Abstract
Women with breast cancer often experience weight gain during and after treatment, significantly increasing risk for recurrence as well as all-cause mortality. Based on a growing body of evidence, meditative movement practices may be effective for weight management. First, we describe the effects of stress on factors associated with weight gain for breast cancer survivors. Then, a model is proposed that utilizes existing evidence to suggest how meditative movement supports behavioral, psychological, and neurohormonal changes that may explain weight loss. Application of the model suggests how a novel “mindful-body-wisdom” approach may work to help reduce weight for this at-risk group.
Keywords: breast cancer, eating behavior, exercise, model, obesity, psychological disturbance
Background
Increasing rates of breast cancer along with improvements in prognosis mean that the number of survivors is growing across all US populations, regardless of race, ethnicity, and socioeconomic status. As of January 2014, it was estimated that there were 14.5 million cancer survivors, representing approximately 4 percent of the population, with female breast as the most common cancer site representing 41 percent of female survivors (National Cancer Institute, 2014). Weight gain is a common and persistent problem for many breast cancer survivors (BCSs) (Kroenke et al., 2005). A review of 23 studies that assessed weight gain in BCSs (17 of which reported on women previously diagnosed at stages I–III) found that 50–96 percent of women with breast cancer experience significant weight gain during treatment and many, including some women who remain weight stable during treatment, report progressive weight gain in the months and years after diagnosis (Vance et al., 2011). Among those who gain weight, average increases range from 2.5 to 6.2 kg (Demark-Wahnefried et al., 1997; Heideman et al., 2009; Nagaiah et al., 2010). In one of the largest cohorts studied, with over 3000 BCSs participating in the Women’s Healthy Eating and Living (WHEL) study, weight gain was found to be positively associated with chemotherapy of any type, but less likely among the oldest and the most obese at diagnosis (body mass index (BMI) ≥ 35 kg/m2). Even in this very large study, however, only 10 percent returned to pre-diagnosis weight, reinforcing that despite conditions that vary the risk, weight gain is highly prevalent among stage I–III BCSs (Saquib et al., 2007). The bulk of the studies do not report on weight gain for women diagnosed with metastatic cancer, nor address concerns about weight management (Saquib et al., 2007; Vance et al., 2011). Further references to BCSs in this review regarding patterns associated with weight gain and suggestions for interventions to address the need of weight management, then, primarily address women in the nonmetastatic stages, working toward recovery and prevention of recurrence, past primary treatment.
Overweight and obesity at diagnosis, later weight gain, and sedentary behavior add to the risk of recurrence and all-cause mortality for BCSs (Caan et al., 2006; Chlebowski et al., 2002; Cleveland et al., 2007; Ewertz et al., 2011; Nichols et al., 2009; Patterson et al., 2010), making physical activity (PA) and weight loss for overweight and obese BCSs important targets of behavioral interventions.
Currently, there is a burgeoning interest in meditative movement (MM) practices among BCSs, including Tai Chi (TC), Yoga, and Qigong (QG). Evidence is accruing that MM may nudge the scales in a favorable direction, reducing weight and/or BMI in a number of populations without the typical, perceived hard work of vigorous PA and radical intentional reductions in dietary energy intake. For example, a number of studies have shown reductions in BMI or weight loss in diabetic populations in response to TC (Chen et al., 2010) or QG (Liu et al., 2011), and women with binge eating disorders, post-menopausal women, and adults with metabolic syndrome have reduced BMI in response to Yoga (Lee et al., 2012; McIver et al., 2009; Manchanda et al., 2013). There is some indication that this pattern may also hold for BCSs, a population particularly at risk for consequences of overweight and obesity (Janelsins et al., 2011; Larkey et al., 2012). It is possible that, with the added impetus for recovering from fatigue and the hope for avoiding recurrence, BCSs might be a population particularly ready to adopt new behaviors and be more successful at changing and maintaining a new health routine.
Our purpose in this article is to first summarize a set of biobehavioral factors that interact, explaining how weight gain occurs so frequently in BCSs, and then propose mechanisms for how this specific category of practices, MM, may provide an opportunity for discovering a novel path to weight loss.
Breast cancer and weight gain
The cascade of events during the diagnosis and treatment phases for women with breast cancer leads to weight gain for most, and then the problem persists into the post-treatment survivorship phase. Explanations for weight gain related to diet and PA levels are particularly relevant for this group of vulnerable women. Higher calorie consumption has been implicated as a possible reason for weight gain during breast cancer treatment (Demark-Wahnefried et al., 1997; Heideman et al., 2009; Rock et al., 1999). PA levels decline after diagnosis, with greater decline among women treated with chemotherapy and radiation or those who are obese (Irwin et al., 2003). Changes in resting energy expenditure during breast cancer treatment (Harvie et al., 2004) additionally contribute to the energy imbalance by reducing the energy requirements for individuals and resulting in weight gain even among those who maintain the same level of consumption and activity. Chemotherapy, especially those containing docetaxel and hormonal therapies, appears to exacerbate the problem (even more so for pre-menopausal women) (Demark-Wahnefried et al., 2001; Vance et al., 2011).
Beyond the critical energy balance relationships, there is an emerging picture of neuroendocrine factors associated with stressful life events that appear to not only spur weight gain for those who are highly stress reactive (i.e. respond to stress with sudden increases in cortisol that may, in turn, trigger eating) (Epel et al., 2001) but also contribute to weight maintenance after treatment ends. Although the relationships are complex and not fully understood, we focus here on a few of the established relationships, focusing on factors that have either been demonstrated as part of the profile of BCSs or for sedentary, stressed, overweight individuals—and in many cases, these profiles are similar. What follows is a description of how a number of psychological and behavioral factors interact with neuroendocrine markers related to BCSs and weight in stressful conditions (particularly the diagnosis and treatment for breast cancer), generally showing how “feeling physically poor” and “feeling emotionally poor” work together to make healthy behaviors and positive psychological states even less likely, thus sustaining the cycle.
Women with breast cancer experience significant stress during diagnosis and treatment of breast cancer (Andersen et al., 1994; Shapiro et al., 2002), even after treatment completion (Shapiro et al., 2002). In particular, continuous psychological stress and emotional distress such as anxiety, depression, and fear are shown to be prevalent in BCSs (Han et al., 2005), conditions that have a substantial negative influence on biological, physiological, and behavioral factors. Prolonged or frequent stress over diagnosis and treatment of cancer gives rise to disruption of the hypothalamic–pituitary–adrenal (HPA) axis (Elenkov and Chrousos, 2002; Tsigos and Chrousos, 2002) resulting in metabolic disruption (Alokail et al., 2013; Tyrka et al., 2012) including increased insulin resistance and its concomitant metabolic abnormalities (Duggan et al., 2010). These patterns indicative of metabolic syndrome, along with sedentariness, are common in BCSs (Irwin et al., 2009).
Chronic stress is further associated with changes in cortisol patterns. Higher levels of cortisol and flatter diurnal rhythms have been observed in women with breast cancer (Abercrombie et al., 2003; Sephton et al., 2000), and early stage survivors with fatigue show low waking levels of cortisol with flattened slopes (Bower et al., 2005). The increased sensitization of the HPA axis by psychological stress and associated cortisol disruption contributes to physiological and behavioral declines such as poor sleep and fatigue (the most common symptom in cancer patients) (Blesch et al., 1991; Bower et al., 2005). The rise in cortisol secondary to chronic stress has been strongly associated with weight gain (Vicennati et al., 2009).
Emotional distress prevalent among BCSs (Han et al., 2005; Von Ah and Kang, 2008) may also be linked to emotional eating, a key factor associated with weight gain. Somatic dissociation (i.e. a stepping back from body awareness, often associated with extreme stress) accompanying the experience of breast cancer (Landmark and Wahl, 2002) may persist into the survivorship phase (Cohen et al., 1998). A tendency to remove oneself from somatic experiences and reduction in body awareness may be related to various forms of disordered eating, including binge and emotional eating patterns (Katterman et al., 2014).
The high prevalence of fatigue among BCSs may diminish activities over daily life or lead to increasingly sedentary behaviors. These declined activities or sedentary behaviors may cause isolation and depression, resulting in further decrease in activity (Flechtner and Bottomley, 2003). This fatigue cycle can further influence emotional eating and weight gain (Demark-Wahnefried et al., 2012; Gerber et al., 2011).
HPA axis disruption also contributes to immune and inflammatory factor dysregulation. Women with breast cancer and BCSs have shown significantly high C-reactive protein (CRP) and interleukin (IL)-6 (Al Murri et al., 2006; Bower et al., 2002, 2003, 2011a; Howren et al., 2009; Salgado et al., 2003).CRP, an indicator of systemic inflammation, is known to be elevated in people who are overweight, and in a high proportion of BCSs (Al Murri et al., 2006), both pre- and post-menopausal Bower et al. 2011b. It has been shown that CRP binds to plasma leptin (the hunger-suppressing hormone associated with overweight) and impairs leptin signaling, suggesting CRP is not only a marker for inflammation-related co-morbidities, but may be involved in regulation of adiposity through leptin resistance and hunger cues (Chen et al., 2006). Therefore, CRP linked to the stress response (Owen et al., 2003; Steptoe et al., 2007; Yudkin et al., 2000) may hold a clue to the continued weight gain of BCSs and would be important to explore.
Many breast cancer patients and survivors, even years after completing treatment, continue to experience sleep dysfunctions (Vargas et al., 2010) that trigger inflammation theorized to mediate emotional distress (e.g. anxiety, depression) (Irwin et al., 2013) and fatigue. These persistent symptoms are all associated with overweight (Patel and Hu, 2008) and are potentially related to continued elevation of cortisol and CRP with depressed leptin into the evening hours (when cortisol should be decreasing to allow sleep, and leptin should be increasing to curb hunger prior to sleep) (Spiegel et al., 2004). Lower sleep duration is correlated with weight and weight gain (with progressively higher weight gain with each hour less sleep than 7 hours per night), perpetuating the problem through the cycle of biochemical imbalances that arise as sleep worsens. This “perfect storm” seems to be a cycle that is difficult to break (Patel et al., 2006).
Given the number of emotional, psychological, and behavioral factors and neuroendocrine disruptions for some BCSs contributing to gaining and maintaining overweight, it appears that a multi-factor approach to turn this tide is needed. In the next section, we discuss traditional approaches and then consider an approach to weight management for BCSs that addresses the stress response as a first step and that initiates a mind–body type of PA to begin shifting emotional distress, sleep patterns, and neurohormonal balance.
Weight loss for BCSs
PA and energy balance
Most weight loss interventions for the general population aim to alter energy balance by increasing energy expenditure through increasing the level of PA or reducing energy intake, or both. Weight loss programs in general depend heavily upon nutrition education, external control, and behavior change models and are difficult to sustain with or without PA (Howard et al., 2006; Rogers et al., 2005; Teixeira et al., 2011). Randomized trials assessing the effectiveness of popular diets among healthy individuals report modest weight loss only among a minority of individuals who are able to maintain a high level of adherence to these restrictive diets (Dansinger et al., 2005). Furthermore, there is generally poor sustainability in adherence to diets over time among study participants. While the overall patterns of activity intensity and activity-duration-to-inactivity ratios are considered critical, it has also been shown that participation in regular PA is associated with weight maintenance (Physical Activity Guidelines Advisory Committee, 2008).
Dietary counseling interventions produce modest weight losses that diminish over time in general populations (Dansinger et al., 2007) as well as cancer survivors (Mosher et al., 2013). Weight loss interventions among BCSs have been less studied, but again, generally follow the model of increasing PA and/or lowering energy intake to reduce weight and improve body composition (Christy et al., 2011; Demark-Wahnefried et al., 2007; Greenlee et al., 2013; McTiernan et al., 1998; Travier et al., 2014) with benefits also shown for strength training (Schmitz et al., 2010).
Exercise has been declared an important “medicine” for a number of symptoms and improving quality of life among cancer survivors (Schmitz et al., 2010) while addressing weight gain; there are many good reasons for promoting these lifestyle behaviors among BCSs. While there is limited evidence that certain dietary patterns, particularly low fat intake, are associated with breast cancer recurrence (Prentice et al., 2006; Rock et al., 2004), PA has been shown to reduce risk for recurrence among BCSs (Chen et al., 2011; Holmes et al., 2005; Irwin et al., 2008; Patterson et al., 2010).
The recent American College of Sports Medicine (ACSM) guidelines (Schmitz et al., 2010) recommend that cancer survivors follow PA guidelines for the general population (Physical Activity Guidelines Advisory Committee, 2008), with adaptations to fit the disease profile. Reduction in insulin resistance is common with PA initiation; insulin resistance has been associated with carcinogenesis through stimulation of insulin/insulin-like growth factor (IGF)-1 signaling pathways that promote cell proliferation and suppress apoptosis (Gallagher and LeRoith, 2010). Furthermore, hormonal profiles predicting recurrence are more favorable among survivors with lower BMI and those who are physically active (McTiernan et al., 2006), further making PA an important intervention target, both for overall weight management as well as for a number of symptoms. Specifically among BCSs, PA has been shown to have a number of positive effects on health and fitness in BCSs independent of weight loss, including improvements in upper and lower body strength, fatigue, depression, body image, and health-related quality of life (Duijts et al., 2011; Speck et al., 2010). Only a limited number of trials have demonstrated significant BMI loss, albeit small, among cancer survivors, with successes only achieved with nearly a year of intensive PA and dietary interventions (Demark-Wahnefried et al., 2007; Duijts et al., 2011; Speck et al., 2010). In a 10-month PA and dietary intervention study, patients gained weight back with no significant differences between control and study groups at the 2-year follow-up (Christy et al., 2011). Exploratory work is needed to evaluate a novel approach to weight management among this high-risk population.
MM: a potential intervention for weight loss for BCSs
MM is defined by Larkey et al. (2009) as practices that combine movement or postures, with meditative states and a focus on the breath to achieve deep states of relaxation, including forms such as QG, TC, or Yoga. Women who have been diagnosed with breast cancer are increasingly exploring complementary and alternative options during and after treatment, including mind/body exercise practices such as MM (e.g. Yoga, TC, QG) (Boon et al., 2007; Carpenter et al., 2009; Fouladbakhsh and Stommel, 2010; Matthews et al., 2007). Although these practices are often less exertive than conventional aerobic exercise, there is a growing body of evidence that MM—mind/body practices that also utilize movement—may reduce a number of symptoms and improve quality of life for BCSs (Jahnke et al., 2010; Janelsins et al., 2011; Larkey et al., 2014; Lee et al., 2010; Lengacher et al., 2009; Moadel et al., 2007; Oh et al., 2012a, 2012b; Speed-Andrews et al., 2010). These changes in symptom reduction and quality of life in response to MM practices are, in and of themselves, important outcomes that have been addressed in the current literature for each of these practices, Yoga, TC, and QG. We turn our attention now to the potential for MM to affect a critical factor associated with breast cancer recurrence, weight loss.
Although, to date, there are only two studies that indicate that MM affects weight loss among BCSs (Larkey et al., 2012; Janelsins, Davis, Wideman et al, 2011), there are a number of dynamics inherent in MM practices that address the very factors that may be related to weight loss. Thus, although it might not appear that lower levels of exertion associated with many of the MM practices would predict a change in weight, consideration of these factors beyond the PA intensity suggests other influences may be operating.
A number of recent studies indicate that weight, or more specifically BMI, may be reduced in response to MM practices (Cade et al., 2010; Liu et al., 2010; Manchanda et al., 2000), particularly for those who are overweight or obese (Chen et al., 2010), even when compared to non-MM exercise controls (Chen et al., 2010; Cheung et al., 2005; Dechamps et al., 2009).
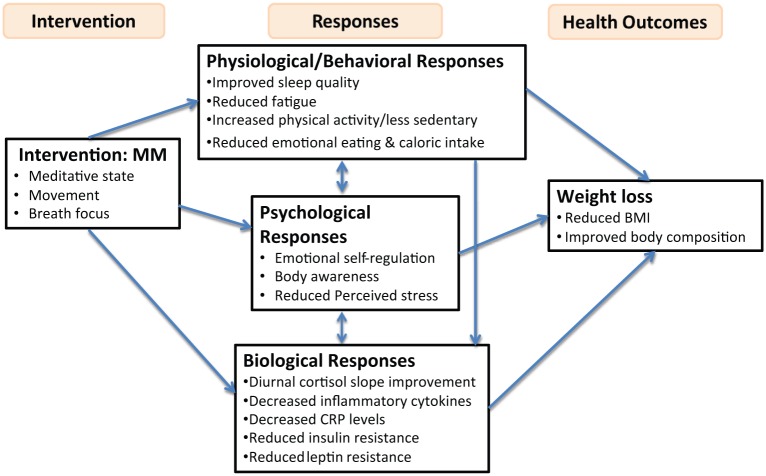
Findings from a recent pilot study (Larkey et al., 2012) indicate that with a low-intensity QG/TC intervention, BMI can be significantly reduced compared to a similar, low-intensity exercise that excludes the meditative and breath focus aspects of the QG/TC intervention and this low-intensity exercise may serve as a bridge to more moderate-intensity activities. Furthermore, a trend in the direction of decreased BMI was shown for BCSs in a recently published pilot study of TC compared to a sedentary psychosocial support control, also showing a significant difference in insulin levels (stable levels in TC) (Janelsins et al., 2011). Why might this be? We propose a model that suggests how MM components (PA, meditative states, and breath focus) may affect a number of factors that shift the burden of stress (Figure 1) and propose how MM may provide a multi-factor program for weight loss among overweight/sedentary BCSs with a previous diagnosis of up-to-stage-III breast cancer. For purposes of the presentation of this model and the relationships proposed, the factors discussed in the text are intended to be applied to BCSs. Although the formal definition of a survivor includes those from the time of diagnosis, we use the term, survivor in this case, to refer to those who have completed primary treatment (i.e. surgery, radiation, chemotherapies) and the literature supporting the proposed relationships is primarily focused on the post-treatment survivorship phase.
Figure 1.
Proposed model of effects of meditative movement on weight.
PA component of MM
QG and TC have been reported to be low to moderate level of intensity (Manzaneque et al., 2004; Taylor-Piliae and Froelicher, 2004) while Yoga practices may vary even more greatly up to high intensity (Ross and Thomas, 2010), and thus, there is a wide variety in the level of intensity in the MM practices showing up in intervention research. MM exercises have been associated with an increase in adherence to PA programs (Dechamps et al., 2007), and overall patterns of increased activity in daily life, even beyond the MM practice (Larkey et al., 2009). While PA alone is considered to be a necessary lifestyle intervention to promote weight loss, MM may bring additional elements to bear on the constellation of critical factors. We suggest that increases in overall PA resulting from participation in MM may contribute to weight loss based on the PA increases alone. Even with varying levels of intensity, reduced sedentary behavior and increased overall PA over time have been linked to reduced stress, improvements in CRP levels (Kasapis and Thompson, 2005), improved cortisol patterns, blood glucose and sleep regulation (Chodzko-Zajko et al., 2009), reduced insulin resistance (Mayer-Davis et al., 1998), and lowered BMI with reduced fat/lean ratios.
Meditative states, breathing practice, mood, and weight
A key component of MM is the mindful or meditative state and the relaxation response associated with slow, deep breathing. A growing body of research suggests that mindfulness-based interventions may reduce stress-related emotions (anxiety, depression) (Carlson et al., 2007; Ospina et al., 2007; Shapiro et al., 2002; Von Ah and Kang, 2008), metabolic imbalance (Daubenmier et al., 2012), and disordered eating (Katterman et al., 2014) through an emphasis on body awareness, emotional self-regulation, and attention to inner “wisdom” cues, as well as decreased BMI (Kristeller and Hallett, 1999; Kristeller and Wolever, 2011; Lillis et al., 2009). Mindfulness practices may also help maintain weight loss post-surgery in bariatric patients (Engstrom, 2007), possibly facilitated by reductions in emotional eating and caloric intake.
While breathing practices alone have not been directly tested/associated with weight loss, the calming of emotional states and self-regulation associated with MM breathing practices (Bhattacharya et al., 2002; Joseph et al., 2005; Martarelli et al., 2009) implies potential for changing emotional eating patterns and dietary intake. Both Yoga and TC have been shown to improve metabolic state and psychological symptoms (Cohen et al., 2008; Liu et al., 2010; Ross and Thomas, 2010; Tsang and Fung, 2008). Furthermore, there is evidence that mindfulness practice may reduce waking cortisol levels along with weight loss among obese women (Daubenmier et al., 2011).
Tsang and Fung (2008) propose a model of cascading effects for QG and TC for patients with depression shedding light on several routes through which weight loss may occur, including changes in symptoms (fatigue and sleep) and in serotonin levels, cortisol, and slowed release of glucocorticoids. Although very little has been done to test methods of normalizing cortisol in cancer patients, interventions that reduce stress have been relatively successful. Cortisol levels were reduced in acute response to Yoga practice (Kamei et al., 2000), and normal slopes were restored in a group of breast and prostate cancer patients who practiced mindfulness meditation (Carlson et al., 2004).
In summary, we propose that MM elements (meditative state, movement, breath focus) may increase emotional self-regulation and body awareness, eating behaviors, and PA in favor of weight loss. Diurnal cortisol slope improvements, reduced insulin resistance, and decreased CRP levels (reducing leptin resistance) may be centrally involved in the MM/weight loss relationship as mechanisms of change.
Discussion
Although at first consideration, MM practices may not be seen as adequate exertion to change the metabolic processes expected to change for weight loss results, we suggest that there are other effects of various components of MM that may make a difference through other routes. Overall increase in activity levels as women become less sedentary is a typical result of MM practice. Furthermore, the stress effects on the HPA axis induced by breast cancer diagnosis and treatment may potentially be reversed through normalizing metabolic patterns, and inflammatory and immune biomarkers. At the same time, these markers of biochemical change may also reflect behavioral changes in sleep and emotional eating, turning the tide of the many factors noted to be related to the weight gain in the first place. With the growing evidence for MM practices to be effective for weight loss, and the factors theorized to be associated with these results, there is a need to examine more specifically these practices, and potential mechanisms of change for BCSs, in research designs addressing the full model.
The model of behavioral/physiological, psychological, and biological responses to MM expected to support weight loss among overweight BCSs includes a number of testable relationships. In the conceptual model (see Figure 1), we propose several possible pathways by which MM interventions may impact weight loss. One pathway depicted suggests that the MM intervention leads to improved physiological (behavioral) responses (e.g. improved sleep quality), which then lead to weight loss. Evidence for MM intervention effects on sleep and the relationship of sleep and weight have been established, but the throughput of these relational factors has not been directly tested. This portion of the model could be tested using multiple regression analyses or path analyses to examine the role of physiological responses as mediators of the effect of MM on weight loss. Mediation models could also be used to examine the impact of MM interventions on psychological responses (e.g. emotional self-regulation), which then lead to weight loss, another pathway depicted in the conceptual model. Additionally, multiple mediator models could be used to examine the effect of an MM intervention on physiological and psychological responses simultaneously and whether one of these responses has an impact on weight loss while controlling for the effect of the other. Relevant research questions include “does MM have a stronger impact on physiological or psychological responses?” and “are physiological or psychological responses the stronger mediator of MM’s impact on weight loss?” Finally, three path mediation models could be used to test the effects of the MM intervention on physiological/psychological responses which then lead to biological responses (e.g. diurnal cortisol slope improvement) and ultimately weight loss.
Finally, we recognize that one of the most powerful benefits that could accrue to finding MM effects on weight loss is the potential for preventing recurrence. As with any model that is in the early stages of being tested, the various components related to weight loss need to be tested first to better understand the relationships. If findings confirm this model, the next step would be to design studies for the longer term to examine effects on recurrence mediated by MM forms of activity.
Conclusion
A model is proposed describing MM as an intervention venue for overweight BCSs that may provide an advantage over the typically prescribed diet and exercise solutions for some individuals, and asserting that this is a ripe area for testing in rigorous research. MM interventions may not only directly and indirectly address the usual culprits in weight gain (e.g. sedentariness and dietary intake) but also may begin to shift a number of underlying psychological and bio-mechanisms related to stress, potentially having more systematic and longer term effects.
Footnotes
Funding: This research was partially funded by the National Institutes of Health, National Center for Complementary and Alternative Medicine, 1U01AT002706-01A2, PI, Larkey.
References
- Abercrombie HC, Kalin NH, Thurow ME, et al. (2003) Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience 117(3): 505–516. [DOI] [PubMed] [Google Scholar]
- Al Murri A, Bartlett J, Canney P, et al. (2006) Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. British Journal of Cancer 94(2): 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alokail M, Al-Daghri N, Abdulkareem A, et al. (2013) Metabolic syndrome biomarkers and early breast cancer in Saudi women: Evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 13(1): 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R. (1994) A biobehavioral model of cancer stress and disease course. American Psychologist 49(5): 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Pandey US, Verma NS. (2002) Improvement in oxidative status with yogic breathing in young healthy males. Indian Journal of Physiology and Pharmacology 46(3): 349–354. [PubMed] [Google Scholar]
- Blanchard CM, Denniston MM, Baker F, et al. (2003) Do adults change their lifestyle behaviors after a cancer diagnosis? American Journal of Health Behaviour 27(3): 246–256. [DOI] [PubMed] [Google Scholar]
- Blesch KS, Paice JA, Wickham R, et al. (1991) Correlates of fatigue in people with breast or lung cancer. Oncology Nursing Forum 18(1): 81–87. [PubMed] [Google Scholar]
- Boon HS, Olatunde F, Zick SM. (2007) Trends in complementary/alternative medicine use by breast cancer survivors: Comparing survey data from 1998 and 2005. BMC Women’s Health 7(1): Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, et al. (2002) Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosomatic Medicine 64(4): 604–611. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, et al. (2003) T-cell homeostasis in breast cancer survivors with persistent fatigue. Journal of the National Cancer Institute 95(15): 1165–1168. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, et al. (2005) Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology 30(1): 92–100. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, et al. (2011a) Fatigue and gene expression in human leukocytes: Increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain, Behavior, and Immunity 25(1): 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, et al. (2011b) Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology 29(26): 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan BJ, Emond JA, Natarajan L, et al. (2006) Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Research and Treatment 99(1): 47–57. [DOI] [PubMed] [Google Scholar]
- Cade W, Reeds D, Mondy K, et al. (2010) Yoga lifestyle intervention reduces blood pressure in HIV-infected adults with cardiovascular disease risk factors. HIV Medicine 11(6): 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, et al. (2007) One year pre-post intervention follow up of, immune, endocrine and blood pressure outcomes of mindfulness based stress reduction (MBSR) in breast and prostate cancer patients. Brain, Behavior, and Immunity 21: 1038–1049. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, et al. (2004) Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology 29(4): 448–474. [DOI] [PubMed] [Google Scholar]
- Carpenter C, Ganz P, Bernstein L. (2009) Complementary and alternative therapies among very long-term breast cancer survivors. Breast Cancer Research and Treatment 116(2): 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li F, Li J, et al. (2006) Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nature Medicine 12(4): 425–432. [DOI] [PubMed] [Google Scholar]
- Chen SC, Ueng KC, Lee SH, et al. (2010) Effect of t’ai chi exercise on biochemical profiles and oxidative stress indicators in obese patients with type 2 diabetes. The Journal of Alternative and Complementary Medicine 16(11): 1153–1159. [DOI] [PubMed] [Google Scholar]
- Chen X, Lu W, Zheng W, et al. (2011) Exercise after diagnosis of breast cancer in association with survival. Cancer Prevention Research 4(9): 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B, Lo J, Fong D, et al. (2005) Randomised controlled trial of qigong in the treatment of mild essential hypertension. Journal of Human Hypertension 19(9): 697–704. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Aiello E, McTiernan A. (2002) Weight loss in breast cancer patient management. Journal of Clinical Oncology 20(4): 1128. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko W, Proctor D, Fiatarone Singh M, et al. (2009) Exercise and physical activity for older adults. Medicine & Science in Sports & Exercise 41(7): 1510–1530. [DOI] [PubMed] [Google Scholar]
- Christy SM, Mosher CE, Sloane R, et al. (2011) Long-term dietary outcomes of the FRESH START intervention for breast and prostate cancer survivors. Journal of the American Dietetic Association 111(12): 1844–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland RJ, Eng SM, Abrahamson PE, et al. (2007) Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiology Biomarkers & Prevention 16(9): 1803–1811. [DOI] [PubMed] [Google Scholar]
- Cohen B, Chang A, Grady D, et al. (2008) Restorative yoga in adults with metabolic syndrome: A randomized, controlled pilot trial. Metabolic Syndrome and Related Disorders 6(3): 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MZ, Kahn DL, Steeves RH. (1998) Beyond body image: The experience of breast cancer. Oncology Nursing Forum 25(5): 835–841. [PubMed] [Google Scholar]
- Dansinger M, Gleason J, Griffith J, et al. (2005) Comparison of the Atkins, Ornish, weight watchers, and zone diets for weight loss and heart disease risk reduction: A randomized trial. The Journal of the American Medical Association 293(1): 43–53. [DOI] [PubMed] [Google Scholar]
- Dansinger M, Tatsioni A, Wong JB, et al. (2007) Meta-analysis: The effect of dietary counseling for weight loss. Annals of Internal Medicine 147(1): 41–50. [DOI] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, et al. (2011) Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity 2011: Article ID 651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lin J, Blackburn E, et al. (2012) Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 37(7): 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechamps A, Gatta B, Bourdel-Marchasson I, et al. (2009) Pilot study of a 10-week multidisciplinary Tai Chi intervention in sedentary obese women. Clinical Journal of Sport Medicine 19(1): 49–53. [DOI] [PubMed] [Google Scholar]
- Dechamps A, Lafont L, Bourdel-Marchasson I. (2007) Effects of Tai Chi exercises on self-efficacy and psychological health. European Review of Aging and Physical Activity 4(1): 25–32. [Google Scholar]
- Demark-Wahnefried W, Campbell KL, Hayes SC. (2012) Weight management and its role in breast cancer rehabilitation. Cancer 118(S8): 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. (2007) Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. Journal of Clinical Oncology 25(19): 2709–2718. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Peterson BL, Winer EP, et al. (2001) Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. Journal of Clinical Oncology 19(9): 2381–2389. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Rimer BK, Winer EP. (1997) Weight gain in women diagnosed with breast cancer. Journal of the American Dietetic Association 97(5): 519–529; quiz 527–528. [DOI] [PubMed] [Google Scholar]
- Duggan C, Irwin ML, Xiao L, et al. (2010) Associations of insulin resistance and adiponectin with mortality in women with breast cancer. Journal of Clinical Oncology 29(1): 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijts SFA, Faber MM, Oldenburg HSA, et al. (2011) Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors? A meta-analysis. Psycho-Oncology 20(2): 115–126. [DOI] [PubMed] [Google Scholar]
- Elenkov I, Chrousos G. (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences 966(1): 290–303. [DOI] [PubMed] [Google Scholar]
- Engstrom D. (2007) Eating mindfully and cultivating satisfaction: Modifying eating patterns in a bariatric surgery patient. Bariatric Nursing and Surgical Patient Care 2(4): 245–250. [Google Scholar]
- Epel E, Lapidus R, McEwen B, et al. (2001) Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26(1): 37–49. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Jensen MB, Gunnarsdóttir K, et al. (2011) Effect of obesity on prognosis after early-stage breast cancer. Journal of Clinical Oncology 29(1): 25–31. [DOI] [PubMed] [Google Scholar]
- Flechtner H, Bottomley A. (2003) Fatigue and quality of life: Lessons from the real world. The Oncologist 8(Suppl. 1): 5–9. [DOI] [PubMed] [Google Scholar]
- Fouladbakhsh JM, Stommel M. (2010) Gender, symptom experience, and use of complementary and alternative medicine practices among cancer survivors in the US cancer population. Oncology Nursing Forum 37(1): E7–E15. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. (2010) The proliferating role of insulin and insulin-like growth factors in cancer. Trends in Endocrinology and Metabolism 21(10): 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber LH, Stout N, McGarvey C, et al. (2011) Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive Care in Cancer 19(10): 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee HA, Crew KD, Mata JM, et al. (2013) A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity 21: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WT, Collie K, Koopman C, et al. (2005) Breast cancer and problems with medical interactions: Relationships with traumatic stress, emotional self-efficacy, and social support. Psycho-Oncology 14(4): 318–330. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Campbell I, Baildam A, et al. (2004) Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Research and Treatment 83(3): 201–210. [DOI] [PubMed] [Google Scholar]
- Heideman W, Russell N, Gundy C, et al. (2009) The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. European Journal of Cancer 45(1): 119–126. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, et al. (2005) Physical activity and survival after breast cancer diagnosis. The Journal of the American Medical Association 293(20): 2479–2486. [DOI] [PubMed] [Google Scholar]
- Howard BV, Manson JE, Stefanick ML, et al. (2006) Low-fat dietary pattern and weight change over 7 years: The women’s health initiative dietary modification trial. The Journal of the American Medical Association 295(1): 39–49. [DOI] [PubMed] [Google Scholar]
- Howren M, Bryant M, Lamkin D, et al. (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine 71(2): 171–186. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Crumley D, McTiernan A, et al. (2003) Physical activity levels before and after a diagnosis of breast carcinoma. Cancer 97(7): 1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Smith AW, McTiernan A, et al. (2008) Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. Journal of Clinical Oncology 26(24): 3958–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez-Reeves M, et al. (2009) Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: The Yale exercise and survivorship study. Cancer Epidemiology, Biomarkers & Prevention 18(1): 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Ganz P, et al. (2013) Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior, and Immunity 30(15): S58–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke R, Larkey LK, Rogers CE, et al. (2010) Comprehensive review of health benefits of Qigong and Tai Chi. American Journal of Health Promotion 24(6): e1–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Davis PG, Wideman L, et al. (2011) Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clinical Breast Cancer 11(3): 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph C, Porta C, Casucci G, et al. (2005) Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46(4): 714–718. [DOI] [PubMed] [Google Scholar]
- Kamei T, Toriumi Y, Kimura H, et al. (2000) Decrease in serum cortisol during yoga exercise is correlated with alpha wave activation. Perceptual and Motor Skills 90(3 Pt. 1): 1027–1032. [DOI] [PubMed] [Google Scholar]
- Kasapis C, Thompson PD. (2005) The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. Journal of the American College of Cardiology 45(10): 1563–1569. [DOI] [PubMed] [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM, et al. (2014) Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eating Behaviors 15(2): 197–204. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Hallett CB. (1999) An exploratory study of a meditation-based intervention for binge eating disorder. Journal of Health Psychology 4(3): 357–363. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Wolever RQ. (2011) Mindfulness-based eating awareness training for treating binge eating disorder: The conceptual foundation. Eating Disorders 19(1): 49–61. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Chen WY, Rosner B, et al. (2005) Weight, weight gain, and survival after breast cancer diagnosis. Journal of Clinical Oncology 23(7): 1370–1378. [DOI] [PubMed] [Google Scholar]
- Landmark BT, Wahl A. (2002) Living with newly diagnosed breast cancer: A qualitative study of 10 women with newly diagnosed breast cancer. Journal of Advanced Nursing 40(1): 112–121. [DOI] [PubMed] [Google Scholar]
- Larkey LK, Jahnke R, Etnier J, et al. (2009) Meditative movement as a category of exercise: Implications for research. Journal of Physical Activity & Health 6(2): 230–238. [DOI] [PubMed] [Google Scholar]
- Larkey LK, Roe D, Weihs K, et al. (2014) Randomized controlled trial of Qigong/Tai Chi easy on cancer-related fatigue in breast cancer survivors. Annals of Behavioral Medicine. DOI: 10.1007/s12160-014-9645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkey LK, Weihs C, Lopez AM, et al. (2012) BMI Reductions in a Qigong/Tai Chi Easy Trial with Breast Cancer Survivors. In: Poster at Society for Behavioral Medicine, New Orleans, LA, April 24. [Google Scholar]
- Lee JA, Kim JW, Kim DY. (2012) Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause 19(3): 296–301. [DOI] [PubMed] [Google Scholar]
- Lee MS, Chen KW, Ernst E. (2010) Supportive cancer care with qigong. In: Cho WCS. (ed.) Supportive Cancer Care with Chinese Medicine. New York: Springer, pp. 77–94. [Google Scholar]
- Lengacher CA, Johnson-Mallard V, Post-White J, et al. (2009) Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncology 18(12): 1261–1272. [DOI] [PubMed] [Google Scholar]
- Lillis J, Hayes S, Bunting K, et al. (2009) Teaching acceptance and mindfulness to improve the lives of the obese: A preliminary test of a theoretical model. Annals of Behavioral Medicine 37(1): 58–69. [DOI] [PubMed] [Google Scholar]
- Liu X, Miller D, Burton NW, et al. (2010) A preliminary study of the effects of Tai Chi and Qigong medical exercise on indicators of metabolic syndrome, glycaemic control, health-related quality of life, and psychological health in adults with elevated blood glucose. British Journal of Sports Medicine 44(10): 704–709. [DOI] [PubMed] [Google Scholar]
- Liu X, Miller YD, Burton NW, et al. (2011) Qi-gong mind-body therapy and diabetes control: A randomized controlled trial. American Journal of Preventive Medicine 41(2): 152–158. [DOI] [PubMed] [Google Scholar]
- McIver S, O’Halloran P, McGartland M. (2009) Yoga as a treatment for binge eating disorder: A preliminary study. Complementary Therapies in Medicine 17(4): 196–202. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Ulrich C, Kumai C, et al. (1998) Anthropometric and hormone effects of an eight-week exercise-diet intervention in breast cancer patients: Results of a pilot study. Cancer Epidemiology Biomarkers & Prevention 7(6): 477–481. [PubMed] [Google Scholar]
- McTiernan A, Wu LL, Chen C, et al. (2006) Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 14(9): 1662–1677. [DOI] [PubMed] [Google Scholar]
- Manchanda S, Mehrotra U, Makhija A, et al. (2013) Reversal of early atherosclerosis in metabolic syndrome by Yoga—A randomized controlled trial. Journal of Yoga & Physical Therapy 3: Article 132. [Google Scholar]
- Manchanda S, Narang R, Reddy K, et al. (2000) Retardation of coronary atherosclerosis with yoga lifestyle intervention. The Journal of the Association of Physicians of India 48(7): 687–694. [PubMed] [Google Scholar]
- Manzaneque JM, Vera FM, Maldonado EF, et al. (2004) Assessment of immunological parameters following a qigong training program. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 10(6): CR264–R270. [PubMed] [Google Scholar]
- Martarelli D, Cocchioni M, Scuri S, et al. (2009) Diaphragmatic breathing reduces exercise-induced oxidative stress. Evidence-Based Complementary and Alternative Medicine 2011: Article ID 932430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AK, Sellergren SA, Huo D, et al. (2007) Complementary and alternative medicine use among breast cancer survivors. The Journal of Alternative and Complementary Medicine 13(5): 555–562. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, D’Agostino R, Jr., Karter AJ, et al. (1998) Intensity and amount of physical activity in relation to insulin sensitivity: The insulin resistance atherosclerosis study. The Journal of the American Medical Association 279(9): 669–676. [DOI] [PubMed] [Google Scholar]
- Moadel AB, Shah C, Wylie-Rosett J, et al. (2007) Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: Effects on quality of life. Journal of Clinical Oncology 25(28): 4387–4395. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Lipkus I, Sloane R, et al. (2013) Long-term outcomes of the FRESH START trial: Exploring the role of self-efficacy in cancer survivors’ maintenance of dietary practices and physical activity. Psycho-Oncology 22: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaiah G, Hazard HW, Abraham J. (2010) Role of obesity and exercise in breast cancer survivors. Oncology 24(4): 342–346. [PubMed] [Google Scholar]
- National Cancer Institute (2014) Estimated US cancer prevalence counts: Who are our cancer survivors in the U.S.? Available at: http://cancercontrol.cancer.gov/ocs/statistics/statistics.html(accessed October 2014).
- Nichols HB, Trentham-Dietz A, Egan KM, et al. (2009) Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiology Biomarkers & Prevention 18(5): 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Butow PN, Mullan BA, et al. (2012a) A critical review of the effects of medical qigong on quality of life, immune function, and survival in cancer patients. Integrative Cancer Therapies 11(2): 101–110. [DOI] [PubMed] [Google Scholar]
- Oh B, Butow PN, Mullan BA, et al. (2012b) Effect of medical qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Supportive Care in Cancer 20(6): 1235–1242. [DOI] [PubMed] [Google Scholar]
- Ospina MB, Bond K, Karkhaneh M, et al. (2007) Meditation Practices for Health: State of the Research (No. 07-E010). Rockville, MD: Agency for Healthcare Research and Quality. [PMC free article] [PubMed] [Google Scholar]
- Owen N, Poulton T, Hay FC, et al. (2003) Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain, Behavior, and Immunity 17(4): 286–295. [DOI] [PubMed] [Google Scholar]
- Patel SR, Hu FB. (2008) Short sleep duration and weight gain: A systematic review. Obesity 16(3): 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, White D, et al. (2006) Association between reduced sleep and weight gain in women. American Journal of Epidemiology 164(10): 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RE, Cadmus LA, Emond JA, et al. (2010) Physical activity, diet, adiposity and female breast cancer prognosis: A review of the epidemiologic literature. Maturitas 66(1): 5–15. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee (2008) Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Prentice RL, Caan B, Chlebowski RT, et al. (2006) Low-fat dietary pattern and risk of invasive breast cancer. The Journal of the American Medical Association 295(6): 629–642. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Newman V, et al. (1999) Factors associated with weight gain in women after diagnosis of breast cancer. Journal of the American Dietetic Association 99(10): 1212–1218, 1221. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Thomson CA, et al. (2004) Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. Journal of Clinical Oncology 22(12): 2379–2387. [DOI] [PubMed] [Google Scholar]
- Rogers LQ, Shah P, Dunnington G, et al. (2005) Social cognitive theory and physical activity during breast cancer treatment. Oncology Nursing Forum 32(4): 807–815. [DOI] [PubMed] [Google Scholar]
- Ross A, Thomas S. (2010) The health benefits of yoga and exercise: A review of comparison studies. The Journal of Alternative and Complementary Medicine 16(1): 3–12. [DOI] [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, et al. (2003) Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. International Journal of Cancer 103(5): 642–646. [DOI] [PubMed] [Google Scholar]
- Saquib N, Flatt SW, Natarajan L, et al. (2007) Weight gain and recovery of pre-cancer weight after breast cancer treatments: Evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Research and Treatment 105(2): 177–186. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, et al. (2010) American college of sports medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise 42(7): 1409–1426. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, et al. (2000) Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute 92(12): 994–1000. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Schwartz GER, Santerre C. (2002) Meditation and positive psychology. In: Snyder CR, Lopez SJ. (eds) Handbook of Positive Psychology. New York: Oxford University Press, pp. 632–645. [Google Scholar]
- Speck RM, Courneya KS, Mâsse LC, et al. (2010) An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Journal of Cancer Survivorship 4(2): 87–100. [DOI] [PubMed] [Google Scholar]
- Speed-Andrews AE, Stevinson C, Belanger LJ, et al. (2010) Pilot evaluation of an Iyengar yoga program for breast cancer survivors. Cancer Nursing 33(5): 369–381. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L’Hermite-Balériaux M, et al. (2004) Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology & Metabolism 89(11): 5762–5771. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. (2007) The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity 21(7): 901–912. [DOI] [PubMed] [Google Scholar]
- Taylor-Piliae R, Froelicher ES. (2004) The effectiveness of Tai Chi exercise in improving aerobic capacity: A meta-analysis. Journal of Cardiovascular Nursing 19(1): 48–57. [DOI] [PubMed] [Google Scholar]
- Teixeira P, Patrick H, Mata J. (2011) Why we eat what we eat: The role of autonomous motivation in eating behaviour regulation. Nutrition Bulletin 36(1): 102–107. [Google Scholar]
- Travier N, Fonseca-Nunes A, Javierre C, et al. (2014) Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Medical Oncology 31(1): 783. [DOI] [PubMed] [Google Scholar]
- Tsang HWH, Fung KMT. (2008) A review on neurobiological and psychological mechanisms underlying the anti-depressive effect of qigong exercise. Journal of Health Psychology 13(7): 857–863. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos G. (2002) Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research 53(4): 865–871. [DOI] [PubMed] [Google Scholar]
- Tyrka A, Walters O, Price L, et al. (2012) Altered response to neuroendocrine challenge linked to indices of the metabolic syndrome in healthy adults. Hormone and Metabolic Research 44(7): 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V, Mourtzakis M, McCargar L, et al. (2011) Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obesity Reviews 12(4): 282–294. [DOI] [PubMed] [Google Scholar]
- Vargas S, Wohlgemuth WK, Antoni MH, et al. (2010) Sleep dysfunction and psychosocial adaptation among women undergoing treatment for non-metastatic breast cancer. Psycho-Oncology 19(6): 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicennati V, Pasqui F, Cavazza C, et al. (2009) Stress-related development of obesity and cortisol in women. Obesity 17(9): 1678–1683. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Kang D. (2008) Correlates of mood disturbance in women with breast cancer: Patterns over time. Journal of Advanced Nursing 61(6): 676–689. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, et al. (2000) Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 148(2): 209–214. [DOI] [PubMed] [Google Scholar]