experiences had lower serum creatinine level than those who did not report growing psychologically. Our results suggest that posttraumatic growth may serve as a protective factor in relation to consequent health outcomes.
The results of the logistic regression analysis in our study showed that the integrity of the body concept and posttraumatic growth, together with pre-discharge serum creatinine, was able to predict graft rejection during the 3-year period after transplantation. The predictive value of plasma creatinine for kidney graft rejection was also proved in the study of Dickenmann et al. (2002). Furthermore, as discussed above, the lower level of integrity of the drawings may refer to the difficulties of the body image re-integration and psychological defenses of withdrawal. Less integrated drawings may indicate the lower sense of separate identity and lower sense of the boundaries of the body. Together with the feelings of isolation, lower self-esteem, and energy levels, these defenses could interfere with proper therapy adherence. Previous research also suggests that body image dissatisfaction may relate to biological processes and can influence physical health through the complex pathways connecting psychological factors and physical illness (Černelič-Bizjak and Jenko-Pražnikar, 2014).
The psychological processes (low energy level, withdrawal, less effort, etc.) represented by the less integrated self-figure drawings may contribute to inadequate coping and consequent deficits in physiological functioning. As part of a system of circular associations, unsatisfactory somatic function signals (e.g. serum creatinine level) may have a further negative inducing impact on the above-mentioned psychological processes, thus further decreasing the patient’s energy level, self-esteem, and their capacity for coping.
Finally, our study has several limitations (small sample size, single-center study, limited demographic patient population). Furthermore, the interactions of psycho-immunological mechanisms in these processes are particularly multifarious. At last, it was not possible to make psychopathological comparisons since our patients’ actual anxiety and depression levels fell within the normal range a few days after transplantation. The lack of clinical anxiety and depression might be due to the supportive counseling session provided to all patients after transplantation. A proof for this therapeutic effect has been justified by post-transplant state anxiety falling within the normal range, even for those patients whose scores were in the clinical range on the trait anxiety scale.
Although our study has several limitations, the results support the body image and self-reconstruction problems and may help to signal kidney graft rejection, especially when combined with somatic predictors, such as serum creatinine levels which can contribute to poorer renal outcome. In order to explore and prevent these difficulties, the use of such psychometric tools such as our Combined Self-Test and Organ Drawing Test may be useful clinical aids. As a non-verbal tool, its use is comfortable for inpatient care and renders possible qualitative and quantitative interpretations. Besides the contribution to better understand the complex psychosomatic nature of the transplantation process, our study may also promote the development of supportive techniques which can enhance recovery in kidney transplant patients. This psychosocial intervention could be an effective means of addressing emotional problems (the psychological integration of the newly acquired kidney, fear of rejection), reduce emotional distress, and improve health behaviors among patients with kidney transplantation.
Acknowledgments
The authors would like to thank Nagy Ernest for his help in reviewing and editing the English version of this paper.
Footnotes
Declaration of conflicting interests: None declared.
Funding: This research was supported by the TÁMOP-4.2.2.A-11/1/KONV-2012-0035 Program.
References
- Achille MA, Ouellette A, Fournier S, et al. (2006) Impact of stress, distress and feelings of indebtedness on adherence to immunosuppressants following kidney transplantation. Clinical Transplantation 20: 301–306. [DOI] [PubMed] [Google Scholar]
- Achille MA, Oullette A, Fournier S, et al. (2004) Impact of transplant-related stressors and feelings of indebtedness on psychosocial adjustment following kidney transplantation. Journal of Clinical Psychology in Medical Settings 11: 63–73. [Google Scholar]
- Basch SH. (1973) The intrapsychic integration of a new organ—A clinical study of kidney transplantation. Psychoanalytic Quarterly 42: 364–384. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, et al. (1961) An inventory for measuring depression. Archives of General Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- Bower JE, Kemeny ME, Taylor SE, et al. (1998) Cognitive processing, discovery of meaning, CD4 decline, and AIDS-related mortality among bereaved HIV-seropositive men. Journal of Consulting and Clinical Psychology 66: 979–986. [DOI] [PubMed] [Google Scholar]
- Broadbent E, Ellis CJ, Gamble G, et al. (2006) Changes in patient drawings of the heart identify slow recovery after myocardial infarction. Psychosomatic Medicine 68: 910–913. [DOI] [PubMed] [Google Scholar]
- Calia R, Lai C, Aceto P, et al. (2011) Preoperative psychological factors predicting graft rejection in patients undergoing kidney transplant: A pilot study. Transplantation Proceedings 43: 1006–1009. [DOI] [PubMed] [Google Scholar]
- Castelnuovo-Tedesco P. (1983) Organ transplant, body image, psychosis. Psychoanalytic Quarterly 42: 349–363. [PubMed] [Google Scholar]
- Černelič-Bizjak M, Jenko-Pražnikar Z. (2014) Impact of negative cognitions about body image on inflammatory status in relation to health. Psychology & Health 29: 264–278. [DOI] [PubMed] [Google Scholar]
- Consoli E. (2012) Person-centered approach in the medicine of organ transplants. Available at: http://www.psicoanalisi.it/psicoanalisi/osservatorio/articoli/osservaing1132.htm (accessed 10 July 2014).
- Daleboudt GMN, Broadbent E, Berger SP, et al. (2011) Illness perceptions in patients with systemic lupus erythematosus and proliferative lupus nephritis. Lupus 20: 290–298. [DOI] [PubMed] [Google Scholar]
- De Pasquale C, Pistorio ML, Sorbello M, et al. (2010) Body image in kidney transplantation. Transplantation Proceedings 42: 1123–1126. [DOI] [PubMed] [Google Scholar]
- Dickenmann MJ, Nickeleit V, Tsinalis D, et al. (2002) Why do kidney grafts fail? A long-term single-center experience. Transplant International 15: 508–514. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. (1998) Embodying psychological thriving: Physical thriving in response to stress. Journal of Social Issues 54: 301–322. [Google Scholar]
- Fukunishi I, Sugawara Y, Takayam T, et al. (2002) Association between pretransplant psychological assessments and posttransplant psychiatric disorders in living-related transplantation. Psychosomatics 43: 49–54. [DOI] [PubMed] [Google Scholar]
- Gouda GT. (1989) Body Schema and Body Image. Amsterdam: Swets & Zeitlinger. [Google Scholar]
- Handler L. (1967) Anxiety indexes in the Draw-a-Person test: A scoring manual. Journal of Projective Techniques & Personality Assessment 31: 46–57. [DOI] [PubMed] [Google Scholar]
- Hjorth CW, Harway M. (1981) The body-image of physically abused and normal adolescents. Journal of Clinical Psychology 37: 863–866. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf MA, Ninaber MK, Willems LNA, et al. (2012) “Feelings are facts”: Illness perceptions in patients with lung cancer. Respiratory Medicine 106: 170–1176. [DOI] [PubMed] [Google Scholar]
- Horwitz EB, Kowalski J, Theorell T, et al. (2006) Dance/movement therapy in fibromyalgia patients: Changes in self-figure drawings and their relation to verbal self-rating scales. Art Psychotherapy 33: 11–25. [Google Scholar]
- Ilić S, Avramović M. (2002) Psychological aspects of living donor kidney transplantation. Medicine and Biology 9: 195–200. [Google Scholar]
- Joralemon D. (1995) Organ wars: The battle for body parts. Medical Anthropology Quarterly 9: 335–356. [DOI] [PubMed] [Google Scholar]
- Kaba E, Thompson DR, Burnard P, et al. (2005) Somebody else’s heart inside me: A descriptive study of psychological problems after a heart transplantation. Issues in Mental Health Nursing 26: 611–625. [DOI] [PubMed] [Google Scholar]
- Kahill S. (1984) Human figure drawing in adults: An update of the empirical evidence. Canadian Psychology 25(4): 269–292. [Google Scholar]
- Kemph JP. (1967) Psychotherapy with patients receiving kidney transplant. American Journal of Psychiatry 126: 623–629. [DOI] [PubMed] [Google Scholar]
- Kuenemund A, Zwick S, Rief W, et al. (2014) (Re-)defining the self—Enhanced posttraumatic growth and event centrality in stroke survivors: A mixed-method approach and control comparison study. Journal of Health Psychology. Available at: http://hpq.sagepub.com/content/early/2014/05/29/1359105314535457 (accessed 10 July 2013). [DOI] [PubMed]
- Látos M, Barabás K, Lázár G, et al. (2012) Mental representation of the new organ and posttransplant patient’s anxiety as related to kidney function. Transplantation Proceedings 44: 2143–2145. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. (1999) Interpreting Projective Drawings: A Self Psychological Approach. New York: Routledge. [Google Scholar]
- Lev-Wiesel R, Drori D. (2000) The effect of social status upon the self concept of elderly widows and wives assessed by human figure drawings. Art Psychotherapy 27: 263–267. [Google Scholar]
- Machover K. (1949) Personality Projection in the Drawing of the Human Figure. Springfield, IL: Charles C. Thomas. [Google Scholar]
- Machover K. (1951) Drawing of the human figure: A method of personality investigation. In: Anderson HH, Anderson GL. (eds) An Introduction to Projective Techniques. New York: Prentice Hall, pp. 341–369. [Google Scholar]
- Nankivell BJ, Alexander SI. (2010) Rejection of the kidney allograft. New England Journal of Medicine 363: 1451–1462. [DOI] [PubMed] [Google Scholar]
- Nesci DA, Favale C, Foco M, et al. (2001) Psychodynamic evaluation of kidney transplant patients enrolled in a new immunosuppressive drug trial. Transplantation Proceedings 33: 1907–1908. [DOI] [PubMed] [Google Scholar]
- Opelz G, Döhler B. (2012) Association of HLA mismatch with death a functioning graft after kidney transplantation: A collaborative transplant study report. American Journal of Transplantation 12: 3031–3038. [DOI] [PubMed] [Google Scholar]
- Pérez-San-Gregorio MA, Martín-Rodríguez A, Galán-Rodríguez A, et al. (2009) Living and deceased transplanted patients one year later: Psychosocial differences just after surgery. International Journal of Clinical Health Psychology 9: 429–438. [Google Scholar]
- Pérez-San-Gregorio MA, Martín-Rodriquez A, Díaz-Dominiquez R, et al. (2006) The influence of posttransplant anxiety on the long-term health of patients. Transplantation Proceedings 38: 2406–2408. [DOI] [PubMed] [Google Scholar]
- Petrie KJ, Weinman J. (2012) Patients’ perceptions of their illness: The dynamo of volition in health care. Current Directions in Psychological Science 21(1): 60–65. [Google Scholar]
- Reynolds L, Broadbent E, Ellis CJ, et al. (2007) Patients’ drawings illustrate psychological and functional status in heart failure. Journal of Psychosomatic Research 63: 525–532. [DOI] [PubMed] [Google Scholar]
- Scanner MA. (2005) Living with a stranger’s organ—Views of the public and transplant recipients. Annals of Transplantation 10: 9–12. [PubMed] [Google Scholar]
- Schipper K, Abma TA, Koops C, et al. (2013) Sweet and sour after renal transplantation: A qualitative study about the positive and negative consequences of renal transplantation. British Journal of Health Psychology 19(3): 580–591. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Blank TO, Bellizzi KM, et al. (2014) The relationship of coping strategies, social support, and attachment style with posttraumatic growth in cancer survivors. Journal of Health Psychology 17: 1033–1040. [DOI] [PubMed] [Google Scholar]
- Segatto BL, Sabiston CM, Harvey WJ, et al. (2013) Exploring relationships among distress, psychological growth, motivation, and physical activity among transplant recipients. Disability and Rehabilitation 35: 2097–2103. [DOI] [PubMed] [Google Scholar]
- Shimazono Y. (2013) Accommodating a “foreign” organ inside the body: Post-transplant bodily experiences of Filipino kidney recipients. Ars Vivendi Journal 3: 24–50. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. (1970) Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tedeschi R, Calhoun L. (1996) The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress 9: 455–471. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Liu X. (2012) Posttraumatic growth of injured patients after motor vehicle accidents: An interpretative phenomenological analysis. Journal of Health Psychology 17: 297–308. [DOI] [PubMed] [Google Scholar]
- Witkin HA. (1962) Articulation of the body concept. In: Witkin HA, Dyk RB, Faterson HF, et al. (eds) Psychological Differentiation. New York: Wiley, pp. 115–133. [Google Scholar]
- Yorulmaz H, Bayraktar S, Özdilli K. (2010) Posttraumatic growth in chronic kidney failure disease. Procedia Social and Behavioral Sciences 5: 2313–2319. [Google Scholar]