Abstract
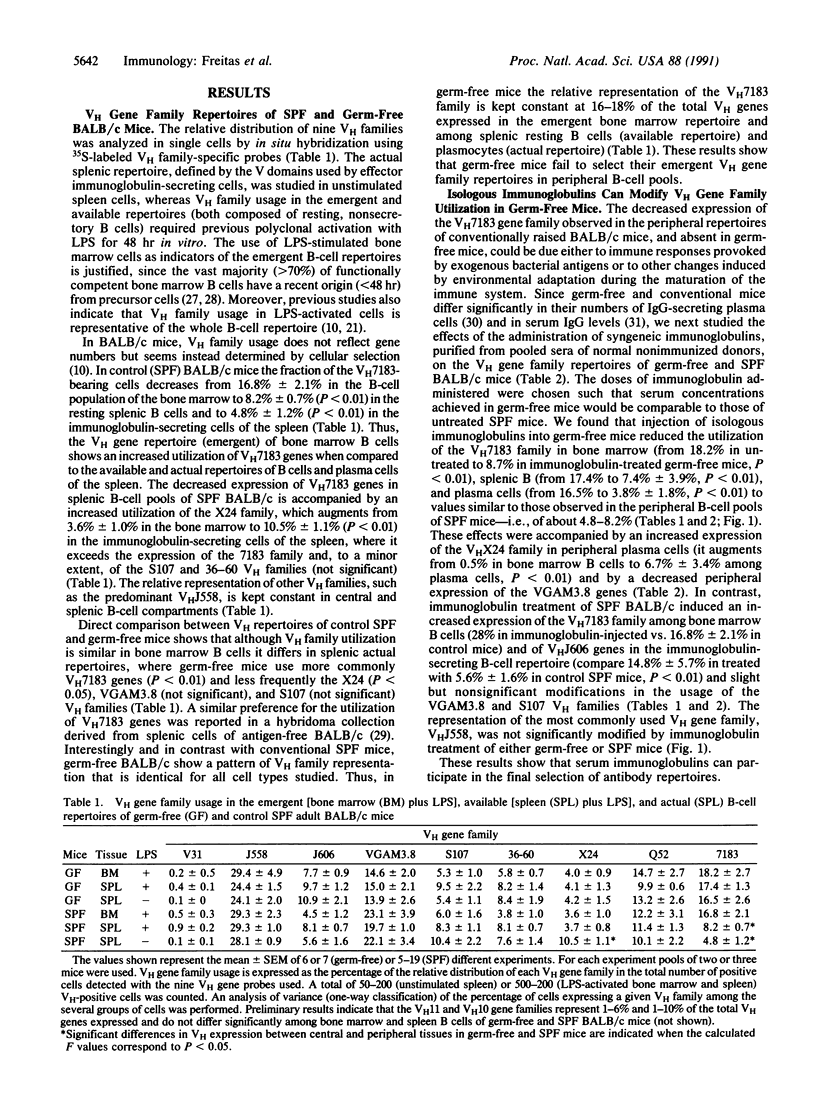
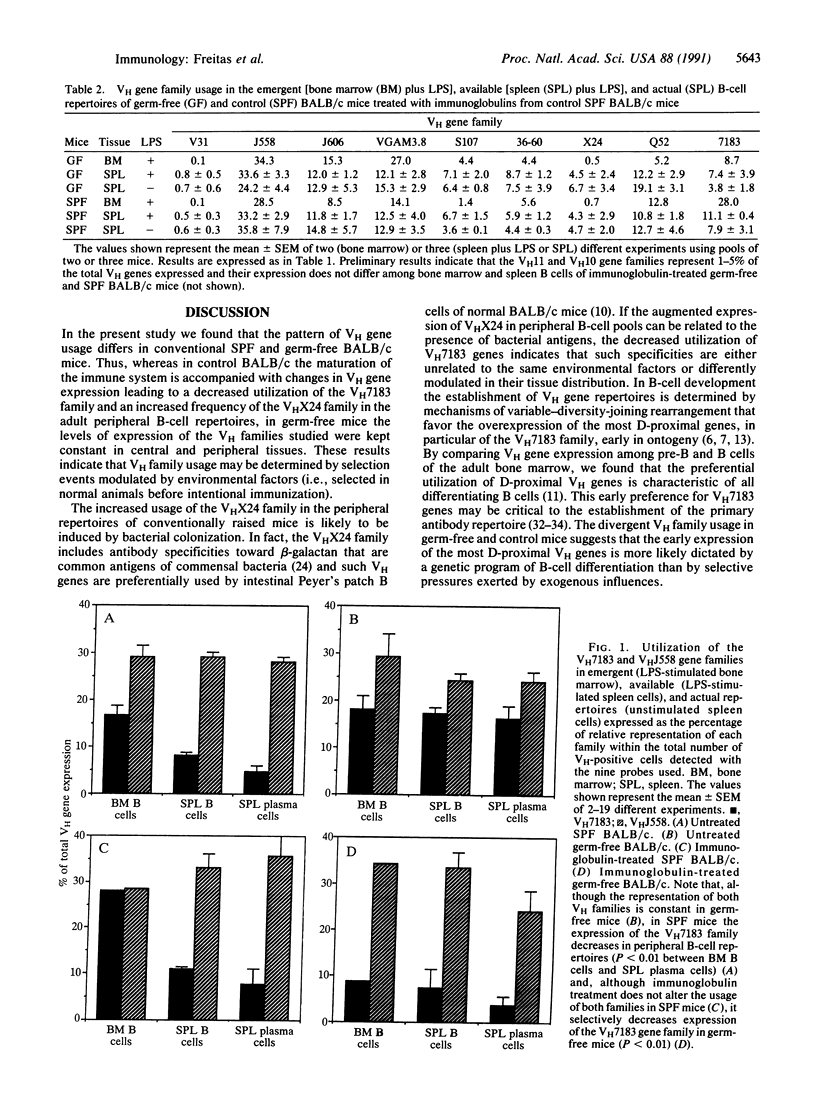
In B-cell development, expression of immunoglobulin heavy-chain variable-region (VH) gene repertoires is determined by genetic mechanisms that favor rearrangement of the most D-proximal genes, resulting in overutilization of the VH7183 gene family early in ontogeny and in differentiating B cells of the adult bone marrow. Maturation of the immune system is accompanied by a decreased expression of VH7183 genes in the peripheral immunocompetent B-cell pool of adult animals. By comparing VH gene family expression in the bone marrow (emergent) and peripheral (available and actual) B-cell repertoires of germ-free and conventionally raised BALB/c mice, we found that peripheral selection of VH gene family utilization does not occur in germ-free animals. Reconstitution of germ-free mice with normal serum immunoglobulins purified from syngeneic donors reestablishes selection of VH7183-expressing B cells. Our results indicate that preimmune B-cell repertoires are selected in normal animals by environmental antigens and serum immunoglobulins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Bernabé R. R., Coutinho A., Cazenave P. A., Forni L. Suppression of a "recurrent" idiotype results in profound alterations of the whole B-cell compartment. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6416–6420. doi: 10.1073/pnas.78.10.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos N. A., Kimura H., Meeuwsen C. G., De Visser H., Hazenberg M. P., Wostmann B. S., Pleasants J. R., Benner R., Marcus D. M. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989 Dec;19(12):2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- Bos N. A., Meeuwsen C. G. B cell repertoire in adult antigen-free and conventional neonatal BALB/c mice. I. Preferential utilization of the CH-proximal VH gene family PC7183. Eur J Immunol. 1989 Oct;19(10):1811–1815. doi: 10.1002/eji.1830191008. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989 Aug;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Andrade L., Lembezat M. P., Coutinho A. Selection of VH gene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2(1):15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Lembezat M. P., Coutinho A. Expression of antibody V-regions is genetically and developmentally controlled and modulated by the B lymphocyte environment. Int Immunol. 1989;1(4):342–354. doi: 10.1093/intimm/1.4.342. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Lembezat M. P., Rocha B. Selection of antibody repertories: transfer of mature T lymphocytes modifies VH gene family usage in the actual and available B cell repertories of athymic mice. Int Immunol. 1989;1(4):398–408. doi: 10.1093/intimm/1.4.398. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Sidman C. L. VH gene family repertoires of "viable motheaten" (mev) mice. Eur J Immunol. 1990 May;20(5):1033–1037. doi: 10.1002/eji.1830200513. [DOI] [PubMed] [Google Scholar]
- Grandien A., Coutinho A., Andersson J., Freitas A. A. Endogenous VH gene family expression in immunoglobulin-transgenic mice: evidence for selection of antibody repertoires. Int Immunol. 1991 Jan;3(1):67–73. doi: 10.1093/intimm/3.1.67. [DOI] [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg D., Andersson A., Carlsson L., Forsgren S. Establishment and functional implications of B-cell connectivity. Immunol Rev. 1989 Aug;110:89–103. doi: 10.1111/j.1600-065x.1989.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. Comparison of the fetal and adult functional B cell repertoires by analysis of VH gene family expression. J Exp Med. 1988 Aug 1;168(2):589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. VH gene family repertoire of resting B cells. Preferential use of D-proximal families early in development may be due to distinct B cell subsets. J Immunol. 1989 Oct 15;143(8):2752–2760. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986 Dec;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Kofler R. A new murine Ig VH gene family. J Immunol. 1988 Jun 1;140(11):4031–4034. [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. J., Barber P. C. Fixation of Thy-1 in nervous tissue for immunohistochemistry: a quantitative assessment of the effect of different fixation conditions upon retention of antigenicity and the cross-linking of Thy-1. J Histochem Cytochem. 1983 Feb;31(2):263–274. doi: 10.1177/31.2.6131917. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Mechanisms of clonal abortion tolerogenesis. I. Response of immature hapten-specific B lymphocytes. J Exp Med. 1978 Nov 1;148(5):1161–1170. doi: 10.1084/jem.148.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986 Oct;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Pereira P., Forni L., Larsson E. L., Cooper M., Heusser C., Coutinho A. Autonomous activation of B and T cells in antigen-free mice. Eur J Immunol. 1986 Jun;16(6):685–688. doi: 10.1002/eji.1830160616. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Klotz J. L., Bond M. W., Nahm M., Davie J. M., Hood L. Multiple VH gene segments encode murine antistreptococcal antibodies. J Exp Med. 1984 Jan 1;159(1):179–192. doi: 10.1084/jem.159.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinach P., Holmberg N. Ca-stimulated Mg dependent ATPase activity in a plasma membrane enriched fraction of bovine corneal epithelium. Curr Eye Res. 1987 Feb;6(2):399–405. doi: 10.3109/02713688709025193. [DOI] [PubMed] [Google Scholar]
- Reininger L., Kaushik A., Izui S., Jaton J. C. A member of a new VH gene family encodes antibromelinized mouse red blood cell autoantibodies. Eur J Immunol. 1988 Oct;18(10):1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- Rocha B., Penit C., Baron C., Vasseur F., Dautigny N., Freitas A. A. Accumulation of bromodeoxyuridine-labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990 Aug;20(8):1697–1708. doi: 10.1002/eji.1830200812. [DOI] [PubMed] [Google Scholar]
- Rossi F., Dietrich G., Kazatchkine M. D. Anti-idiotypes against autoantibodies in normal immunoglobulins: evidence for network regulation of human autoimmune responses. Immunol Rev. 1989 Aug;110:135–149. doi: 10.1111/j.1600-065x.1989.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Sheehan K. M., Brodeur P. H. Molecular cloning of the primary IgH repertoire: a quantitative analysis of VH gene usage in adult mice. EMBO J. 1989 Aug;8(8):2313–2320. doi: 10.1002/j.1460-2075.1989.tb08358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Rajewsky K. Mechanism of neonatally induced idiotype suppression and its relevance for the acquisition of self-tolerance. Immunol Rev. 1984 Jun;79:103–117. doi: 10.1111/j.1600-065x.1984.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Tutter A., Riblet R. Conservation of an immunoglobulin variable-region gene family indicates a specific, noncoding function. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7460–7464. doi: 10.1073/pnas.86.19.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D., Reis M. H., Albanese C., Costantini F., Baltimore D., Imanishi-Kari T. Altered repertoire of endogenous immunoglobulin gene expression in transgenic mice containing a rearranged mu heavy chain gene. Cell. 1986 Apr 25;45(2):247–259. doi: 10.1016/0092-8674(86)90389-2. [DOI] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization is regulated by a locus outside of the VH region. J Exp Med. 1988 Apr 1;167(4):1499–1504. doi: 10.1084/jem.167.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Malynn B. A., Alt F. W. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med. 1988 Jul 1;168(1):417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]



