Abstract
The genus Pinguicula (Lentibulariaceae) consists of about 100 carnivorous species, also known as butterworts. Eleven taxa are endemic to Italy, which represents a biodiversity hotspot for butterworts in Europe. The aim of our study was to provide a phylogenetic framework for the Italian endemics, in order to: a) investigate the relationships between species in this group; b) evaluate their actual taxonomic value. To achieve this, we analysed all the taxa endemic to Italy, along with several other species, by means of ITS nrDNA analysis. Our results clarify the relationships between Italian endemics and other Pinguicula taxa identifying a basal polytomy defined by five clades. All of the Italian endemics (with the exception of P. lavalvae) fall within a single large clade, which includes P. vulgaris and allied species. Among them, P. poldinii represents the most isolated lineage. Other taxa show strong molecular similarities and form a single subclade, although their taxonomic ranks can be retained. Pinguicula lattanziae sp. nov., seemingly endemic to Liguria (NW Italy), is also described.
Introduction
The genus Pinguicula L. (butterworts; Lentibulariaceae—Lamiales) includes about 100 species [1]. Butterworts are carnivorous plants that capture their prey by means of mucilaginous and sticky leaves, a rather simple trap compared to the other genera within the family, i.e. Genlisea A.St.-Hil. and Utricularia L. [2]. The geographic range of Pinguicula has two main areas of diversity, one in the Holarctic and the other in the Neotropic floristic kingdoms [3]. Only a few species cover a large geographic range (e.g. P. vulgaris L. or P. alpina L.), while many others are endemic to more restricted areas (e.g. P. balcanica Casper in the Balkans) or narrow endemics (e.g. P. sehuensis Bacch., Cannas & Peruzzi in a single mountain of Sardinia). Most of the European species grow in moist rocky habitat, with a few exceptions thriving in bogs or damp meadows (e.g. P. corsica Bern. & Gren. ex Gren. & Godr. or P. lusitanica L.), however all species require a humid environment [4].
Traditionally, butterworts have been grouped according to features of their generative and vegetative rosettes. Pinguicula species show either a temperate growth type, forming hibernacula to overcome the cold season, or a tropical growth type, with an overwintering vegetative rosette [3]. Moreover, the generative and vegetative rosettes can be different in shape and/or size, allowing a distinction between homoblastic (homophyllous) and heteroblastic (heterophyllous) species [5]. Another important diagnostic feature in butterworts is their chromosome number, and five different basic chromosome numbers, x = 6, 8, 9, 11, 14, have been identified [6]. Casper [7] divided the genus into three subgenera and 15 sections, but his taxonomy resulted artificial in several cases [8]. Compared with other families, a good molecular phylogenetic knowledge is available in literature for Lentibulariaceae [2, 9, 10, 11] and for its three genera: Pinguicula, Genlisea [12] and Utricularia [13].
Phylogenetic reconstructions in Pinguicula have been published by Cieslak et al. [8], by means of plastid matK and trnK group II intron (cpDNA), and by Degtjareva et al. [14] by means of ITS region (nuclear internal transcribed spacers and small ribosomal gene, ITST, nrDNA). The ITST resulted much more informative than plastid markers, and was also used in a couple of studies focused on the species from Northern hemisphere [15] and focused on central American and Cuban taxa [16, 17]. In the above-cited studies, a few Italian endemic species, such as P. fiorii Tammaro & Pace (endemic to Majella, Abruzzo [18]), P. poldinii J.Steiger & Casper (endemic to NE Italy [19]), were already included. However, after these studies, many new taxa were described from Italy, such as P. vallis-regiae F.Conti & Peruzzi endemic to Camosciara (Abruzzo, central Italy), P. vulgaris L. subsp. anzalonei Peruzzi & F.Conti endemic to central Italy (Latium), P. vulgaris subsp. ernica Peruzzi & F.Conti endemic to Ernici Mountains (Abruzzo, central Italy), P. vulgaris subsp. vestina F.Conti & Peruzzi endemic to Gran Sasso (Abruzzo, central Italy) [20], P. apuana Ansaldi & Casper and P. mariae Casper endemic to Apuan Alps (Tuscany, central Italy) [21], P. christinae Peruzzi & Gestri endemic to N Apennine (northern Italy) [22], P. lavalvae Innangi & Izzo [23] endemic to Mts. Picentini (Campania, southern Italy), and P. sehuensis Bacch., Cannas & Peruzzi endemic to Sardinia [1]. With a total of 11 endemic taxa [24, 25], Italy clearly represents a biodiversity hotspot for this genus in Europe (Fig 1). To date, the biogeographic and evolutionary history of butterworts is still not completely resolved [3, 14]. Hence, the aim of our study was to provide a phylogenetic framework for the Italian endemics, in order to: a) investigate the relationships between species in this group; b) evaluate their actual taxonomic value. To achieve this, we analysed all butterworts endemic to Italy, along with several other species by means of nuclear molecular markers and literature data. The molecular marker chosen for this study was the ITST (nrDNA), which proved to be the best tool for our purposes. Our marker choice is supported by: (1) high variability and discriminating capabilities of this molecular marker among Pinguicula taxa as already shown by Degtjareva et al. [14], Kondo & Shimai [15], Shimai & Kondo [16] and Shimai et al. [17]; (2) a complete GenBank nrDNA-Pinguicula database to be used for an exhaustive phylogenetic reconstruction; (3) completeness of information as a consequence of the biparental inheritance of nrDNA [6, 26, 27, 28]. In fact, cpDNA markers may cause confusion in inferring phylogenetic relationships in potentially hybrid/introgressed/polypoid taxa (e.g. [29]) because, with few exceptions (e.g. [30, 31]) they are maternally inherited [32, 33, 34] and hybridization phenomena easily go undetected. In addition, while cpDNA markers were successful in resolving phylogenetic relationships among Central American species [8], their variability resulted very low within P. sect. Pinguicula (including most of the species studied here), possibly due to more recent speciation events.
Fig 1. Flower diversity of Pinguicula species occurring in Italy and surrounding areas.
a = P. alpina; b = P. apuana; c = P. balcanica; d = P. christinae; e = P. corsica; f = P. fiorii; g = P. hirtiflora; h = P. lavalvae; k = P. poldinii; i = P. leptoceras; j = P. mariae; l = P. reichenbachiana; m = P. sehuensis; n = P. vallis-regiae; o = P. vulgaris subsp. anzalonei; p = P. vulgaris subsp. ernica; q = P. vulgaris subsp. vestina; r = P. vulgaris subsp. vulgaris.
Materials and Methods
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLoS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, new names contained in this work have been submitted to IPNI, from where they will be made available to the Global Names Index. The IPNI LSIDs can be resolved and the associated information viewed through any standard web browser by appending the LSID contained in this publication to the prefix http://ipni.org/. The online version of this work is archived and available from the following digital repositories: PubMed Central, LOCKSS, ResearchGate (https://www.researchgate.net/), ARPI (https://arpi.unipi.it/).
Ethics statement
The plant tissues (a small portion of leaf) analyzed are from herbarium specimens. These specimens are stored in Herbaria. In addition, when the plants were sampled, no specific permissions were required for this. Finally, the sampling has been performed without damaging the populations or the species in the case of narrow endemics with a single known population.
Pinguicula sampling
The plant tissues (i.e. small portion of leaf) analyzed were sampled from herbarium specimens. These source of material and Herbaria is summarized in Table 1.
Table 1. List of Pinguicula accessions sequenced in the molecular study, available karyological information, voucher information, origin, ITST—GC content (%) and GenBank accession no.
| Code | Taxon | 2n (ref.) | Origin (lat./long.) (date) | Voucher (Herbarium) (DNA code) | ITST | |
|---|---|---|---|---|---|---|
| GC% | GenBank no. | |||||
| P1 | Pinguicula apuana Casper & Ansaldi | 64, 128 [35] | Italy, Tuscany, Apuan Alps, M. Corchia (44.03, 10.29) (24 June 2004) | M. Ansaldi, G. Bedini, G. Cataldi (PI) (Ei 2) | 65.6 | LN887909 |
| P2 | P. apuana | 64, 128 [35] | Italy, Tuscany, Apuan Alps, M. Corchia (44.03, 10.29) (24 June 2004) | M. Ansaldi, G. Bedini, G. Cataldi (PI) (Ei 3) | 65.6 | LN887910 |
| P3 | P. cf. apuana | 64 [36] | France, Maritime Alps, Bendola (43.97, 7.58) (19 June 2013) | M. Pires (CBNMed) (Ei 23) | 65.6 | LN887911 |
| P4 | P. cf. apuana | 64 [36] | France, Maritime Alps, Bendola (43.97, 7.58) (19 June 2013) | M. Pires (CBNMed) (Ei 24) | 65.6 | LN887912 |
| P5 | P. balcanica Casper | 32 [7] | Bulgaria, M. Pirin, 2000 m (41.61, 23.54) (26 June 2006) | L. Peruzzi, D. Uzunov, G. Caruso (PI) (Ei 4) | 66 | LN887913 |
| P6 | P. balcanica | n.a. | Bulgaria, M. Vitosha, 1700–1800 m (42.59, 23.27) (23 June 2006) | L. Peruzzi (PI) (Ei 5) | 66 | LN887914 |
| P7 | P. christinae Peruzzi & Gestri | n.a. | Italy, Tuscany, N Apennine, Foce di Campolino, 1600 m (44.10, 10.64) (4 July 2011) | L. Peruzzi, G. Gestri (PI) (Ei 6) | 65.6 | LN887915 |
| P8 | P. christinae | 64 [7] | Italy, Tuscany, N Apennine, Lama Rossa, 1460 m (44.21, 10.38) (2 June 2011) | G. Gestri (PI) (Ei 7) | 65.4 | LN887916 |
| P9 | P. christinae | n.a. | Italy, Tuscany, N. Apennine, Val di Luce, 1620 m (44.12, 10.63) (13 June 2010) | G. Gestri (PI) (Ei 9) | 65.6 | LN887917 |
| P10 | P. cf. christinae | n.a. | Italy, Liguria, N. Apennine, Val d’Aveto, M. Aiona (44.47, 9.45) (21 June 2014) | G. Gestri (PI) (Ei 35) | 65.6 | LN887918 |
| P11 | P. corsica Bernard & Gren. ex Gren. & Godr. | n.a. | France, Corsica, Val d’Asco (42.42, 8.96) (25 September 2013) | G. Bacchetta 2051318 (CAG) (Ei 31) | 63.9 | LN887919 |
| P12 | P. corsica | n.a. | France, Corsica, Val d’Asco (42.42, 8.96) (25 September 2013) | G. Bacchetta 2051303 (CAG) (Ei 32) | 63.9 | LN887920 |
| P13 | P. corsica | n.a. | France, Corsica, Val d’Asco (42.42, 8.96) (25 September 2013) | G. Bacchetta 2051312 (CAG) (Ei 33) | 63.9 | LN887921 |
| P14 | P. corsica | n.a. | France, Corsica, Val d’Asco (42.42, 8.96) (25 September 2013) | G. Bacchetta 2051316 (CAG) (Ei 34) | 63.9 | LN887922 |
| P15 | P. fiorii Tammaro & Pace | n.a. | Italy, Abruzzo, Majella, Fara S. Martino (42.08, 14.18) (23 June 1988) | F. Conti 12720 (APP) (Ei 15) | 65.6 | LN887923 |
| P16 | P. fiorii | n.a. | Italy, Abruzzo, Majella, Valle dell’Orfento (42.17, 14.08) (28 May 2013) | L. Peruzzi (PI) (Ei 25) | 65.6 | LN887924 |
| P17 | P. fiorii | n.a. | Italy, Abruzzo, Majella, Valle dell’Orfento (42.17, 14.08) (28 May 2013) | L. Peruzzi (PI) (Ei 26) | 65.6 | LN887925 |
| P18 | P. hirtiflora Ten. | n.a. | Italy, Campania, Mts. Picentini, Matrunolo gorge (40.83, 14.92) (June 2010) | M. Innangi, A. Izzo VM217 (NAP) (Ei 38) | 56 | LN887926 |
| P19 | P. hirtiflora | n.a. | Italy, Campania, Amalfi peninsula, Vecite gorge (40.67, 14.66) (8 July 2010) | M. Innangi, A. Izzo VV14 (NAP) (Ei 39) | 56 | LN887927 |
| P20 | P. hirtiflora | 28 [7] | Italy, Campania, Sorrento peninsula, Mt. S’Angelo Tre Pizzi (40.65, 14.50) (17 June 2010) | M. Innangi, A. Izzo MSA523 (NAP) (Ei 36) | 56 | LN887928 |
| P21 | P. hirtiflora | 28 [7] | Italy, Calabria, Rossano Calabro (39.57, 16.64) (2011) | (CLU) (Ei 40) | 55.5 | LN887929 |
| P22 | P. hirtiflora | 28 [7] | Italy, Calabria, Rossano Calabro (39.57, 16.64) (16 June 2012) | M. Innangi, A. Izzo, P. Sbragia n.s. (NAP) | 55.5 | LN887930 |
| P23 | P. hirtiflora | n.a. | Greece, N Pindo, along the road from Grevena to Metsovo, 1000 m (39.79, 21.27) (27 June 2006) | L. Peruzzi, D. Uzunov, G. Caruso (PI) (Ei 11) | 55.4 | LN887931 |
| P24 | P. hirtiflora | 28 [67] | France, Maritime Alps, Val Roya, gorges de Bergue, along the road from Fontan to St. Dalmas de Tende, 500 m (44.01, 7.56) (30 April 2007) | L. Peruzzi, K.F. Caparelli (PI) (Ei 12) | 55.4 | LN887932 |
| P25 | P. lavalvae Innangi & Izzo | n.a | Italy, Campania, Mts. Picentini, Sabato valley (40.79, 14.97) (June 2010) | M. Innangi, A. Izzo VS15 (NAP) | 56 | LN887933 |
| P26 | P. lavalvae | n.a. | Italy, Campania, Mts. Picentini, Sabato valley (40.79, 14.97) (June 2010) | M. Innangi, A. Izzo VS21 (NAP) (Ei 37) | 56 | LN887934 |
| P27 | P. mariae Casper | 32 [7] | Italy, Tuscany, Apuan Alps, Isola Santa (44.06, 10.31) (27 April 2004) | M. Ansaldi (PI) (Ei 13) | 65.7 | LN887935 |
| P28 | P. poldinii J.Steiger & Casper | 32 [7] | Italy, Friuli Venezia Giulia, San Francesco (Pordenone), on the left side of Arzino (46.33, 12.93) (29 April 2013) | L. Peruzzi, G. Gestri, B. Pierini (PI) (Ei 27) | 63.4 | LN887936 |
| P29 | P. poldinii | 32 [7] | Italy, Friuli-Venezia Giulia, San Francesco (Pordenone), on the right side of Arzino (46.30, 12.93) (29 April 2013) | L. Peruzzi, G. Gestri, B. Pierini (PI) (Ei 28) | 63.4 | LN887937 |
| P30 | P. reichenbachiana Schindl. | 32 [37] | France, Maritime Alps, Val Roya, Gorges de Bergue, along the road from Fontan to St. Dalmas de Tende, 500 m (44.01, 7.56) (30 April 2007) | L. Peruzzi, K.F. Caparelli (PI) (Ei 14) | 65.6 | LN887938 |
| P31 | P. seuhensis Bacch., Cannas & Peruzzi | 16 [1] | Italy, Sardinia, Cengia del Monte Tonneri, Seui (39.89, 9.38) (3 May 2014) | G. Bacchetta 471313 (CAG) (Ei 29) | 63.8 | LN887939 |
| P32 | P. seuhensis | 16 [1] | Italy, Sardinia, Cengia del Monte Tonneri, Seui (39.89, 9.38) (3 May 2014) | G. Bacchetta 471314 (CAG) (Ei 30) | 63.8 | LN887940 |
| P33 | P. vallis-regiae F.Conti & Peruzzi | n.a. | Italy, Abruzzo, Villetta Barrea (41.78, 13.90) (2 July 2004) | F. Conti 11290 (APP) (Ei 16) | 65.5 | LN887941 |
| P34 | P. vulgaris L. subsp. anzalonei Peruzzi & F.Conti | n.a. | Italy, Lazio, Mt. Simbruini, Piscicarello di Jenne (41.90, 13.14) (26 May 2005) | F. Conti, F. Bartolucci, A. Bernardini 21422 (APP) (Ei 17) | 65.8 | LN887942 |
| P35 | P. vulgaris subsp. ernica Peruzzi & F.Conti | 64 [38] | Italy, Abruzzo, Mt. Ernici, Zompo Lo Schioppo (41.84, 13.39) (4 July 2004) | F. Conti, F. Bartolucci, M. Iocchi 21421 (APP) (Ei 18) | 65.8 | LN887943 |
| P36 | P. vulgaris subsp. vestina F. Conti & Peruzzi | 64 [38] | Italy, Abruzzo, Gran Sasso, Valle del Rio Arno (42.49, 13.54) (6 July 2005) | F. Conti, F. Bartolucci 10920 (APP) (Ei 19) | 65.8 | LN887944 |
| P37 | P. vulgaris subsp. vulgaris | n.a. | Italy, Abruzzo, Monti della Laga (42.65, 13.39) (23 June 2005) | F. Conti, F. Bartolucci 20984 (APP) (Ei 21) | 65.9 | LN887945 |
| P38 | P. vulgaris subsp. vulgaris | n.a. | Italy, Abruzzo, Monti della Laga, Pizzo di Moscio (42.65, 13.39) (13 August 2002) | F. Conti 2177 (APP) (Ei 22) | 65.9 | LN887946 |
A total of 38 accessions, corresponding to 13 species, was sampled. A single accession from Italy (P. cf. christinae) exhibited peculiar morphological features and its taxonomic position has been investigated in the present study (see morphometric analyses in S1 File).
Most of the sampled taxa are endemic to Italy, except for those accessions coming from Corsica (P. corsica), France (P. cf. apuana from Bendola, P. hirtiflora from Val Roya, P. reichenbachiana Schindl. also from Val Roya), Bulgaria (P. balcanica Casper) and Greece (P. hirtiflora). Sequence data of further 26 Pinguicula species (73 total accessions), many of them with a range not covering Italy, were obtained from GenBank, based on the works of Degtjareva et al. [14] and Kondo & Shimai [15] (Table 2).
Table 2. List of GenBank ITST accessions used for the phylogenetic analysis of Pinguicula taxa (taxon, distribution, GenBank no., reference).
| Taxon | Origin | GenBank no. | Reference |
|---|---|---|---|
| Outgroup | |||
| Antirrhinum majus L. subsp. cirrhigerum (Ficalho) Franco | Morocco, Doukkala-Abda, El Jadida | EU677200 | [39] |
| A. majus subsp. linkianum (Boiss. & Reut.) Rothm. | Spain, La Coruña, Cedeira | EU677214 | [39] |
| A. majus subsp. litigiosum (Pau) Rothm. | Spain, Teruel, Griegos | EU677216 | [39] |
| A. majus subsp. majus | Spain, Gerona, La Molina | EU677219 | [39] |
| A. majus subsp. tortuosum (Vent.) Rouy | Morocco, West Rif, Talembot | EU677242 | [39] |
| Kigelia africana (Lam.) Benth. | USA, County Arboretum & Botanic Garden of Los Angels | AY178638 | [40] |
| Sistergroup to Pinguicula L. | |||
| Genlisea violacea St.-Hil. | Brazil, Itacambira, Mato Grosso | AB212116 | [15] |
| Utricularia intermedia Hayne | Russia, Tver’ Prov. | DQ225109 | [14] |
| U. minor L. | Japan, Higashi-Hiroshima, Hiroshima | AB212118 | [15] |
| Pinguicula accessions | |||
| P. agnata Casper | Germany, Botanical Garden of Jena | DQ441602 | [14] |
| P. alpina L. | Slovakia, Mala Fatra, Terchová, | AB198341 | [15] |
| P. alpina (A) | Romania, Transylvanian Alps, W Sinaia | DQ222969 | [14] |
| P. alpina (B) | Italy, Trentino Alto-Adige, Siusi Alps | DQ438092 | [14] |
| P. alpina (C) | Switzerland, Canton Bern, between Habkern and Grünenberg-Pass | DQ438100 | [14] |
| P. balcanica Casper | Bulgaria, S of Sofia | DQ222954 | [14] |
| P. balcanica | Greece, Vardoússia, Fokída | AB198342 | [15] |
| P. bohemica Krajina | Czech Republic, SE of Ĉeska Lípa | DQ441597 | [14] |
| P. bohemica | Czech Republic, Ĉeska Lípa | AB198343 | [15] |
| P. caerulea Walter | USA, Georgia, SW of Folkston | DQ222963 | [14] |
| P. corsica Bernard & Gren. ex Gren. & Godr. | France, Corsica, Gorges de la Restonica | AB198344 | [15] |
| P. corsica (A) | France, Corsica, Lac de Melo | DQ222955 | [14] |
| P. corsica (B) | France, Corsica, Lac de Melo | DQ438098 | [14] |
| P. corsica (C) | France, Corsica, Lac de Melo | DQ438090 | [14] |
| P. crystallina Sm. | Cyprus, Tróodos | AB198363 | [15] |
| P. crystallina (A) | Cyprus | DQ222965 | [14] |
| P. crystallina (B) | Cyprus, Kakopetria, Ayios Nicolaos | DQ438082 | [14] |
| P. dertosensis (Cañig.) Mateo & M.B.Crespo | Spain, Tarragona, Sierra de Caro/Sierra de Fortalesa | DQ441598 | [14] |
| P. dertosensis | Spain, Ports de Beceit, Terragona | AB198345 | [15] |
| P. fiorii Tammaro & L.Pace | Italy, Abruzzo, Majella, Bocca di Valle | DQ222952 | [14] |
| P. fiorii | Italy, Abruzzo, Valle dell’Orfento | AB198346 | [15] |
| P. grandiflora Lam. subsp. grandiflora | France, Pyrenees, Lac de Fabregés, | AB198347 | [15] |
| P. grandiflora subsp. grandiflora f. grandiflora (A) | France, Pyrenees, Dept. Hautes | DQ222958 | [14] |
| P. grandiflora subsp. grandiflora f. grandiflora (B) | France, Dept. Ain | DQ438099 | [14] |
| P. grandiflora subsp. grandiflora f. grandiflora (C) | Spain, Picos de Europa, Rio Care | DQ438091 | [14] |
| P. grandiflora subsp. grandiflora f. pallida (Gaudin) Casper (A) | France, Dept. Ain | DQ222957 | [14] |
| P. grandiflora subsp. grandiflora f. pallida (B) | France, Dept. Ain | DQ438097 | [14] |
| P. grandiflora subsp. rosea (Mutel) Casper | France, Goncelin, Isère | AB198348 | [15] |
| P. grandiflora subsp. rosea (A) | France, Dept. Isère, between Concelin and Sollières NE of Grenoble | DQ222956 | [14] |
| P. grandiflora subsp. rosea (B) | France, Dept. Isère, Col du Granier | DQ438081 | [14] |
| P. hirtiflora Ten. | Italy, Campania, Vietri sul Mare | AB198364 | [15] |
| P. hirtiflora (A) | Italy, Campania, Salerno | DQ222966 | [14] |
| P. hirtiflora (B) | Greece, Thessalia, Mount Olympus | DQ438083 | [14] |
| P. leptoceras Rchb. | Italy, Liguria, Col di Tende | DQ222947 | [14] |
| P. leptoceras | France, Col de Tende | AB198349 | [15] |
| P. longifolia Ramond ex DC. subsp. caussensis Casper | France, Gorges du Tarn | AB198350 | [15] |
| P. longifolia subsp. caussensis (A) | France, Gorge du Tarn | DQ222948 | [14] |
| P. longifolia subsp. caussensis (B) | France, Gorge du Tarn | DQ438095 | [14] |
| P. longifolia subsp. caussensis (C) | France, Gorge du Tarn | DQ438088 | [14] |
| P. longifolia subsp. longifolia | Spain, Huesca, Valle de Ordesa | AB198351 | [15] |
| P. longifolia subsp. longifolia (A) | France, Pyrenees | DQ222959 | [14] |
| P. longifolia subsp. longifolia (B) | France, Central Pyrenees | DQ438089 | [14] |
| P. longifolia subsp. reichenbachiana (Schindler) Casper | France, Maritime Alps, Roya Valley | AB198352 | [15] |
| P. longifolia subsp. reichenbachiana (A) | France, Maritime Alps, Roya Valley | DQ222950 | [14] |
| P. longifolia subsp. reichenbachiana (B) | France, Maritime Alps, Roya Valley | DQ438094 | [14] |
| P. longifolia subsp. reichenbachiana (C) | France, Maritime Alps, Roya Valley | DQ438087 | [14] |
| P. lusitanica L. | Spain, Rio de la Miel near Algeciras | DQ222960 | [14] |
| P. lusitanica | England, Brokenhurst, Hampshire | AB198365 | [15] |
| P. lutea Walter | USA, Alabama, S. Elsauer | DQ222962 | [14] |
| P. macroceras Link | Japan, Nagano, Todai-gawa | AB198353 | [15] |
| P. macroceras subsp. nortensis J.Steiger & Rondeau | USA, northernmost California, del Norte County | DQ222951 | [14] |
| P. moranensis Kunth | Germany, Botanical Garden of Jena | DQ222967 | [14] |
| P. mundi Blanca Jamilena Ruíz Rejón & Reg.Zamora | Spain, Albacete, near border to Prov. Jaen | DQ441599 | [14] |
| P. mundi | Spain, Albacete, Nacimiento del Río Mundo | AB198354 | [15] |
| P. nevadensis (H.Lindb.) Casper | Sapin, Sierra Nevada | AB198355 | [15] |
| P. planifolia Chapm. | USA, Florida, Apalachicola Forest near Sumatra | DQ441601 | [14] |
| P. poldinii J.Steiger & Casper | Italy, Friuli Venezia Giulia, Campone | DQ441600 | [14] |
| P. poldinii | Italy, Friuli Venezia Giulia, Val d’Arzino | AB198356 | [15] |
| P. primuliflora C.E.Wood & R.K.Godfrey | USA, Florida, S Crestvien | DQ222964 | [14] |
| P. ramosa Miyoshi | Japan, Tochigi, Koshin-zan | AB198357 | [15] |
| P. vallisneriifolia Webb | Spain, Sierra de Segura | AB198358 | [15] |
| P. vallisneriifolia (A) | Spain, Sierra de Cazorla, Cueva de la Magdalena | DQ222953 | [14] |
| P. vallisneriifolia (B) | Spain, Sierra de Cazorla, Cueva de la Magdalena | DQ438084 | [14] |
| P. variegata Turcz. | Russia, Khabarovskiy kray, near Okhotsk | DQ222968 | [14] |
| P. variegata | Russia, Sakhalin Island | AB198359 | [15] |
| P. villosa L. | USA, Alaska, Broad Pass, Kantwell | AB198360 | [15] |
| P. villosa (A) | Cultivated material (no origin) | DQ222961 | [14] |
| P. villosa (B) | Norway, Soer-Troendelag | DQ438096 | [14] |
| P. villosa (C) | Sweden, Abisko | DQ438085 | [14] |
| P. vulgaris L. | Slovakia, Velká Fatra, Martín | AB198361 | [15] |
| P. vulgaris (A) | Iceland | DQ222949 | [14] |
| P. vulgaris (B) | Germany, Altenberga near Jena | DQ438086 | [14] |
| P. vulgaris (C) | Switzerland, Canton Bern | DQ438093 | [14] |
DNA extraction, PCR amplification and sequence analyses
Total DNA was extracted from approx. 3 mg of fresh leaf or 2 mg of dried leaf material using ZR Plant/Seed DNA MicroPrep (ZYMORESEARCH), according to the manufacturer’s protocol, then resuspended in 20 μL sterile distilled water. The concentration was estimated by quantifying 1 μl of DNA extract using a Qubit dsDNA HS Assay Kit with the Qubit ver. 2 Fluorometer (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA).
ITST was amplified by using plant universal primers that were reported in the literature {Forward-18S(3’) 5’-GGA GAA GTC GTA ACA AGG TTT CCG-3′, and Reverse-26S(5′)-internal 5’-TTC GCT CGC CGT TAC TAA GGG-3’} [28] and selective internal primers designed on Pinguicula sp. accessions from GenBank (Pingui_18Sfor 5’-AAG GAT CAT TGT CGA DWY Y-3’ and Pingui_26Srev 5’-TGR GGT CGC RRR IGT TGG CR-3’). The use of nested-PCR with selective primers for Pinguicula accessions can be very useful to remove possible contaminators (e.g. pollen) occurring on the flypaper traps. All oligos were synthetized by Macrogen Inc., the annealing temperature was 55°C for the two primer pairs. The volume of each polymerase chain reaction (PCR) was 25 μl, with c. 10–20 ng of DNA template, 2.5 μl of 10× DreamTaq Buffer (Thermo Fisher Scientific Inc.), 0.5 μl each of the 2.5 mM nucleotides (Promega), 0.125 μl of 50mM primer and 0.25 μl DNA DreamTaq polymerase (5Uμl–1) (Thermo Fisher Scientific Inc.). Amplifications of recalcitrant DNA templates were performed by using nested-PCRs with internal selective primers or KAPA3G Plant PCR Kit (KAPABIOSYSTEMS). The cycling parameters of the PCRs were performed according to the manufacturer’s instructions in a SimpliAmp thermal cycler (Applied Biosystems, Thermo Fisher Scientific Inc.). Amplification products were purified, using the NucleoSpin® Gel and PCR Clean-up (Macherey-Nagel) following the manufacturer’s protocols. An aliquot of approximately 70 ng of purified DNA template was used with the Bright Dye Terminator Cycle Sequencing Kit (ICloning) following the procedure according to Di Maio & De Castro [41] and analysed with a 3130 Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific Inc.).
Several sequences with slippage events for a mononucleotide repeat (polyC/G) were cloned with the CloneJET PCR Cloning Kit (Thermo Fisher Scientific Inc.) following the protocol of the manufacturer. Transformation was carried out using StrataClone SoloPack Competent Cells (Agilent Technologies). Bacteria were cultured in LB medium at 37°C for 30 min and then on LB agar plates containing 100 ug/ml ampicillin. Eight randomly selected clones from each transformation were amplified and sequenced with the pJET2.1 forward and pJET2.1 reverse primers (Thermo Fisher Scientific Inc.) which matching in the flanking regions of the pJET1.2/blunt vector polylinker.
Complete sequences of both strands of each PCR product were processed using the AB DNA Sequencing Analysis ver. 5.2 (Applied Biosystems, Life Technologies), and visually checked using Chromas Lite ver. 2.1.1. software (Chromas Lite version 2.1, http://technelysium.com.au/?page_id=13). Then, sequences were aligned using ClustalW ver. 1.4 software [42] as daughter processes of BioEdit ver. 7.2.5 software [43]. The aligned sequences were visually inspected to correct gap distributions devoid of biological meaning. The sequences obtained in this study are available from GenBank and accession numbers are provided in Table 1.
Phylogenetic analyses
One hundred and twenty accessions were analysed using two phylogenetic approaches: Bayesian inference (BI) and maximum parsimony (MP). The trees generated by these methods were checked for congruence. Outgroups were chosen after several preliminary analyses considering the literature data [7, 14, 15]: Kigelia africana (Lam.) Benth. {GenBank (GB): AY178638}, Antirrhinum majus L. (GB: EU677219) and some of its subspecies {i.e. A. majus subsp. linkianum (Boiss. & Reut.) Rothm., GB: EU677214; A. majus subsp. cirrhigerum (Ficalho) Franco, GB: EU677200; A. majus subsp. tortuosum (Vent.) Rouy, GB: EU677242; A. majus subsp. litigiosum (Pau) Rothm., GB: EU677216}. Representative of Pinguicula sister group were also included: Genlisea violacea St.-Hil. (GB: AB212116), Utricularia intermedia Hayne (GB: DQ225109) and U. minor L. (GB: AB212118).
The BI method for phylogenetic reconstruction was implemented using MrBayes ver. 3.2.2 software [44, 45] and jModelTest ver. 2.1.4 software to obtain the best nucleotide substitutions model [46, 47]. Preliminary analyses were performed to obtain the optimal BI settings. Bayesian Markov Chain Monte Carlo (MCMC) algorithm was run for 15,000,000 generations discarding the first 12% generations (burninfrac = 0.12). Four runs were performed and two heated chains were used. Bayesian posterior probabilities (PP) were obtained from the 50% majority rule consensus of 52,800,000 trees (13,200,000 trees from each runs). The general time reversible (GTR) + proportion of invariant sites (I) + gamma distribution (G) model was used in the analyses (set nst = 6 rates = invgamma), according to the results obtained with jModelTest under the Akaike Information Criterion (AIC) [48]. The same model was obtained using also the correction for sample size (AICc).
MP reconstruction were performed with TNT ver. 1.1 software [49, 50]. Settings included: gaps scored as missing data; maximum storage space of 99,000 trees; a tree storage space per iteration of 100; 100 iterations branch swapping via tree bisection-reconnection. In addition, we used also the "new technology search" (which is guaranteed to find at least one shortest tree), setting 10 hits to the shortest length and an initial level of 100. Bootstrap values [51] were calculated from 1,000 replicates.
Results
Molecular analysis
The alignment of ITST sequences of all Pinguicula accessions was relatively straightforward due to not complex variation among sequences (598–625 bp, P. caerulea Walter-P. agnata Casper/P. moranensis Kunth). In the 38 accessions of Pinguicula sequenced in the present study, the length of the ITST ranged from 607 (P. hirtiflora) to 617 bp (P. vulgaris s.l.). The sister group taxa had relatively variable sequences in length, with ITS sequences ranging from 501 bp (Utricularia intermedia) to 650 bp (Genlisea violacea).
After introducing the necessary gaps, the ITST alignment resulted in a matrix of 695 characters, of which 200 constant and 495 variable (425 potentially parsimony-informative). The mean G+C content of the ITS matrix was 63.5%. Among the Pinguicula species sequenced in this study, the lowest G+C content was found in some P. hirtiflora accessions (P23, P24) (55.4%) and the highest in P. balcanica (P5, P6) (66%) (Table 1).
Phylogenetic reconstructions
Inferred phylogenies from the BI and MP analyses of the ITST datasets produced a very similar topology with similar statistical support (Figs 2 and 3). The MP analysis yielded 492 most-parsimonious trees with a consistency index (CI) of 0.57 and retention index (RI) of 0.89. The strict consensus tree was 1592 steps long. The bootstrap support showed high values (BS > 70%) for the 72% of the nodes (Figs 2 and 3). The Bayesian tree with posterior probabilities (PP) is also shown in Figs 2 and 3. An unresolved basal structure for some Pinguicula groups is detected, albeit many clades are well supported (Figs 2 and 3).
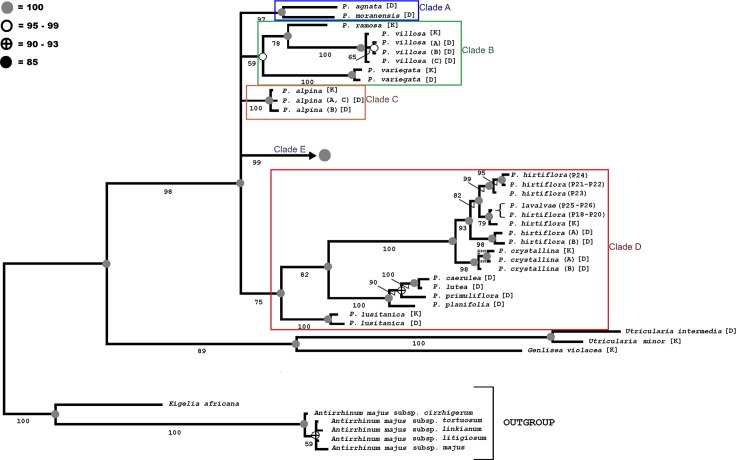
Fig 2. Bayesian phylogenetic tree of Pinguicula (clades A-D), reconstructed using ITST sequences.
Circles on the nodes show posterior probabilities (PP) from the Bayesian analysis under the GTR+I+G model (15,000,000 generations, burn-in 12%). Grey circles: PP = 100; white circles: PP = 99–95; black/white circles: PP = 90–93; black circle: PP = 85. Branches present in the MP strict consensus tree is marked with a dashed line (MP trees = 2720, steps = 1605, CI = 0.57, RI = 0.89). Bootstrap percentages (1000 replicates) are given below the branches. D or K letters after the taxon refer to the accessions obtained from Degtjareva et al. [14] or Kondo & Shimai [15], respectively (Table 2). See Table 1 for further details about our Pinguicula accessions.
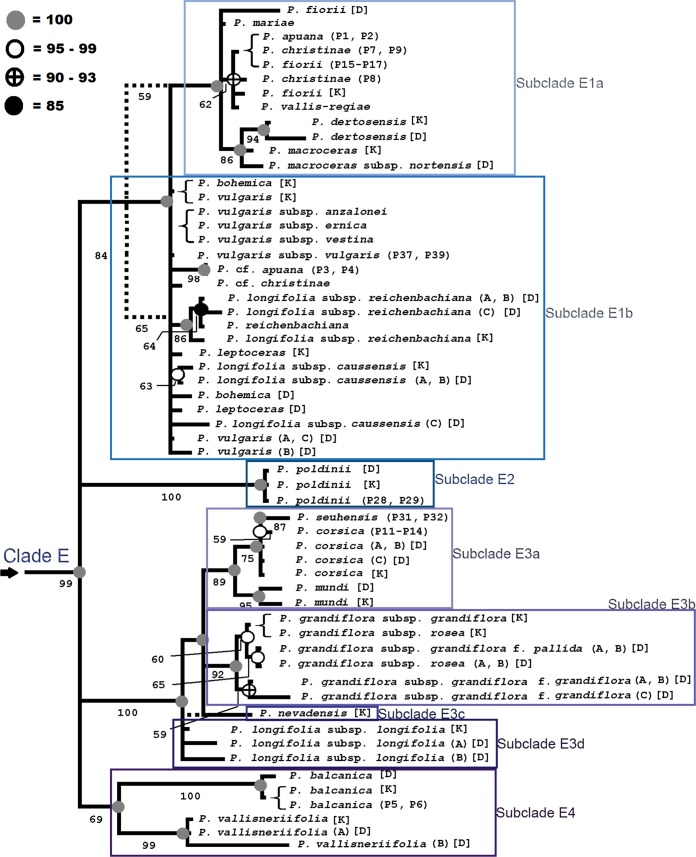
Fig 3. Bayesian phylogenetic tree of Pinguicula (clade E), reconstructed using ITST sequences.
Circles on the nodes show posterior probabilities (PP) from the Bayesian analysis under the GTR+I+G model (15,000,000 generations, burn-in 12%). Grey circles: PP = 100; white circles: PP = 99–95; black/white circles: PP = 90–93; black circle: PP = 85. Branches present in the MP strict consensus tree is marked with a dashed line (MP trees = 2720, steps = 1605, CI = 0.57, RI = 0.89). Bootstrap percentages (1000 replicates) are given below the branches. D or K letters after the taxon refer to the accessions obtained from Degtjareva et al. [14] or Kondo & Shimai [15], respectively (Table 2). See Table 1 for further details about our Pinguicula accessions.
A basal polytomy defined by five clades can be detected. The basally collapsed branches consist of three smaller groups (Clade A = P. agnata+P. moranensis; Clade B = P. ramosa Miyoshi+P. villosa L.+P. variegata Turcz. and Clade C = lineage of P. alpina) and a well-supported lineage (Clade D) where P. hirtiflora/P. lavalvae/P.crystallina are sister to Pinguicula species from the south-east United States of America (P. caerulea+P. lutea Walter+P. primuliflora C.E.Wood & R.K.Godfrey+P. planifolia Chapm.) and the European western-Atlantic P. lusitanica. In the next node (Clade E), which is not as well supported as other clades, four subclades can be identified. The first subclade (E1) includes P. vulgaris and allied taxa, with internal further subdivisions, including many Italian endemics (e.g. P. apuana, P. fiorii, and P. christinae), and other taxa related to P. vulgaris. The second subclade (E2) includes a single lineage with the Italian endemic P. poldinii. The third subclade (E3) includes further subdivisions of species with mostly Iberian origin, that will be discussed in more detail. Finally, the fourth subclade (E4) includes P. balcanica and P. vallisneriifolia Webb.
Discussion
Comparisons to previous phylogenetic reconstructions
Despite we could not include all taxa comprised in the genus Pinguicula, our sample size and array of species was sufficient to make phylogenetic assessments on the Italian endemic taxa (Figs 2 and 3). Comparing the phylogenies obtained with the ITST data published by Degtjareva et al. [14] and by Kondo & Shimai [15] and the matK-trnK sequences published by Cieslak et al. [8], the overall topology of Pinguicula species is generally consistent, although showing several differences.
Cieslak et al. [8] in their matK-trnK analysis, identified five main clades, partly overlapping with our results. Among the main similarities, the deep branching position of species belonging to our clade D (P. lusitanica and P. crystallina) and the overall placement of taxa allied to P. vulgaris in a single clade (corresponding to our clade E, Fig 3). Among the incongruences, P. mundi and P. corsica are placed on different lineages, whereas in our and in the previous ITST studies [14, 15] the aforementioned species are always placed in the same clade. Pinguicula primuliflora and P. lutea are included in the same clade of P. lusitanica, whereas in our analysis they form a distinct clade (B). The position of P. alpina is also in contrast with our results (clade C, Fig 2), since Cieslak et al. [8] place it in an isolated position within East Asian species (our deep branching clade D). The differences between our results and those published by Cieslak et al. [8] can be explained by the use of different molecular markers, as already mentioned in the Introduction.
No major differences are detected in the tree topologies with respect to previously published ITST studies. Kondo & Shimai [15] detected P. crystallina and P. hirtiflora as sister to all the remaining Pinguicula species. On the contrary, we found P. lusitanica, a species occurring on western coast of the Atlantic Ocean, from northern Morocco to Scotland [52], as belonging to the same, earliest branching, clade (D) (Fig 2). The rest of the phylogenetic reconstruction of Kondo & Shimai [15] is largely consistent with our finding, but included only two Italian endemics (P. fiorii and P. poldinii).
Generally, the topologies found by Degtjareva et al. [14] are also consistent with our findings. Similarly to Kondo & Shimai [15] and Cieslak et al. [8], they identified P. crystallina and P. hirtiflora as sister group to all other Pinguicula species. Pinguicula lusitanica, once again, is placed outside this clade. Pinguicula alpina is found in the same clade as P. moranensis, but these species belong to different clades in our results (A and C, respectively) (Fig 2).
The taxonomical evaluation of P. lavalvae
All the species within clade D (Fig 2) show a tropical growth type [3], and have been classified within P. sect. Cardiophyllum [5]. Among them, P. crystallina occurs east of the Aegean sea in southern Turkey and Cyprus, while P. hirtiflora occurs west of the Aegean sea in the Balkans and in separate populations in southern Italy, mainly in Campania and a single population in Calabria [53, 54, 55, 56]. A large degree of variability and complexity is known in this group, with several infraspecific taxa described during the years for P. hirtiflora [5], and a recently described narrow-endemic to Turkey related to P. crystallina, i.e. P. habilii Yıldırım, Şenol & Pirhan [57], not included in our analysis. Given their unique basic chromosome number (x = 14) [5], it has been hypothesised that P. crystallina/hirtiflora may be the result of an ancient hybrid event involving a species similar to P. lusitanica (x = 6) and a species similar to P. corsica (x = 8). This hypothesis seems partly supported by our investigation, given the relative closeness of the P. crystallina/hirtiflora complex to P. lusitanica. Our data also confirm that P. crystallina and P. hirtiflora are separate species [7, 14].
It is interesting to note that there is a remarkable ITST molecular variation within P. hirtiflora. In particular, the accessions from Calabria (southern Italy) (P21-P22, Fig 2 and Table 1) fall in a clade distinct from P. hirtiflora (and P. lavalvae) from Campania (P18-P20 and P25-P26, respectively, Fig 2 and Table 1), as does also the alien population from south-eastern France, Val Roya (P24, Fig 2 and Table 1) [54]. This likely attests (a) for a Balkan origin of the population from Calabria, (b) for a Balkan (or Calabrian) artificial origin for the alien population occurring in south-eastern France, known since the 2000s [54]. It can be speculated that the newly found alien populations of P. hirtiflora in the Czech Republic [58] and Switzerland [56] also derive from commercialized Balkan populations.
Pinguicula lavalvae was recently described as a narrow endemic to Sabato Valley, Mts. Picentini (Campania, southern Italy) [23]. The authors compared it with P. hirtiflora from different portions of its range, distinguishing P. lavalvae for several phenotypic features of the corolla and calyx. Despite this, the latter species showed a complete sequence identity with the accessions of topotypical P. hirtiflora from Campania [59]. The ITS sequence, along with the geographic vicinity of the two taxa, points to an assignment of P. lavalvae to an intraspecific rank within P. hirtiflora. Indeed, Fleischmann [56] considered P. lavalvae as simply one of the many white-flowered variants (P. hirtiflora f. pallida) of P. hirtiflora, as those that are found in the Balkans and elsewhere in Italy [60]. Nevertheless, the ITST variation in all accessions from Campania compared to the Balkans and an ecological niche shift of these populations (M. Innangi et al. in preparation) highlights the necessity of more investigation before establishing taxonomical ranks.
Subclade E1a: the clade comprising most Apennine endemics
A single clade unites four of the Pinguicula sect. Pinguicula species endemic to the Apennines: P. apuana, P. christinae, P. fiorii, and P. vallis-regiae. Only one GenBank accession of P. fiorii is falling outside this clade (Fig 3), but it must be noted that this accession was obtained from seeds [14], and may well represent a misidentification or confusion of materials. This clade includes also another Italian endemic species, the tetraploid P. mariae. On a slightly different branch within the subclade, there are two other octoploid species, P. dertosensis (Cañig.) Mateo & M.B.Crespo, endemic to south-eastern Spain [61, 62] and P. macroceras Pall. Ex Link, endemic to northern America and Japan [7]. The four species endemic to Apennines have very similar sequences, which are identical in some case (i.e. P. apuana, P. christinae P7 and P9, and P. fiorii P15-P17), clearly pointing out to a common origin. However, given the clear qualitative and quantitative combination of character-states that has been used to distinguish these taxa, coupled with allopatry [20, 21, 22], we deem reasonable to maintain them at species level. As a matter of fact, the discriminating resolution of ITS may not be enough within such level of variability. Thus, further intrapopulation approaches could be necessary. The collocation in this clade of P. vallis-regiae, morphologically very similar to P. poldinii [20], was quite unexpected. Indeed, the latter species represents a well distinct lineage in the tree (see further in the text).
Both P. christinae and P. fiorii, show a certain morphological affinity with P. balcanica (endemic to Balkan Peninsula) (see [22, 18], respectively). According to our results, such relationships can be excluded, given the position of P. balcanica in a completely different clade (E4, Fig 3), as sister to the heterophyllous (i.e. bearing spring and summer rosettes significantly differing in shape and dimensions) P. vallisneriifolia, a peculiar Spanish endemic [61, 62]. Finally, P. apuana was compared by Ansaldi & Casper [21] with P. leptoceras Rchb., endemic to Alps [7] and with the circumboreal P. vulgaris [7]. The relationship with these species can also be excluded, given that both P. leptoceras and P. vulgaris fall in a different clade (E1b, Fig 3).
Subclade E1b: the clade of P. vulgaris and its allies
Albeit this clade is weakly supported and highly polytomic (Fig 3), several phylogenetic inferences can be provided. Pinguicula vulgaris, a widespread Circumboreal octoploid species, forms a clade together with similar taxa such as P. bohemica Krajina, a tetraploid/octoploid endemic to the Czech Republic [5, 63], the heterophyllous tetraploid P. reichenbachiana (endemic to the Maritime Alps), P. longifolia subsp. caussensis Casper (endemic to southern France) and the tetraploid Alpine endemic P. leptoceras [7], provisionally including the plants recognised as “P. arvetii” in SW Alps [64]. According to Conti & Peruzzi [20], P. vulgaris segregated into geographical races at its range borders. In the case of three of them, in central Italy, taxonomic recognition at subspecies level was proposed: P. vulgaris subsp. anzalonei, P. vulgaris subsp. ernica and P. vulgaris subsp. vestina. It is interesting to note that the three taxa share identical sequences, but collectively differ from other P. vulgaris accessions for two SNP’s (Single Nucleotide Polymorphism). This likely attests for their common origin, but given their clear diagnosability and allopatry, also in this case we deem the original subspecific rank as appropriate.
Concerning the accession of P. cf. apuana from Bendola (SE France), our results clearly highlight for a significant molecular differentiation of this population, within the clade including P. vulgaris and its allies. The taxonomic position of this population needs further investigation. As concerns P. cf. christinae from Val D’Aveto (Liguria), also falling in the same clade as P. vulgaris accessions, morphological evidence was gathered to support its difference from both P. christinae and P. vulgaris. For further detail, see floral morphometric analysis in S1 File. The Pinguicula population occurring in Val d’Aveto (Liguria), at first sight, shows morphological affinity with P. christinae, but also some character-states intermediate or similar to those of P. vulgaris s.l. Our molecular results highlight a phylogenetic affinity with the latter species, but also a molecular differentiation of this population in the ITST sequences (three SNP’s). In addition, our morphometric study of floral features clearly attests for a distinctive combination of character-states in these plants (S1 File). Accordingly, and given the allopatry of this population from both P. vulgaris s.l. and P. christinae, we describe here this population as a new narrow endemic species:
Pinguicula lattanziae Peruzzi & Gestri sp. nov. (urn:lsid:ipni.org:names:XXXXXXXX-X)
Type: Liguria, N Apennine: Monte Aiona, 1600–1700 m a.s.l., 21 June 2014, G. Gestri et C. Gavazzi s.n. (holotype, PI).
The new taxon is close to P. christinae and P. vulgaris, but distinct for its narrower corolla upper lobes (2.5 ± 0.76 mm, not 4.72 ± 0.81 mm and 3.44 ± 0.69 mm, respectively) and narrower corolla median lobe (2.08 ± 0.42 mm, not 3.31 ± 0.67 mm and 3.83 ± 0.65 mm, respectively). It shares with P. vulgaris the corolla opening angle (≤ 90°) and the spur length (5–7 mm). This new species is dedicated to Edda Lattanzi (Rome), expert in Italian flora, in occasion of her 85th birthday.
Subclade E2: the status of Pinguicula ser. Prealpicae
Casper in Ansaldi & Casper [21] formally described Pinguicula ser. Prealpicae, to accommodate P. poldinii (type species), P. mariae and–putatively–P. vallis-regiae. The shared character-states used to justify this proposal were: homophyllous rosettes (i.e. spring and summer rosettes not significantly differing in shape and dimensions), widely open corollas with long spur and tetraploid status. The same authors explicitly exclude P. reichenbachiana from this series for being heterophyllous, regardless of other morphological similarities. According to our phylogenetic results, P. mariae and P. vallis-regiae represent two distinct lineages, placed in a large clade where also P. apuana, P. christinae, and P. fiorii occur (subclade E1a, Fig 3). On the contrary, the type species P. poldinii lies in a very distinct, albeit unresolved, lineage (subclade E2, Fig 3), to which eventually should be restricted the application of the ser. Prealpicae. However, this is not justified at all by morphology, and this highlights that certain characters in Pinguicula (i.e. homophyllous vs. heterophyllous rosettes, chromosome number) are useful taxonomic markers at species level, but cannot be safely used per se to establish systematic/evolutionary relationships.
Subclade E3: the phylogenetic placement of P. sehuensis and allied species, with some remarks on P. longifolia s.l.
The discovery of a new Pinguicula in Sardinia was defined as “the most important finding of a butterwort during the past 50 years in Europe” (J.S. Casper, in litt.). Pinguicula sehunensis was recently described by Bacchetta et al. [1] as morphologically close to P. dertosensis, P. nevadensis (H.Lindb.) Casper–both endemic to Spain [61, 62]–and the western European P. grandiflora Lam. [7]. According to our results, P. sehuensis, endemic to Sardinia, shares instead a common origin with P. corsica, endemic to Corsica (Fig 3). This phylogenetic structure parallels the biogeographic closeness between the two species, which also share a diploid status. Whereas P. dertosensis is placed in a completely different subclade (E2a, Fig 3), P. nevadensis and P. grandiflora belong to the same clade including also P. corsica+P. sehuensis, together with P. mundi Blanca, Jamilena, Ruiz Rejón & Reg.Zamora, also endemic to south-eastern Spain [61, 62]. The position of P. mundi as sister species to P. corsica and P. sehuensis is biogeographically significant, as until 25 Ma, in the late Oligocene, Sardinia and Corsica started to drift from modern Provence and reached their current geographical position in the middle Miocene (ca. 15 Ma) [64]. The ploidy level and ITS sequences of both P. corsica and P. sehuensis suggest that both species derived from a common diploid ancestor in the geologically and ecologically stable environments of the Hercynian massif of Corsica and Sardinia sometime between 25 and 15 Ma. Accordingly, and following the criteria proposed by Siljak-Yakovlev & Peruzzi [65], it is possible to hypothesize for P. corsica and P. sehuensis the status of patro-schizoendemic taxa.
Pinguicula grandiflora s.l., P. nevadensis and P. longifolia subsp. longifolia fall within the same subclade, and they are tetraploid. As argued for P. grandiflora, a species with Lusitanian distribution, its ploidy level and current distribution has been shaped by palaeoclimatic Quaternary events [66]. Our results further suggest that Quaternary glaciations played a prominent role in the evolution of polyploid butterworts currently scattered and isolated in the mountains.
Finally, some remarks can be done for P. longifolia s.l. The three different subspecies of P. longifolia are clearly paraphyletic, as already demonstrated by Cieslak et al. [8]. While P. longifolia subsp. reichenbachiana, endemic to few locations on the Maritime Alps in France and Italy, was already considered as a distinct species by several authors [1, 20, 21, 22], this is not the case for P. longifolia subsp. caussensis. The latter taxon, endemic to Les Causses in southern France, is clearly not related to P. longifolia s.str. and it has several quali-quantitative features that distinguish it as much as it happens for P. reichenbachiana [67, 68]. Degtjareva et al. [14] already pointed out the necessity of redefining the taxonomic state of P. longifolia subspecies. Thus, we deem appropriate to change its taxonomic status to species level as P. caussensis (Casper) Innangi, De Castro & Peruzzi stat. nov. (Bas.: P. longifolia subsp. caussensis Casper, Biblioth. Bot. 31(127–128): 154. 1966).
Conclusions
We can summarize our results as follows:
We identified a basal polytomy defined by five clades in Pinguicula. All of the Italian endemic taxa (with the exception of P. lavalvae) fall within the large clade E, which corresponds to P. sect. Pinguicula (type species: P. vulgaris).
Among Italian endemics, P. poldinii represents the most distinct lineage. Other species, such as P. apuana, P. christinae, P. fiorii, P. mariae, and P. vallis-regiae show strong molecular similarities and form a single subclade, although their taxonomic rank can be retained. The subspecies of P. vulgaris endemic to Italy (i.e. P. vulgaris subsp. anzalonei, P. vulgaris subsp. ernica, and P. vulgaris subsp. vestina) all share similar sequences, but are sufficiently different from subsp. vulgaris and are allopatric, thus can be considered as valid taxa. Pinguicula sehuensis is closely related to P. corsica, but it is clearly a good taxon that highlights interesting biogeographic patterns. Pinguicula lavalvae shares sequences with P. hirtiflora from Campania and could be potentially considered a subspecies of the latter, but the whole group of P. hirtiflora needs further investigation before a final taxonomic setting can be proposed. Finally, this study contributed to the identification of a new narrow Italian endemic systematic unit in Clade E, i.e. P. lattanziae sp. nov., a still undescribed species from SE France, and the change in taxonomical status from P. longifolia subsp. caussensis to P. caussensis.
In conclusion, our research allowed broadening the knowledge of taxonomic and evolutionary trends in the whole genus Pinguicula, with a special focus on the Italian endemics. The genus Pinguicula is complex and evolutionarily interesting, thus further research, possibly uniting different disciplines (e.g. population genetics, morphometrics, ecology and karyology), is still needed to clarify the taxonomic value of several taxa and implement the knowledge of evolutionary and biogeographic patterns in the whole genus.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bacchetta G, Cannas M, Peruzzi L. A new diploid butterwort species (Pinguicula, Lentibulariaceae) from Sardinia. Phytotaxa. 2014; 186: 279–286. [Google Scholar]
- 2.Müller KF, Borsch T, Legendre L, Porembski S, Barthlott W. Recent progress in understanding the evolution of carnivorous Lentibulariaceae (Lamiales). Pl Biol. 2006; 8: 748–757. [DOI] [PubMed] [Google Scholar]
- 3.Legendre L. The genus Pinguicula L. (Lentibulariaceae): an overview. Acta Bot Gallica. 2000; 147: 77–95. [Google Scholar]
- 4.Steiger J. Pinguicula (Lentibulariaceae): the Cool Climate Species of the Northern Hemisphere—Morphology, Biology, Cultivation. In: Schlauer J, Meyers-Rice B, editors. Second Conference of the International Carnivorous Plant Society; 1998 May 30-Jun 1; Bonn, Germany. P. 12–13.
- 5.Shuka L, Xhulaj M, Kashta L, Casper SJ. The genus Pinguicula (Lentibulariaceae) in Albania—a critical review. Wulfenia. 2007; 14: 15–65. [Google Scholar]
- 6.Casper SJ, Stimper R. Chromosome numbers in Pinguicula (Lentibulariaceae): survey, atlas, and taxonomic conclusions. Plant Syst Evol. 2009; 277: 21–60. [Google Scholar]
- 7.Casper SJ. Monographie der Gattung Pinguicula L. Biblioth Bot. 1966; 127: 1–209. [Google Scholar]
- 8.Cieslak T, Polepalli JS, White A, Muller K, Borsch T, Barthlott W, et al. Phylogenetic analysis of Pinguicula (Lentibulariaceae): chloroplast DNA sequences and morphology support several geographically distinct radiations. Amer J Bot. 2005; 92: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 9.Jobson RW, Playford J, Cameron KM, Albert VA. Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. Syst Bot. 2003; 28: 157–171. [Google Scholar]
- 10.Müller KF, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W. Evolution of carnivory in Lentibulariaceae and Lamiales. Pl Biol. 2004; 6: 477–490. [DOI] [PubMed] [Google Scholar]
- 11.Veleba A, Bures P, Adamec L, Smarda P, Lipnerova I, Horova L. Genome size and genomic GC content evolution in the miniature genome-sized family Lentibulariaceae. New Phytol. 2014; 203: 22–28. 10.1111/nph.12790 [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann A, Schäferhoff B, Heubl G, Rivadavia F, Barthlott W, Müller KF. Phylogenetics and character evolution in the carnivorous plant genus Genlisea A.St.-Hil. (Lentibulariaceae). Molec Phylogen Evol. 2010; 56: 768–783. [DOI] [PubMed] [Google Scholar]
- 13.Müller KF, Borsch T. Phylogenetics of Utricularia (Lentibulariaceae) and molecular evolution of the trnK in a lineage with high substitutional rates. Plant Syst Evol. 2005; 250: 39–67. [Google Scholar]
- 14.Degtjareva GV, Casper SJ, Hellwig FH, Schmidt AR, Steiger J, Sokoloff DD. 2006. Morphology and nrITS phylogeny of the genus Pinguicula L. (Lentibulariaceae), with special attention to embryo evolution. Pl Biol. 2006; 8: 778–790. [DOI] [PubMed] [Google Scholar]
- 15.Kondo KF, Shimai H. Phylogeny analysis of the northern Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) sequence. Acta Phytotax Geobot. 2006; 57: 155–164. [Google Scholar]
- 16.Shimai H, Kondo K. Phylogenetic analysis of Mexican and Central American Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) region. Chromosome Bot. 2007; 2: 67–77. [Google Scholar]
- 17.Shimai H, Masuda Y, Panfet Valdés CM, Kondo K. Phylogenetic analysis of Cuban Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) region. Chromosome Bot. 2007; 2: 151–158. [Google Scholar]
- 18.Tammaro F, Pace L. Il genere Pinguicula L. (Lentibulariaceae) in Italia Centrale ed istituzione di una nuova specie P. fiorii Tamm. et Pace. Inform Bot Ital. 1987; 19: 429–436. [Google Scholar]
- 19.Casper SJ, Steiger J. A new Pinguicula (Lentibulariaceae) from the pre-alpine region of Northern Italy (Friuli-Venezia Giulia): Pinguicula poldinii Steiger et Casper spec. nov. Wulfenia. 2001; 8: 27–37. [Google Scholar]
- 20.Conti F, Peruzzi L. Pinguicula (Lentibulariaceae) in central Italy: taxonomic study. Ann Bot Fenn. 2006; 43: 321–337. [Google Scholar]
- 21.Ansaldi M, Casper SJ. Pinguicula mariae Casper nova spec. and Pinguicula apuana Casper et Ansaldi nova spec.—A contribution to the occurrence of the genus Pinguicula L. (Lentibulariaceae) in the Apuan Alps (Italy). Wulfenia. 2009; 16: 1–31. [Google Scholar]
- 22.Peruzzi L, Gestri G. A new butterwort species (Pinguicula, Lentibulariaceae) from Northern Apennine (Italy). Pl Biosyst. 2013; 147: 692–703. [Google Scholar]
- 23.Innangi M, Izzo A. Pinguicula lavalvae (Lentibulariaceae), a new endemic butterwort from southern Italy diagnosed with the aid of geometric morphometrics. Pl Biosyst. 2015; 149: 990–999. [Google Scholar]
- 24.Peruzzi L, Conti F, Bartolucci F. An inventory of vascular plants endemic to Italy. Phytotaxa. 2014; 168: 1–75. [Google Scholar]
- 25.Peruzzi L, Domina G, Bartolucci F, Galasso G, Peccenini S, Raimondo FM, et al. An inventory of the names of vascular plants endemic to Italy, their loci classici and types. Phytotaxa. 2015; 196: 1–217. [Google Scholar]
- 26.Vijayan K, Zhang W-J, Tsou C-H. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Amer J Bot. 2009; 96: 1348–1360. [DOI] [PubMed] [Google Scholar]
- 27.Hodač L, Scheben AP, Hojsgaard D, Paun O, Hörandl E. ITS polymorphisms shed light on hybrid evolution in apomictic plants: a case study on the Ranunculus auricomus Complex. PLoS ONE. 2014; 9(7): e103003 10.1371/journal.pone.0103003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Castro O, Di Maio A, Lozada García JA, Piacenti D, Vázquez-Torres M, De Luca P. Plastid DNA sequencing and nuclear SNP genotyping help resolve the puzzle of central American Platanus. Ann Bot. 2013; 112: 589–602. 10.1093/aob/mct134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gargiulo R, Del Guacchio E, Caputo P. Phylogenetic reconstruction of Asperula sect. Cynanchicae (Rubiaceae) reveals a mosaic of evolutionary histories. Taxon. 2015; 64: 754–769. [Google Scholar]
- 30.Mogensen HL. The hows and whys of cytoplasmic inheritance in seed plants. Amer J Bot. 1998; 83: 383–404. [Google Scholar]
- 31.Hansen H, Escobar LK, Gilbert LE, Jansen RK. Paternal, maternal, and biparental inheritance of the chloroplast genome in Passiflora (Passifloraceae): implications for phylogenetic studies. Amer J Bot. 2007; 94: 42–46. [DOI] [PubMed] [Google Scholar]
- 32.Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperms. Amer J Bot. 1988; 75: 1443–1458. [Google Scholar]
- 33.Birky CW Jr. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci USA. 1995; 92: 11331–11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greiner S, Sobanski J, Bock R. Why are most organelle genomes transmitted maternally? Bioessays. 2014; 37: 80–94. 10.1002/bies.201400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casper SJ, Stimper R. New and revised chromosome numbers in Pinguicula (Lentibulariaceae). Haussknechtia. 2006; 11: 3–8. [Google Scholar]
- 36.Marhold K, Kučera J. IAPT/IOPB chromosome data 21. Taxon. 2016. 65 (3): 673–676. [Google Scholar]
- 37.Doulat E. Recherches caryologiques sur quelques Pinguicula. Compt Rend Hebd Séances Acad Sci Ser D. 1947; 225: 354–356. [Google Scholar]
- 38.Caparelli KF, Conti F, Peruzzi L. Reports 1677–1678. In: Kamari G, Blanché C, Garbari F, editors. Mediterranean chromosome number reports 18. Flora Medit. 2008; 18: 594–595.
- 39.Vargas P, Carrio E, Guzman B, Amat E, Guemes J. A geographical pattern of Antirrhinum (Scrophulariaceae) speciation since the Pliocene based on plastid and nuclear DNA polymorphisms. J Biogeogr. 2009; 36: 1297–1312. [Google Scholar]
- 40.Gutierrez R Jr, Freeman CE. A molecular phylogeny of the family Martyniaceae (Order Lamiales) using the ITS region of nrDNA. Unpublished 2003; reference from GenBank database.
- 41.Di Maio A, De Castro O. SSR-patchwork: an optimized protocol to obtain a rapid and inexpensive SSR library using first-generation sequencing technology. Appl Pl Sci. 2013; 1: 1200158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp. 1999; 41: 95–98. Available at: http://www.mbio.ncsu.edu/bioedit/bioedit.html. Accessed October 2015. [Google Scholar]
- 44.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001; 17: 754–755. Available at: http://mrbayes.sourceforge.net. Accessed October 2015. [DOI] [PubMed] [Google Scholar]
- 45.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19: 1572–1574. Available at: http://mrbayes.sourceforge.net. Accessed October 2015. [DOI] [PubMed] [Google Scholar]
- 46.Guindon S; Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol. 2003; 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 47.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012; 9: 772 Available at: https://github.com/ddarriba/jmodeltest2. Accessed October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974; 19: 716–723. [Google Scholar]
- 49.Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999; 15: 415–428. [DOI] [PubMed] [Google Scholar]
- 50.Nixon KC. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics. 1999; 15: 407–406. [DOI] [PubMed] [Google Scholar]
- 51.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985; 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 52.Heslop-Harrison Y. Pinguicula L. J Ecol. 2004; 92: 1071–1118. [Google Scholar]
- 53.Mikeladse T. Der crystallina-hirtiflora-Sippenkomplex in der Gattung Pinguicula, mit besonderer Berücksichtigung von P. crystallina. Jena: Friedrich-Schiller-Universität; 1996. [Google Scholar]
- 54.Peruzzi L, Passalacqua NG, Cesca G. Pinguicula crystallina Sibth. et Smith subsp. hirtiflora (Ten.) Strid (Lentibulariaceae) in Calabria (Southern Italy). Cytotaxonomical study and ex situ conservation in the Botanic Garden of Calabria University. Carniv Pl Newsl. 2004; 33: 68–74. [Google Scholar]
- 55.Sbragia P, Sibilio G, Innangi M, Di Febbraro M, Trifuoggi M, Guida M. et al. Rare species habitat suitability assessment and reliability evaluation of an expert-based model: a case study of the insectivorous plant Pinguicula crystallina Sibth. & Smith subsp. hirtiflora (Ten.) Strid (Lentibulariaceae). Pl. Biosyst. 2014 [Google Scholar]
- 56.Fleischmann A. The intricate Pinguicula crystallina/hirtiflora-complex. Carniv Pl Newslett. 2015; 44: 48–61. [Google Scholar]
- 57.Yıldırım H, Şenol SG, Pirrhan AFP. Pinguicula habilii (Lentibulariaceae), a new carnivorous species from South-West Anatolia, Turkey. Phytotaxa. 2012; 64: 46–58. [Google Scholar]
- 58.Pyšek P, Danihelka J, Sádlo J, Chrtek J, Chytrý M, Jarošík V, et al. Catalogue of alien plants of the Czech Republic (2nd edition): checklist update, taxonomic diversity and invasion patterns. Preslia. 2012; 84: 155–255. [Google Scholar]
- 59.Peruzzi L. History of the name Pinguicula hirtiflora Ten. (Lentibulariaceae), or on the uncertainties of Michele Tenore. Carniv Pl Newsl. 2006; 35: 89–90. [Google Scholar]
- 60.Innangi M, Izzo A, La Valva V. Revisione dello status IUCN per alcuni taxa inclusi nella Lista Rossa della Regione Campania. Delpinoa 2007; 49: 77–88. [Google Scholar]
- 61.Blanca G, Ruíz-Rejon M, Zamora R. Taxonomic revision of the genus Pinguicula L. in the Iberian peninsula. Folia Geobot. 1999; 34: 337–361. [Google Scholar]
- 62.Blanca G. Pinguicula L In: Paiva J, Sales F, Hedge IC, Aedo C, Aldasoro JJ, Castroviejo S, Herrero A, Velayos M, editors. Flora Iberica 14. Madrid: Real Jardín Botanico, CSIC; 2001. p. 81–96. [Google Scholar]
- 63.Studnička M. Tučnice Česká. Studie kriticky ohroženého druhu Pinguicula bohemica se zřetelem na možnost jeho záchrany Botanická Zahrada Liberec; 2013. [Google Scholar]
- 64.Thompson JD. Plant evolution in the Mediterranean. Oxford: Oxford University Press; 2005. [Google Scholar]
- 65.Siljak-Yakovlev S, Peruzzi L. Cytogenetic characterization of endemics: past and future. Pl Biosyst. 2012; 146: 694–702. [Google Scholar]
- 66.Beatty GE, Provan J. Phylogeographical analysis of two cold-tolerant plants with disjunct Lusitanian distributions does not support in situ survival during the last glaciation. J Biogeogr. 2014; 41: 2185–2193. [Google Scholar]
- 67.Schmidt AR. Sequenzanalysen an Europäischen Pinguicula-Arten unter Verwendung der internal transcribed spacer (ITS) Region der ribosomalen Kern-DNA (dissertation). Jena: Friedrich-Schiller-Universität; 1998.
- 68.Degtjareva G, Casper J, Hellwig F, Sokoloff D. Seed morphology in the genus Pinguicula (Lentibulariaceae) and its relation to taxonomy and phylogeny. Bot Jahrb Syst. 2004; 125: 431–452. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.