Abstract
Introduction
The objective of this study was to assess the safety and feasibility of in-bed cycling started within the first 4 days of mechanical ventilation (MV) to inform a future randomized clinical trial.
Methods
We conducted a 33-patient prospective cohort study in a 21-bed adult academic medical-surgical intensive care unit (ICU) in Hamilton, ON, Canada. We included adult patients (≥ 18 years) receiving MV who walked independently pre-ICU. Our intervention was 30 minutes of in-bed supine cycling 6 days/week in the ICU. Our primary outcome was Safety (termination), measured as events prompting cycling termination; secondary Safety (disconnection or dislodgement) outcomes included catheter/tube dislodgements. Feasibility was measured as consent rate and fidelity to intervention. For our primary outcome, we calculated the binary proportion and 95% confidence interval (CI).
Results
From 10/2013-8/2014, we obtained consent from 34 of 37 patients approached (91.9%), 33 of whom received in-bed cycling. Of those who cycled, 16(48.4%) were female, the mean (SD) age was 65.8(12.2) years, and APACHE II score was 24.3(6.7); 29(87.9%) had medical admitting diagnoses. Cycling termination was infrequent (2.0%, 95% CI: 0.8%-4.9%) and no device dislodgements occurred. Cycling began a median [IQR] of 3 [2, 4] days after ICU admission; patients received 5 [3, 8] cycling sessions with a median duration of 30.7 [21.6, 30.8] minutes per session. During 205 total cycling sessions, patients were receiving invasive MV (150 [73.1%]), vasopressors (6 [2.9%]), sedative or analgesic infusions (77 [37.6%]) and dialysis (4 [2.0%]).
Conclusions
Early cycling within the first 4 days of MV among hemodynamically stable patients is safe and feasible. Research to evaluate the effect of early cycling on patient function is warranted.
Trial Registration
Clinicaltrials.gov: NCT01885442
Introduction
Functional disability can last for many years in critical illness survivors [1,2]. Due to an aging population, and increasing survival from critical illness [3,4], the burden of physical and cognitive disability among patients discharged from the intensive care unit (ICU) is also increasing [5]. A systematic review of 14 randomized clinical trials (RCTs) identified that exercise-based physical therapy (PT) interventions started in the ICU were most effective to improve physical function compared to other strategies such as nutrition or different modes of mechanical ventilation (MV) [6]. This improved function following critical illness may be due to addressing the early and rapid reduction in muscle size and strength that occur within the first 10 days of a patient’s ICU stay [7,8]. Thus, interventions to prevent or reduce muscle size and minimize strength losses within this early time period may help to improve long-term outcomes in ICU survivors.
Rehabilitation interventions started very early in a patient’s ICU stay can improve function at hospital discharge. In a 104-patient RCT, those who received occupational (OT) and PT interventions in the ICU within 1.5 days of starting MV were more likely to be functionally independent at hospital discharge than those started at 7.4 days [9]. Here, the main difference was receipt of 20 minutes of therapy during MV by the intervention group versus no therapy during MV by the control group. However, common ICU interventions like MV can pose barriers to rehabilitation. For example, a recent prospective cohort study reported that the presence of an oral endotracheal tube (ETT) was an important barrier to receipt of mobilization within the first 14 days of MV [10].
In-bed cycling (“cycling”) is a promising early intervention for critically ill patients, with evidence supporting its use later in a patient’s ICU stay. In a 90-patient RCT, those receiving cycling started 14 days after ICU admission versus usual care had better 6-minute walk (6 MWT) distances, greater leg strength, and better Short Form 36 (SF-36) physical function scores at hospital discharge [11]. Commercially-available devices can provide 3 possible activity modes: passive (i.e., fully motorized, no patient initiation), active-assisted (i.e., partially initiated by the patient), or active (i.e., fully initiated by the patient). Cycling can enhance rehabilitation in critically ill patients[12] by providing low-intensity movement, allowing patients’ spontaneous participation in activity, and facilitating rest breaks in severely deconditioned patients.
Cycling started earlier in a patient’s ICU stay may further improve patient outcomes. However, evidence for early cycling is limited to observations of single sessions [13], cycling incorporated in a multi-modal rehabilitation strategy [14,15], a case-control study of cycling with functional electrical stimulation[16], or retrospective review of cycling in routine PT care [17]. In preparation for a larger trial of this intervention, the objective of this study was to evaluate the safety and feasibility of early leg cycling in critically ill patients.
Materials and Methods
We enrolled adult patients (>18 years) from a 21-bed academic medical-surgical ICU in Hamilton, ON, Canada. Immediately upon ICU admission, a research coordinator screened for eligible patients: MV for 0 to ≤4 days, ICU LOS for 0 to ≤7 days, and who were able to ambulate with or without a gait aid before hospitalization. Pre-enrolment, we screened patients for the duration of their eligibility as long as they met the MV and LOS criteria. Table 1 outlines study exclusion criteria and cycling exemptions developed from a systematic review of early mobility activities [18], clinical trials published at the time of study design [9,11,19], and clinical research team consensus. Written informed consent was obtained by a research coordinator from all participants (or their proxy) included in this open-label study.
Table 1. Study Inclusion, Exclusion, and Temporary exemption criteria.
| Inclusion Criteria |
| Mechanically ventilated for 0 to ≤4 days |
| ICU length of stay for 0 to ≤7 days |
| Able to ambulate with or without a gait aid before hospitalization |
| Exclusion Criteria |
| Unable to follow commands in English at baseline |
| Acute condition impairing patient’s ability to cycle (e.g., leg fracture) |
| Neuromuscular weakness affecting the legs (e.g., stroke, Guillain Barre syndrome) |
| Temporary pacemaker |
| Expected hospital mortality >90% |
| Body habitus unable to fit the bike |
| Pregnancy |
| Palliative goals of care |
| Cycling exemptions precluding cycling within the first 4 days of MV |
| Temporary cycling exemptions |
| Respiratory |
| Persistent O2 saturation <88% |
| Cardiovascular |
| Active myocardial ischemia (MI) |
| Unstable or uncontrolled arrhythmia |
| Any increase in vasoactive infusions within the last 4 hours |
| Mean arterial pressure (MAP) <60 or >110 mmHg within the last 2 hours |
| Heart rate (HR) <40 or >140 beats per minute (BPM) within the last 2 hours |
| Receipt of neuromuscular blocking agents within the last 4 hours |
| Uncontrolled pain |
| Severe agitation (Richmond Agitation and Sedation Scale, RASS[48]) >2) within the last 2 hours |
| Change in goals to palliative care |
| Presence of a femoral arterial or venous catheter* |
| Team perception that cycling was not appropriate, despite absence of above exemption criteria |
This table outlines trial inclusion, exclusion, and temporary cycling exemption criteria. We excluded patients if they had persistent temporary cycling exemption criteria within the first 4 days of mechanical ventilation. Once enrolled, we reviewed patients’ clinical status for temporary cycling exemption criteria daily.
*We removed this temporary exemption following published evidence for the safety of rehabilitation activities for femoral catheter in situ.
Intervention
We prescribed 30 minutes of leg cycling with an additional 1 minute cool down, 6 days/week, for the duration of the patient’s ICU stay to a maximum of 28 days. This intervention was in addition to routine PT. If a patient was re-admitted to ICU during the index hospitalization, we re-started cycling. We reviewed patients daily for temporary cycling exemptions (Table 1).
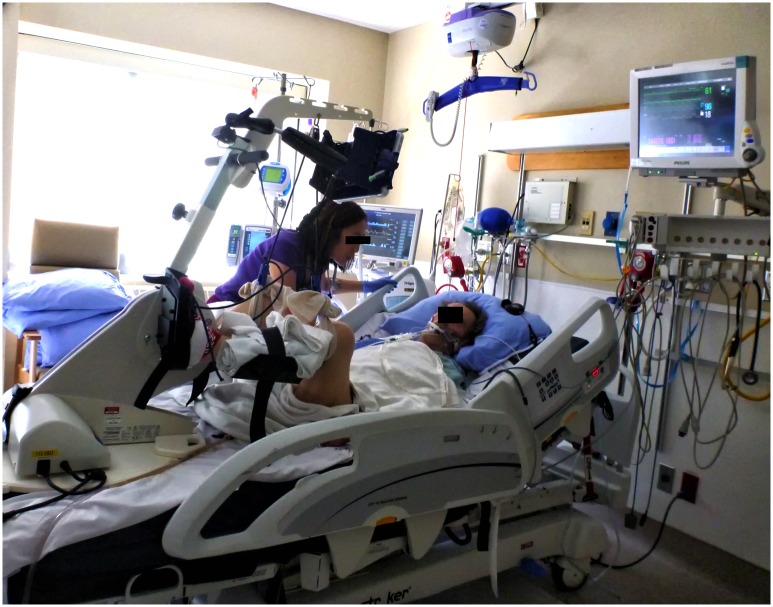
ICU PTs led all cycling sessions. We used a specialized cycle ergometer purchased by our hospital (RT300 supine cycle, Restorative Therapies, Baltimore, MD; Fig 1)[11]. All PTs received a 1-day in-service and had over 6 months of clinical experience with the cycle before starting the study. The participants started with passive cycling at a rate of 5 revolutions/minute (RPM) based on our clinical experience where patients actively cycled at low pedal cadence. If patients initiated active cycling, the PT promoted active participation. The therapists encouraged as much active cycling as possible, and low resistance (0.5 NM) was used during active cycling. Due to a variable level of consciousness throughout their stay, we allowed patients to cycle at a self-selected rate. We chose not to add resistance during the cycling intervention because we would not be able to discern if early cycling at a patient’s self-selected pace or increased resistance influenced outcomes in a future RCT. If the patient stopped cycling actively, the ergometer reverted to passive cycling.
Fig 1. Example of in-bed cycling.
This figure demonstrates a patient in the ICU receiving in-bed cycling and mechanical ventilation. An ICU physiotherapist supervises the in-bed cycling session.
We measured vital signs pre-, during (at 5, 10, 20, and 30 minutes), and post- cycling. During cycling, if the patient had 2 consecutive readings of mean arterial pressure (MAP) <60 or >110 mmHg, heart rate (HR) < 40 or >140 beats per minute (BPM), or SpO2 <88%, despite adjustments to FiO2, we advised PTs to use clinical judgment to stop a cycling session, according to each patient’s individual clinical circumstances in consultation with the ICU team.
We collected the following data: APACHE II score,[20] Charlson Comorbidity Index,[21] and Functional Comorbidity Index [22]. We collected the Katz Activity of Daily Living (ADL) Scale,[23] and Functional Status Score for ICU (FSS-ICU) [24] at study entry from interviews with the proxy or patient. Daily ICU data included Multiple Organ Dysfunction Score (MODS),[25] and exposures including MV, receipt of neuromuscular blockers, vasopressors or inotropes; benzodiazepines, opioids, or propofol; and dialysis. We collected MV, drug exposures and receipt of dialysis as binary variables for each study day.
Primary Outcome and Sample Size Calculation
Our primary outcome was Safety (termination), defined as receipt of 30-minute leg cycling sessions without stopping due to 5 a-priori reasons: 1) unplanned extubation, 2) suspected new unstable or uncontrolled arrhythmia, 3) concern for myocardial infarction (MI), 4) ICU physician request to terminate session, and 5) PT terminated session due to physiologic concerns. We hypothesized that the observed Safety (termination) event rate would not differ, or would be better (i.e., lower) than other early rehabilitation studies (0 to 4%).[18] We estimated we needed 164 cycling sessions to ensure an observed Safety (termination) rate was within an upper 95% confidence interval (CI) of 3% from a point estimate of 4% (7 events) [26]. Assuming a median ICU length of stay of 7 days, 2 days to enroll patients from ICU admission, and 5 cycling sessions per patient, we enrolled 33 patients.
Secondary Outcomes
Safety (disconnection or dislodgement)
Inadvertent ventilator disconnection or device dislodgement (catheters: peripheral venous, arterial, central venous, pulmonary artery, or dialysis; tubes: orogastric, nasogastric, or percutaneous gastrostomy or jejunotomy).
Feasibility
Consent rate >70%[9], ability to provide cycling sessions, and ability to collect physical outcome measures at ICU awakening, ICU discharge, and hospital discharge. We recorded the duration of cycling by the patient and the total duration (including patient setup and equipment take-down) of all sessions. Other outcomes included PT assessment of muscle strength (Medical Research Council sum score (MRC-SS),[27,28], ICU-acquired weakness (MRC-SS <48)[29], hand grip[28,30], quadriceps strength with dynamometry[31], and function (FSS-ICU[24], Physical Function ICU Test-scored (PFIT-s)[32,33], 6MWT[34]). We also collected duration of MV, discharge location, ICU and hospital length of stay (LOS) and mortality.
Analysis
For binary variables, we calculated the binary proportion and 95% CI. For continuous variables, we calculated the mean and standard deviation (SD), or if non-normally distributed, the median and interquartile range [IQR]. We compared continuous variables using a two-sided paired or independent Student’s t-test, as appropriate. We used SAS version 9.2 (Cary, North Carolina) for all analyses and considered p-values ≤0.05 significant.
Ethical Approval
The Hamilton Integrated Research Ethics Board approved this study (13–173; Clinicaltrials.gov: NCT01885442).
We reported our study according to the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) Statement[35] and Template for Intervention Description and Replication (TIDieR)[36] checklist (S1 and S2 Tables).
Results
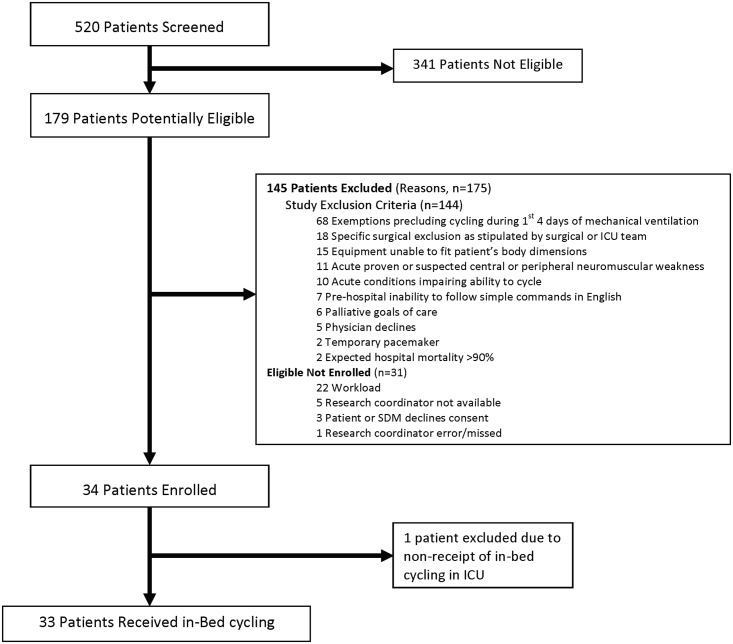
Between October 30, 2013 and August 18, 2014, we enrolled 34 patients (Fig 2). One patient did not receive any cycling during their ICU stay due to persistently high MAP and was excluded from further analysis. Of all patients, 11 (33.0%) received vasopressors or inotropes, 5 (15.1%) received neuromuscular blockers, and 7 (21.2%) received dialysis during their ICU stay. Table 2 outlines patient characteristics.
Fig 2. Patient flow diagram.
This figure outlines patient screening and enrollment in the TryCYCLE study. The 68 persisting temporary exemptions within the first 4 days of mechanical ventilation included: receipt of neuromuscular blocking agents (n = 19), increase in vasoactive infusions (n = 14), femoral arterial or venous catheter in situ (n = 13), active myocardial infarction of unstable/ uncontrolled arrhythmia (n = 8), severe agitation (n = 2), persistent SpO2 <88% (n = 2), mean arterial pressure <60 mmHg or >110 mmHg (n = 1), heart rate <40 or >140 beats per minute (n = 1), other concern (n = 8).
Table 2. Patient Demographics, Baseline Characteristics, and Outcomes.
| Characteristic | N = 33 Patients |
|---|---|
| Age in years, mean (SD) | 65.8 (12.2) |
| Females, n (%) | 16 (48.5) |
| Race, n (%) | |
| White | 29 (87.9) |
| Southeast Indian | 2 (6.1) |
| Black | 1 (3.0) |
| Asian | 1 (3.0) |
| Medical admission, n (%) | 29 (87.9) |
| APACHE II[20], mean (SD) | 24.3 (6.7) |
| Admission diagnosis, n (%) | |
| Respiratory | 19 (57.6) |
| Sepsis | 4 (12.1) |
| Gastrointestinal (non-surgical) | 2 (6.1) |
| Gastrointestinal (surgical) | 2 (6.1) |
| Cardiovascular/vascular | 2 (6.1) |
| Other surgical | 2 (6.1) |
| Renal | 1 (3.0) |
| Other medical | 1 (3.0) |
| Charlson Comorbidity Index[21], mean (SD) | 2.2 (2.0) |
| Functional Comorbidity Index[22], mean (SD) | 2.3 (1.4) |
| Pre-ICU Katz ADL score[23], mean (SD) | 5.5 (1.3) |
| Pre-ICU Functional Status Score for ICU[24], mean (SD) | 33.9 (3.2) |
| Location in hospital before ICU admission, n (%) | |
| Medical or surgical stepdown | 9 (27.3) |
| Other hospital | 8 (24.3) |
| Emergency department | 6 (18.2) |
| Hospital Ward | 6 (18.2) |
| Operating room/ post-operative recovery room | 4 (12.1) |
| Duration of mechanical ventilation (index admission), median [IQR], days | 8 [6, 14] |
| ICU LOS, median [IQR], days | 11 [7, 17] |
| ICU mortality, n (%) | 5 (15) |
| Hospital LOS, median [IQR], days | 31 [16, 42] |
| Hospital mortality, n (%) | 10 (30) |
| Hospital discharge disposition of 23 survivors, n (%) | |
| Home, independent | 7 (30) |
| Home, with home care | 6 (26) |
| Repatriated to another hospital | 4 (17) |
| Inpatient rehabilitation | 3 (13) |
| Assisted living facility | 1 (5) |
| Other | 2 (9) |
This table summarizes patient demographics, baseline characteristics, and patient outcomes. Abbreviations: SD = standard deviation; n = number; LOS = length of stay; APACHE II = Acute Physiology and Chronic Health Evaluation, an 13 item instrument with scores from 0 to 71, higher scores representing higher severity of illness[20]; Charlson Comorbidity Index includes 19 categories of comorbidity, with higher scores representing more comorbidity[21]; Functional Comorbidity Index includes 18 items associated with physical function, with higher scores representing higher comorbid illness[22]; ADL = activities of daily living; Katz score is a 6-item instrument assessing independence in bathing, dressing toileting, transferring, continence, and feeding, with higher scores representing more independence[23].
Safety
The Safety (termination) rate was 2% (95% CI (0.8% to 4.9%) in 205 cycling sessions (4 events: high MAP (n = 2), SpO2 <88% (n = 1), and physician request for termination due to concern for MI (n = 1; subsequent workup revealed no evidence of MI)). There were no unplanned extubations, or Safety (disconnection or dislodgement) events. Of 205 cycling sessions, provided by 5 different PTs, there were 56 respiratory or cardiovascular physiologic changes from baseline (Table 3). In most instances, PTs did not stop cycling early due to these transient changes. There was a statistically, but not clinically significant difference in pre- and post- cycling HR and MAP, and no differences in the remaining vital signs (BP, SpO2, FiO2; S3 Table).
Table 3. Characteristics of a-priori physiologic changes from baseline during in-bed cycling sessions.
| Physiologic changes during cycling sessions, n (%) | N = 56 |
|---|---|
| Respiratory, n (%) | 1(1.8) |
| Sustained O2 desaturation <88%, despite adjustments to FiO2 | 1(1.8) |
| Marked ventilator dysynchrony, despite adjustments | 0 |
| Respiratory distress leading to symptoms of marked dyspnea | 0 |
| Unplanned extubationa | 0 |
| Cardiovascular, n (%) | 55 (98.2) |
| High systolic BP: 20 mmHg more than highest baseline value | 29 (51.8) |
| MAP >110 mmHg (non-sustained) | 22 (39.3) |
| High diastolic BP: 20 mmHg more than highest baseline value | 8 (14.3) |
| Low HR: 20 bpm less than baseline value or 40 bpm (highest) | 4 (7.1) |
| Low systolic BP: 20 mmHg less than lowest baseline value | 4 (7.1) |
| High HR: 20 bpm more than highest baseline value or 140 bpm (lowest) | 3 (5.4) |
| Low diastolic BP: 20 mmHg less than lowest baseline value | 2 (3.6) |
| MAP <60 mmHg | 0 |
| Suspected new unstable/uncontrolled arrhythmiaa | 0 |
| Concern for myocardial ischemiaa | 0 |
Data in this table represent a-priori physiologic changes from baseline. Abbreviations: BP = blood pressure; MAP = mean arterial pressure; HR = heart rate; bpm = beats per minute.
aA-priori Physiologic event leading to immediate termination of in-bed cycling.
Feasibility
Our consent rate was 91.9% (34/37), and median [IQR] time from ICU admission to consent was 2 [1, 3] days. The median [IQR] time from ICU admission to first cycling was 3 [2, 4] days. Patients received a median [IQR] of 5 [3, 8] sessions, and the duration of cycling and total session (including set up and take down) was 30.2 [20.0, 30.7] and 43 [36, 49] minutes, respectively. Of 320 opportunities, 115 (35.9%) sessions were withheld (Table 4) and patients completed the full 30-minute protocol on 138 of 205 (67.3%) occasions. Table 4 outlines details for the 67 sessions stopped early.
Table 4. Summary of reasons for not cyclinga or cycling stopped early.
| Reasons for not cyclinga in 115 sessions | n (%) |
|---|---|
| Medical Conditions | N = 89 (77.3) |
| Neuromuscular blocker within last 4 hours | 37 (32.2) |
| Mean Arterial Pressure <60 or >110 mmHg within the last 2 hours | 22 (19.1) |
| Team perception that in-bed cycling is not appropriate despite absence of explicit reasons | 16 (13.9) |
| Any increase in vasopressor/ inotrope within last 4 hours | 5 (4.3) |
| Femoral arterial or venous catheter | 3 (2.6) |
| Heart Rate <40 or >140 bpm within the last 2 hours | 2 (1.7) |
| Severe agitation (RASS >2 [or equivalent]) within last 2 hours | 2 (1.7) |
| Change in goals to palliative care | 2 (1.7) |
| Active myocardial ischemia, or unstable/ uncontrolled arrhythmia | 1 (0.9) |
| Persistent SpO2 <88% within the last 2 hours | 0 |
| Uncontrolled pain | 0 |
| Other Reasons | N = 32 (27.8) |
| Patient refusedb | 27 (23.5) |
| Patient not available (patient out of the ICU)c | 3 (2.6) |
| Otherd | 2 (1.7) |
| Reasons for cycling stopped early in 67 sessions | N (%) |
| Patient request to stop due to fatigue | 48 (71.6) |
| Patient agitation | 5 (7.4) |
| A priori safety (termination) concerns | 4 (6.0) |
| Perceived patient discomfort | 3 (4.5) |
| Bowel movement during cycling | 2 (3.0) |
| Peripheral intravenous foot catheter interfering with cycling motion | 1 (1.5) |
| Cycle ergometer malfunction | 1 (1.5) |
This table summarizes reasons for not cycling that were recorded during daily review pre-cycling and reasons for stopping cycling early (i.e., before 30 minutes). Abbreviations: RASS = Richmond Agitation and Sedation Scale; bpm = beats per minute; ICU = intensive care unit.
aTotals sum greater than 115 because each session could have more than one reason for not cycling. Data are reasons as a proportion of 115 sessions.
bOf 27 sessions, 10 patients refused 1 or more cycling sessions, and 2 patients accounted for 16 (60%) of all refusals (10 and 6 refusals each). On 6 of these occasions, patients received alternate mobility activities (e.g., sitting at the edge of the bed, sitting in a chair) on the same day.
cPatients were not available due to procedures in the operating room (n = 2) or diagnostic imaging (n = 1).
dOther includes bike unable to fit on bed (n = 1), left foot intravenous catheter interfering with cycling (n = 1).
Cycling Session Characteristics
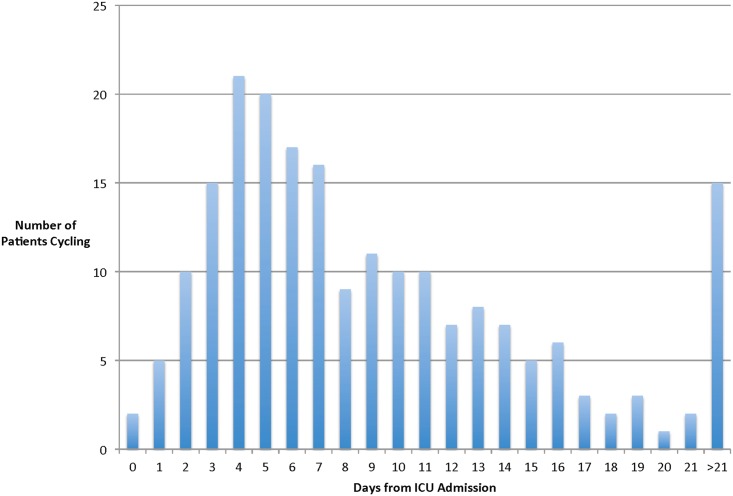
Of 205 cycling sessions, 150 (73.1%) occurred while patients received MV (via oral ETT (n = 144, 96.0%) or via tracheostomy (n = 6, 4.0%)), and the mean (SD) FiO2 was 38%(15). Over half (106 (51.7%) of all sessions occurred within the first 7 days of patients’ admission to the ICU (Fig 3). The mean (SD) MODS score [25] on cycling days was 3.4(2.9). Any infusions of benzodiazepines, opiates, propofol, or any bolus of benzodiazepine, opiate or propofol occurred during 40 (19.5%), 44 (21.5%), 38 (18.5%), and 77 (37.6%) of all sessions, respectively. The mean (SD) RASS score was -1.4 (1.6). Patients received vasopressors or inotropes during 6 (2.9%), and dialysis during 4 (2.0%) of all sessions (continuous renal replacement therapy, n = 3; hemodialysis, n = 1).
Fig 3. Histogram of cycling by day of ICU stay.
This figure outlines the number of patients biking by days since ICU admission. Of 205 in bed cycling sessions, over half (106 (52%)) occurred within the first 7 days of the patient’s ICU admission.
The median [IQR] distance cycled per session and per patient was 1.0 [0.9, 2.2] and 8.7 [5.0, 14.0] km, respectively. The maximum distance cycled per session, and per patient were 9.0 and 41.2 km, respectively. Therapists observed active cycling in 165 (80.5%) of all sessions. On days of in-bed cycling, the 3 most common additional PT interventions were passive range of motion (39 days, 19.0%), bed mobility (32, 15.6%), and airway clearance techniques (28, 13.7%). Sitting at the edge of the bed, standing, and walking occurred on 27 (13.2%), 24 (11.7%), and 7 (3.4%) of in-bed cycling days, respectively. On 86 (42%) days of in-bed cycling, no additional PT interventions occurred. Additional information on non-cycling physiotherapy interventions and cycling details per patient are available in S4 and S5 Tables.
Strength and Functional Outcomes
At ICU discharge, 7/28 (25.0%) of all survivors were walking, which improved to 18/23 (78.3%) by hospital discharge (Table 5). For 20 survivors with paired assessments, patients’ Katz ADL and FSS-ICU scores were significantly lower at hospital discharge than at baseline (Tables 2 and 5, p-value for difference, Katz = 0.004; FSS-ICU = 0.015). At hospital discharge, patients required assistance for at least 2 ADLs as well as for standing or walking.
Table 5. Patient strength and functional outcomes.
| ICU Awakening | ICU Discharge | Hospital Discharge | |
|---|---|---|---|
| N = 28a | N = 26b | N = 20c | |
| Muscle Strength | |||
| Medical Research Council (MRC) Sum Score | 47.9 (9.4) | 47.4 (12.9) | 54.1 (5.3) |
| Total score <48, n (%) | 10 (47.6) | 10 (47.6) | 4 (23.5) |
| Hand Grip Strength, median [IQR] kg | 8.8 [3.0 to 16.5] | 10.8 [3.8 to 18.3] | 16.3 [10.2 to 21.5] |
| Knee Extensor Strength (N) | 73.8 (79.1) | 69.9 (72.7) | 73.2 (85.4) |
| Function | |||
| Katz ADL score | 0.32 (0.94) | 0.73 (1.48) | 3.85 (2.30) |
| Functional Status Score for ICU | 15.0 (8.9) | 19.2 (10.7) | 28.7 (8.2) |
| Physical Function Test for ICU-scored | 4.6 (1.7) | 5.3 (2.1) | 7.2 (1.3) |
| 6 Minute Walk Test (metres) | - | 114 (-) | 343 (-) |
This table outlines the strength and function outcomes recorded at ICU awakening, ICU discharge, and hospital discharge. All values are mean (SD) unless otherwise specified. Abbreviations: IQR = interquartile range; ADL = activities of daily living; ICU = intensive care unit.
aSample size for assessments performed at ICU Awakening: MRC Sum Score and MRC total score <48, 21; Hand grip, 22; Knee extensor strength, 14; Katz ADL score, 28; Functional Status Score for ICU, 23; Physical Function Test for ICU, 26; 6 minute walk test, 0.
bSample size for assessments performed at ICU Discharge: MRC Sum Score and MRC total score <48, 21; Hand grip, 22; Knee extensor strength, 19; Katz ADL score, 26; Functional Status Score for ICU, 25; Physical Function Test for ICU, 26; 6 minute walk test, 1.
cSample size for assessments performed at Hospital Discharge: MRC Sum Score, MRC total score <48, and Hand grip, 17; Knee extensor strength, 14; Katz ADL score, 20; Functional Status Score for ICU and Physical Function Test for ICU, 20; 6 minute walk test, 1.
Discussion
Our results suggest that it is safe and feasible to enroll critically ill, hemodynamically stable MV patients in a rehabilitation study of early cycling. In this study of 33 MV patients, we began cycling within 3 days of ICU admission, session termination was infrequent, and device dislodgements did not occur. On average, patients received 5 cycling sessions of 30 minutes duration, cycled 1 km per session, and cycled a distance of 9 km in total in the ICU.
Our data add to a growing body of literature suggesting early cycling can occur safely with critically ill patients. A single, 20-minute passive cycling session started within the first 72 hours of MV documented no safety concerns while patients received low-dose vasoactive drug infusions, and no increase in cardiac output, or oxygen consumption.[13]. In a retrospective study of cycling incorporated into routine PT interventions in a medical ICU, cycling began within 4 days of MICU admission, and only 1 device dislodgement occurred out of 541 sessions (0.2% event rate).[17] In a case-control study of cycling and functional electrical stimulation initiated within the first 96 hours of MV, 1 transient desaturation occurred in 69 sessions.[13,16]
Due to the dynamic nature of critical illness, patients’ suitability for rehabilitation may vary daily. We designed our study to start cycling within the first 4 days of MV. Of 179 potentially eligible patients, 114 met some (or multiple) exclusion criteria. Of 144 exclusion reasons, almost half (68, 47%) occurred because patients had one or more persistent temporary exemptions precluding cycling within the first 4 days of MV (Fig 2). Most temporary exemptions reflected patients’ acuity: receipt of neuromuscular blocking agents (n = 19), increasing vasoactive medications (n = 14), or active myocardial ischemia/uncontrolled arrhythmia (n = 8). During our study, new studies supporting the safety of rehabilitation (including cycling) with femoral catheters were published[37,38]; we subsequently revised our protocol to remove this exemption. Future studies need to consider the possible impact of temporary exemptions from cycling in ICU patients and their impact on patient outcomes, and document protocol adherence, as in other critical care trials.
Prospective research in early cycling is feasible. We attained a high consent rate (92%), met our target sample size, delivered early cycling, and measured physical function in all survivors at hospital discharge. Previous early rehabilitation studies [9,11,14,39–41] had consent rates varying from 48% [42] to 89% [11], indicating differing receptivity of substitute decision makers to early rehabilitation research. We met our recruitment target, whereas some studies closed early due to slow accrual.[14,40,41] While another early rehabilitation intervention, NMES, had difficulty consistently achieving muscle contractions[40,43], all but 1 patient in this study successfully received cycling. However, of 31 patients eligible not enrolled, 22 (71%) were not enrolled because physiotherapists did not have capacity to manage multiple cycling trial patients concurrently (Fig 2). Investigators need to consider how to best engage front line ICU staff in delivering and/or outcome measure assessment to optimize timely accrual and cycling opportunities.
Further prospective research on the efficacy of early cycling in medical-surgical MV patients is needed. Cycling targets the legs, particularly hip flexors, which are most vulnerable to muscle atrophy and weakness during bed rest [44]. Two RCTs of cycling as part of a multimodal intervention showed no functional differences between intervention and control groups [14,15]. In these studies, cycling occurred later in the patients’ rehabilitation, and it was difficult to discern the unique contribution of cycling to patient outcomes[14,15]. In a retrospective cohort of cycling in a medical ICU, patients received 25 minutes of cycling in 2 of 4 total PT sessions during their stay, but functional status was not reported[17]. Cycling may offer a rehabilitation option for a broad range of ICU patients, particularly those who must be bedbound, have ~75° knee and ~80° available hip flexion[17], are not on active spinal precautions, and have no other orthopedic restrictions (e.g., no weight-bearing). However, further research in this area is needed.
Early mobility is recommended as a front-line non-pharmacological intervention to reduce the incidence and duration of delirium in critically ill patients.[45] However, some mobilization protocols require patients to be interactive [41,46], which may delay the time to start rehabilitation during the early critical time period for muscle size and strength losses. A multicenter prospective cohort study reported the presence of an oral ETT as one of the main barriers to mobilization in 192 MV patients.[10] Only 37% of all patients received any mobilization within the first 14 days of MV, and only in 16% of all potential occasions.[10] In contrast, patients in our cohort started cycling within a median of 3 days of MV, over half of all sessions occurred within the first 7 days of ICU admission (Fig 3), and 70% occurred while patients received MV via ETT.
Limitations and Strengths
We had no control group to determine if cycling improved patient outcomes compared to usual care. Experienced ICU PTs conducted all biking sessions and outcome measures. Compared to other ICU cycling studies,[13,47] few patients cycled while receiving vasopressors or inotropes in our cohort, reflecting the predominant respiratory conditions in our population. We did not systematically record patients’ delirium status or reasons why they refused cycling. We conducted this study in a single centre ICU with a highly collaborative PT department and strong interprofessional critical care research culture.
To our knowledge, this is the largest prospective cohort of early cycling sessions in MV, medical-surgical ICU patients. We included an a-priori sample size calculation focused on safety events, prospectively collected all data, and engaged clinical PTs to lead all cycling sessions. We had a high consent rate, achieved our sample size target, and patients received multiple cycling sessions. Our Safety (termination) event rate of 2% was similar to the RCT of cycling started 2 weeks after ICU admission, where 3.8% (16/425) of sessions terminated early.[11] While we originally excluded patients from cycling if they had femoral catheters in-situ, we revised our protocol during the study to reflect new evidence supporting the safety of mobility activities with femoral catheters.[37,38]
Conclusions
This study suggests that it is safe and feasible for hemodynamically stable MV patients to receive early cycling in the ICU and may inform future RCTs in this field.
Supporting Information
This diagram outlines the TryCYCLE study schema. Abbreviations: MV = mechanical ventilation; ICU = intensive care unit; PT = physiotherapy interventions.
(DOCX)
(DOCX)
(DOCX)
Values in this table represent vital sign recordings during in-bed cycling sessions. All values represent mean (SD). * = p<0.001 difference between pre- and post- cycling heart rate; ** p = 0.004 difference between pre- and post- cycling mean arterial pressure. aSample size for blood pressure measurements at 5, 10, 20, and 30 minutes: n = 201, n = 190, n = 178, and n = 146, respectively. Abbreviations: bpm = beats per minute.
(DOCX)
In this table, we outline additional therapeutic activities occurring on days of in-bed cycling.
(DOCX)
This table outlines the mean (SD) number of cycling sessions, cycling session duration, and distance per cycling session for all patients. Abbreviations: SD = standard deviation.
(DOCX)
(PDF)
(DOCX)
Acknowledgments
We are grateful to Diane Heels-Ansdell for assistance with statistical analysis, Professor Paul Stratford for statistical advice regarding sample size calculation, Lisa Buckingham for database development, Melissa Shears for assistance with data collection and data cleaning, assisting physiotherapists with in-bed cycling sessions, and figure preparation, Julie Reid for assistance with data collection, and Drs. Peter Dodek and Donald Griesdale for thoughtful manuscript suggestions.
This work was performed in the St. Joseph’s Healthcare Intensive Care Unit, Hamilton, ON. Preliminary data from this study were presented at the Society of Critical Care Medicine Congress, January 17–21, 2015, Phoenix, Arizona.
Guarantor
MEK had full access to all the data in the study and she takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects.
Data Availability
Ethics board approval for sharing patient data publicly was not obtained for this study. Data are from the TryCYCLE study whose authors may be contacted for access at khome@mcmaster.ca.
Funding Statement
This work was supported by: Canadian Institutes of Health Research (www.cihr-irsc.gc.ca), Institute of Musculoskeletal Health and Arthritis Operating grant – Priority Announcement: IMHA New investigators - Bridge Funding (131584) MEK DJC MSH KK MM JR AS; Canada Research Chairs MEK DJC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364: 1293–1304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304: 1787–1794. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Penuelas O, Abraira V, et al. (2013) Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 188: 220–230. 10.1164/rccm.201212-2169OC [DOI] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311: 1308–1316. 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
- 5.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A (2005) Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Critical Care Medicine 33: 574–579. [DOI] [PubMed] [Google Scholar]
- 6.Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA (2013) Interventions to improve the physical function of ICU survivors: a systematic review. Chest 144: 1469–1480. 10.1378/chest.13-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. (2013) Acute skeletal muscle wasting in critical illness. JAMA 310: 1591–1600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 8.Vivodtzev I, Devost A, Saey D, Villeneuve S, Bollard G, Gagnon P, et al. (2014) Severe and early quadriceps weakness in mechanically ventilated patients. Critical Care 18: 431 10.1186/cc13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373: 1874–1882. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TEAM Study Investigators, Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, et al. (2015) Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care 19: 81 10.1186/s13054-015-0765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. (2009) Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 37: 2499–2505. [DOI] [PubMed] [Google Scholar]
- 12.Needham DM, Truong AD, Fan E (2009) Technology to enhance physical rehabilitation of critically ill patients. Critical Care Medicine 37: S436–441. [DOI] [PubMed] [Google Scholar]
- 13.Camargo Pires-Neto R, Fogaca Kawaguchi YM, Sayuri Hirota A, Fu C, Tanaka C, Caruso P, et al. (2013) Very early passive cycling exercise in mechanically ventilated critically ill patients: physiological and safety aspects—a case series. PLoS One 8: e74182 10.1371/journal.pone.0074182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayambu G, Boots R, Paratz J (2015) Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med 41: 865–874. 10.1007/s00134-015-3763-8 [DOI] [PubMed] [Google Scholar]
- 15.Dantas CM, Silva PF, Siqueira FH, Pinto RM, Matias S, Maciel C, et al. (2012) Influence of early mobilization on respiratory and peripheral muscle strength in critically ill patients. Rev Bras Ter Intensiva 24: 173–178. [PubMed] [Google Scholar]
- 16.Parry SM, Berney S, Warrillow S, El-Ansary D, Bryant AL, Hart N, et al. (2014) Functional electrical stimulation with cycling in the critically ill: A pilot case-matched control study. J Crit Care 29: 695 e691–697. [DOI] [PubMed] [Google Scholar]
- 17.Kho ME, Martin RA, Toonstra AL, Zanni JM, Mantheiy EC, Nelliot A, et al. (2015) Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit (ICU). Journal of Critical Care 30: e1–5. [DOI] [PubMed] [Google Scholar]
- 18.Adler J, Malone D (2012) Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J 23: 5–13. [PMC free article] [PubMed] [Google Scholar]
- 19.Kho ME, Truong AD, Brower RG, Palmer JB, Fan E, Zanni JM, et al. (2012) Neuromuscular Electrical Stimulation for Intensive Care Unit-Acquired Weakness: Protocol and Methodological Implications for a Randomized, Sham-Controlled, Phase II Trial. Physical Therapy 92: 1564–1579. 10.2522/ptj.20110437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818–829. [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 22.Groll DL, To T, Bombardier C, Wright JG (2005) The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 58: 595–602. 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. Jama 185: 914–919. [DOI] [PubMed] [Google Scholar]
- 24.Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, et al. (2010) Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. Journal of Critical Care 25: 254–262. 10.1016/j.jcrc.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 25.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ (1995) Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 23: 1638–1652. [DOI] [PubMed] [Google Scholar]
- 26.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. (2010) A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 10: 1 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM (2010) Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Medicine 36: 1038–1043. 10.1007/s00134-010-1796-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, et al. (2012) Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle and Nerve 45: 18–25. 10.1002/mus.22219 [DOI] [PubMed] [Google Scholar]
- 29.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. (2002) Paresis acquired in the intensive care unit: a prospective multicenter study. Jama 288: 2859–2867. [DOI] [PubMed] [Google Scholar]
- 30.Ali NA, O'Brien JM Jr., Hoffmann SP, Phillips G, Garland A, Finley JC, et al. (2008) Acquired weakness, handgrip strength, and mortality in critically ill patients. American Journal of Respiratory and Critical Care Medicine 178: 261–268. 10.1164/rccm.200712-1829OC [DOI] [PubMed] [Google Scholar]
- 31.Vanpee G, Segers J, Van Mechelen H, Wouters P, Van den Berghe G, Hermans G, et al. (2011) The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med 39: 1929–1934. [DOI] [PubMed] [Google Scholar]
- 32.Denehy L, de Morton NA, Skinner EH, Edbrooke L, Haines K, Warrillow S, et al. (2013) A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored). Phys Ther 93: 1636–1645. 10.2522/ptj.20120310 [DOI] [PubMed] [Google Scholar]
- 33.Skinner EH, Berney S, Warrillow S, Denehy L (2009) Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc 11: 110–115. [PubMed] [Google Scholar]
- 34.ATS statement: guidelines for the six-minute walk test. (2002) Am J Respir Crit Care Med 166: 111–117. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 35.Des Jarlais DC, Lyles C, Crepaz N, Group T (2004) Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 94: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348: g1687 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 37.Damluji A, Zanni JM, Mantheiy E, Colantuoni E, Kho ME, Needham DM (2013) Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit. J Crit Care. [DOI] [PubMed] [Google Scholar]
- 38.Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM (2014) Safety of physical therapy interventions in critically ill patients: A single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care 29: 395–400. 10.1016/j.jcrc.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 39.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, et al. (2009) Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 13: R161 10.1186/cc8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kho ME, Truong AD, Zanni JM, Ciesla ND, Brower RG, Palmer JB, et al. (2015) Neuromuscular electrical stimulation in mechanically ventilated patients: A randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care 30: 32–39. 10.1016/j.jcrc.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denehy L, Skinner EH, Edbrooke L, Haines K, Warrillow S, Hawthorne G, et al. (2013) Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care 17: R156 10.1186/cc12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brummel NE, Girard TD, Ely EW, Pandharipande PP, Morandi A, Hughes CG, et al. (2014) Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med 40: 370–379. 10.1007/s00134-013-3136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segers J, Hermans G, Bruyninckx F, Meyfroidt G, Langer D, Gosselink R (2014) Feasibility of neuromuscular electrical stimulation in critically ill patients. J Crit Care. [DOI] [PubMed] [Google Scholar]
- 44.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E (1992) Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 73: 2172–2178. [DOI] [PubMed] [Google Scholar]
- 45.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41: 263–306. [DOI] [PubMed] [Google Scholar]
- 46.Korupolu R, Gifford JM, Needham DM (2009) Early mobilization of critically ill patients: Reducing neuromuscular complications after critical care. Contemporary Critical Care 6: 1–12. [Google Scholar]
- 47.Hickmann CE, Roeseler J, Castanares-Zapatero D, Herrera EI, Mongodin A, Laterre PF (2014) Energy expenditure in the critically ill performing early physical therapy. Intensive Care Med 40: 548–555. 10.1007/s00134-014-3218-7 [DOI] [PubMed] [Google Scholar]
- 48.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 289: 2983–2991. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This diagram outlines the TryCYCLE study schema. Abbreviations: MV = mechanical ventilation; ICU = intensive care unit; PT = physiotherapy interventions.
(DOCX)
(DOCX)
(DOCX)
Values in this table represent vital sign recordings during in-bed cycling sessions. All values represent mean (SD). * = p<0.001 difference between pre- and post- cycling heart rate; ** p = 0.004 difference between pre- and post- cycling mean arterial pressure. aSample size for blood pressure measurements at 5, 10, 20, and 30 minutes: n = 201, n = 190, n = 178, and n = 146, respectively. Abbreviations: bpm = beats per minute.
(DOCX)
In this table, we outline additional therapeutic activities occurring on days of in-bed cycling.
(DOCX)
This table outlines the mean (SD) number of cycling sessions, cycling session duration, and distance per cycling session for all patients. Abbreviations: SD = standard deviation.
(DOCX)
(PDF)
(DOCX)
Data Availability Statement
Ethics board approval for sharing patient data publicly was not obtained for this study. Data are from the TryCYCLE study whose authors may be contacted for access at khome@mcmaster.ca.