Abstract
Glucagon-like peptide 1 (GLP-1) can promote islet β cell replication and function, and mesenchymal stem cells (MSCs) can inhibit T cell autoimmunity. The aim of this study was to test the dynamic distribution of infused human MSCs and the therapeutic effect of combined MSCs and liraglutide, a long-acting GLP-1 analog, on preserving β cell function in severe nonobese diabetic (NOD) mice. We found that infused MSCs accumulated in the pancreas at 4 wks post infusion, which was not affected by liraglutide treatment. Liraglutide significantly enhanced the function of MSCs to preserve islet β cells by reducing glucose levels 30 min post glucose challenge and increasing the content and secretion of insulin by islet β cells in severely diabetic mice. Infusion with MSCs significantly reduced insulitis scores but increased the frequency of splenic Tregs, accompanied by a reduction in plasma IFN-γ and TNF-α levels and an elevation of plasma IL-10 and transforming growth factor-β1 (TGF-β1) levels in NOD mice. Although liraglutide mitigated MSC-mediated changes in the frequency of Tregs and the level of plasma IL-10, it significantly increased the plasma TGF-β1 levels in severely diabetic mice. Therefore, our findings suggest that liraglutide could enhance the therapeutic efficacy of MSCs in the treatment of severe type 1 diabetes.
INTRODUCTION
Type 1 diabetes results from the destruction of islet β cells and is characterized by an imbalance of autoreactive Th1 and regulatory T cells (Tregs) (1,2). Although exogenous insulin administration can control hyperglycemia, this intervention is insufficient to prevent long-term complications, including vascular degeneration, neuropathy, retinopathy and nephropathy, leading to cardiovascular diseases, blindness and kidney failure (3). Ideally, therapeutic strategies for intervention in type 1 diabetes should safely combine inhibition of Th1 autoimmunity with preservation and promotion of islet β cell function to reverse hyperglycemia and mitigate long-term hyperglycemia-related complications (4,5).
Mesenchymal stem cells (MSCs) are multipotent nonhematopoietic stromal cells derived from bone marrow, umbilical cord Wharton’s jelly and blood, fat, skeletal muscle and other sites (6,7). MSCs have the capacity of self-renewal and multilineage differentiation to form mesodermal, ectodermal and endodermal tissues as well as insulin-producing cells (8). Furthermore, MSCs can regulate T cell autoimmunity and inflammation by secreting antiinflammatory transforming growth factor-β1 (TGF-β1), IL-10, PGE2 and other substances. In addition, MSCs can induce autoreactive T cell anergy and promote Treg response (9-11). Because of their function and low immunogenicity, allogeneic MSC-based therapies have been tested for their ability to ameliorate autoimmune diseases, including type 1 diabetes (6,7,12). However, the efficacy of MSC-based therapies in reversing hyperglycemia and maintaining long-term euglycemia is limited. Further improvement of its therapeutic efficacy by combining it with another reagent is urgently needed, particularly for severe diabetes.
Glucagon-like peptide 1 (GLP-1) is an incretin mainly produced by intestinal L cells (13). GLP-1 is highly susceptible to degradation by dipeptidyl peptidase IV (DPP-IV) in the body, and its plasma half-life is less than 2 min. GLP-1 can stimulate insulin secretion by islet β cells and inhibit glucagon secretion by islet α cells to reduce blood glucose. A recent study has shown that GLP-1 can promote islet β cell proliferation and neogenesis (14). Furthermore, treatment with a DPP-IV inhibitor to elevate circulating levels of active GLP-1 can modulate T cell immunity and promote Treg response in nonobese diabetic (NOD) mice (15–18). Actually, treatment with a long-acting GLP-1 such as Ex-4 reverses hyperglycemia or delays the onset of diabetes by preserving β cell function or enhancing Treg response in diabetic or perdiabetic mice (19–22). Accordingly, a combination of MSC infusion and treatment with liraglutide, a long-acting GLP-1 analog, could enhance the therapeutic efficacy of MSCs in NOD mice. However, it is unclear whether GLP-1 can affect the distribution of infused MSCs and their immunomodulatory effect during the process of autoimmune diabetes. Furthermore, there is no information on whether treatment with MSCs or a combination of MSCs and GLP-1 can preserve islet β cell function and modulate proinflammatory and antiinflammatory responses in NOD mice with severe type 1 diabetes.
In this study, we analyzed the distribution of human bone marrow–derived MSCs in different organs and evaluated the effects of combined therapies with MSCs and liraglutide on glucose tolerance and β cell function longitudinally in NOD mice with severe diabetes. Furthermore, we characterized the frequency of CD4+, CD8+ and Tregs and plasma proinflammatory and antiinflammatory cytokine levels in severely diabetic NOD mice. The objective of these measurements was to determine the potential effect of combined liraglutide and MSCs on preserving β cell function and modulating autoimmune reponse, and the dynamic distribution of infused MSCs in severe type 1 diabetes.
MATERIALS AND METHODS
Mice
Female NOD/Ltj and C57BL/6 mice at 7–8 wks of age were purchased from the Animal Model Research Center of Nanjing University (Nanjing, China) and housed in a specific pathogen-free facility at a 12-h:12-h light/dark cycle with constant temperature and humidity. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Nanjing University.
Isolation, Expansion and Characterization of MSCs
Written informed consent was obtained from human participants. Human bone marrow–derived MSCs were prepared from healthy donors, as reported previously (23). Briefly, heparinized human bone marrow samples (5 mL each) were obtained from individual donors. Bone marrow mononuclear cells were isolated by density-gradient centrifugation and cultured in 10% fetal bovine serum (FBS, Thermo Fisher Scientific) and Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific) for 48 h. After removal of nonadherent cells, the remaining cells were exposed to fresh medium every 3 d until cell confluency and then passaged. The cells at passages 2-5 were stained with fluorescent antibodies against human cell-surface molecules peridinin chlorophyll protein–CD45, phycoerythrin (PE)-CD14, fluorescein isothiocyanate (FITC)-CD29, FITC-CD44, PE-CD34 and PE-CD105 (eBioscience) and analyzed by flow cytometry using the BD FACSCanto™ flow cytometry system (BD BioSciences). A preparation of CD29+CD44+CD105+CD14-CD34–CD45- MSCs with a purity of >95 was used for treatment.
Diabetes Induction and Treatment
Individual NOD/Ltj mice were injected intraperitoneally with 40 mg/kg streptozocin (Shanghai Shifeng Biological Technology Co.) for five consecutive days to accelerate diabetes development and progression. Individual mice with blood glucose levels ≥ 20.0 mmol/L for two consecutive days were diagnosed with severe diabetes. The severely diabetic mice were randomized into three groups: type 1 diabetes, MSC and MSC + GLP-1. The mice in the type 1 diabetes group were injected intravenously with Hank’s solution (200 μL) and the mice in the MSC group were infused with one dose of 2.0 × 106 MSCs. The mice in the MSC + GLP-1 group were injected intravenously with one dose of 2.0 × 106 MSCs and intraperitoneally with 2 μg liraglutide (Novo Nordisk) daily up to 42 d post MSC infusion. A group of C57BL/6 mice were injected with vehicle and served as healthy controls. Following treatment, the body weights of individual mice were measured and their fasting blood glucose (FBG) levels were examined weekly using a One Touch profile glucometer (Johnson & Johnson).
Intraperitoneal Glucose Tolerance Test and Serum Insulin Levels
The effect of treatment with MSCs or MSC + GLP-1 on glucose tolerance was determined by biweekly intraperitoneal glucose tolerance test (IPGTT) and serum insulin levels. Briefly, individual mice were fasted for 6 h and injected intraperitoneally with glucose (1 g/kg body weight). The level of blood glucose in individual mice was measured before glucose challenge and 30, 60 and 120 min post glucose challenge using the One Touch profile glucometer. The values of the area under the curve (AUC) for the blood glucose level over the experimental period and of ΔG30, the net increase in blood glucose level at 30 min post glucose challenge, were calculated.
Orbital venous blood samples were obtained from some mice (n = 6 per group) before they were euthanized. Fasting plasma insulin levels were determined using a Rat/Mouse Insulin ELISA (enzyme-linked immunosorbent assay) Kit (Millipore). Spleens and pancreatic tissues were collected for flow cytometry and histology, respectively. In addition, lungs, kidneys, livers and intestines were dissected for quantitative measurement of the content of Alu element in human DNA by real-time polymerase chain reaction (RT-PCR) to determine the distribution of infused MSCs in individual mice.
Plasma Cytokine Levels
The concentrations of plasma IFN-γ, IL-4, IL-10, TNF-α and TGF-β1 in individual mice were measured by immunology multiplex assay in a MAGPIX system using the MT17MAG47K-PX25 and TGFBMAG-64K-03 kits (Millipore), according to the manufacturer’s instructions. Assays for each cytokine were performed in duplicate, and the detection limitations for IFN-γ, IL-4, IL-10, TNF-α and TGF-β1 were 7.8, 1.5, 1.8, 2.3 and 12.0 pg/mL, respectively.
Flow Cytometric Analysis
Splenic mononuclear cells were isolated and the frequency of CD4+, CD8+ and CD4+CD25+Foxp3+ Tregs in individual mice was determined by flow cytometry. Briefly, splenic mononuclear cells (1 × 106/tube) were stained in duplicate with allophycocyanin (APC)-conjugated anti-CD3, FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 (eBioscience), and the percentages of splenic CD4+ and CD8+ T cells were determined. Furthermore, splenic mononuclear cells were stained in duplicate with FITC-anti-CD4 and APC-anti-CD25, fixed, permeabilized and stained intracellularly with PE-anti-FoxP3 (eBioscience). The frequency of splenic CD4+CD25+Foxp3+ Tregs in total CD4+ T cells was determined by flow cytometry. The data were analyzed with FlowJo software.
Histology and Immunohistochemistry
The dissected pancreatic tissues were fixed and paraffin-embedded. Pancreatic tissue sections (3 μm) were stained with hematoxylin and eosin (ZSGB-bio), followed by imaging under a Zeiss Axioplan light microscope (Carl Zeiss AG). The contents of inflammatory infiltrates were scored in a blinded manner as 0 = no infiltration, 1 = infiltrates covering 1–10% of the islet area, 2 = 10–25% of the islet area, 3 = 25–50 % of the islet area, and 4 = >50% of the islet area. At least 20 islets from each mouse pancreatic tissue section were evaluated.
The impact of treatment with MSCs or MSC + GLP-1 on the content of islet β cells was determined by immunohistochemistry using anti-insulin antibodies. Briefly, the pancreatic tissue sections (5 μm) were deparaffinized, rehydrated and treated with 3% H2O2 in methanol for 15 min to inactivate endogenous peroxidase activity. The sections were subjected to antigen retrieval and treated with 2% bovine serum albumin (Thermo Fisher Scientific) for 1 h. Subsequently, the sections were incubated with rabbit anti-insulin antibodies (1:600; Santa Cruz Biotech). After being washed, the bound antibodies were detected with horseradish peroxidase–conjugated goat anti-rabbit IgG (ZSGB-bio) and visualized with 0.2% 3,3′-diaminobenzidine (ZSGB-bio). The content of anti-insulin staining in the islets was evaluated using ImageJ software, and at least 20 islets selected randomly from each pancreatic tissue were analyzed.
Preparation of Tissue Samples for Measuring Alu Element in Human DNA by Quantitative RT-PCR
To determine the dynamic distribution of infused MSCs, the dissected pancreatic tissues, livers, kidneys, lungs and intestines were weighed, snap-frozen in liquid nitrogen and stored at –80°C until use. These frozen tissues were homogenized, and their DNA was extracted using the Blood/Cell/Tissue Genome DNA Extract Kit (Tiangen), according to the manufacturer’s instructions. After quantification of DNA concentrations, the levels of Alu DNA in individual samples were determined in triplicate by quantitative RT-PCR assay in a StepOnePlus RT-PCR system (Applied Biosystems) using 100 ng sample DNA as the template, the Alu-specific primers, TaqMan probe (Takara). The sequences of primers were as follows: forward, 5′-CATGGTGAAACCCCGTCTCTA-3′; reverse, 5′-GCCTCAGCCTCCCGAGT A-3′; probe, 5′-FAM-ATTAGCCGGGC GTGGTGGCG-TAMRA-3′. The levels of human Alu DNA were calculated according to the standard curve established by mixing different amounts of human genomic DNA with mouse monocyte genomic DNA.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). The difference among groups was analyzed by analysis of variance and post hoc Fisher’s least significant difference. The difference between the two groups was analyzed by Student t test using SPSS software version 15.0. A P value of < 0.05 was considered statistically significant.
RESULTS
GLP-1 Does Not Affect Migration and Accumulation of MSCs in the Pancreatic Tissues of Severely Diabetic Mice
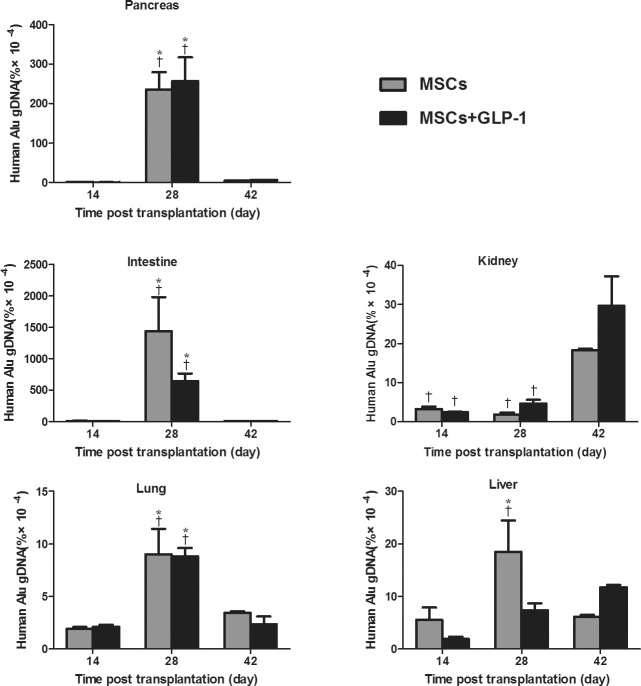
To determine the effect of GLP-1 on the dynamic distribution of infused MSCs in diabetic mice, severely diabetic mice were infused with MSCs, randomized and injected intraperitoneally with vehicle or GLP-1 daily. At 2, 4 and 6 wks post treatment, pancreatic, lung, kidney, liver and intestine tissues were dissected from some mice of each group and the content of human Alu element DNA was determined by qRT-PCR (Figure 1). Alu element DNA was detected in the pancreas, lung, kidney, liver and intestine tissues in both groups at 2 wks post treatment, and the content of Alu element DNA in those tissues peaked at 4 wks post treatment, except in the kidney, where the highest levels of Alu element DNA were detected 6 wks post treatment. Furthermore, the highest levels of Alu element DNA were detected in the intestine, followed by the pancreas, kidney, liver and lung. While the highest levels of Alu element DNA were detected in the livers of mice without GLP-1 treatment at 4 wks post treatment, the peak of Alu element DNA was detected in the livers of mice with GLP-1 treatment at 6 wks post treatment. More interestingly, there was no significant difference in the levels of Alu element DNA in any organ at any time point tested between these two groups of mice. Hence, the infused MSCs migrated and accumulated in the inflammatory pancreatic tissues in severely diabetic mice at 4 wks post treatment, and GLP-1 treatment did not affect the migration and accumulation of infused MSCs in these mice.
Figure 1.
GLP-1 does not affect the migration and accumulation of infused MSCs into the pancreatic tissues of NOD mice. Diabetic NOD mice were infused with MSCs and treated intraperitoneally with GLP-1 or vehicle daily for 42 d. The indicated tissues were dissected from some mice of each group and the content of Alu element DNA was determined by quantitative RT-PCR. Data are expressed as the mean ± SEM of each group of mice (n = 6 per group per time point from three separate experiments. *P < 0.05 versus mice at 14 d post treatment. †P < 0.05 versus mice at 42 d post treatment.
GLP-1 Treatment Enhances the Anti-diabetic Effect of MSCs in Severely Diabetic Mice
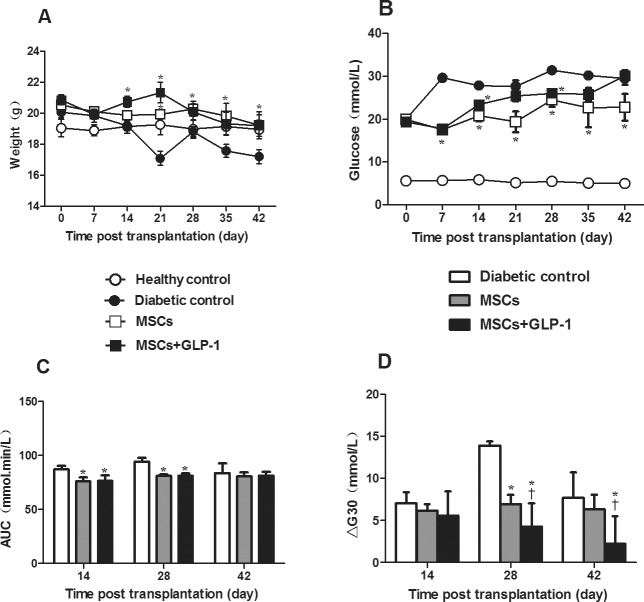
Next, the impact of GLP-1 treatment on the anti-diabetic effect of MSCs was determined in NOD mice. After acceleration of severe diabetes, the mice were randomized and treated with (or without) MSCs or MSC + GLP-1. An additional group of healthy C57BL/6 mice served as controls. First, longitudinal measurement indicated that diabetic mice that had not been treated with MSCs gradually lost body weight, while the mice that had been treated with MSCs alone or together with daily GLP-1 had only slightly reduced body weight (Figure 2A). Body weights in the diabetic controls were significantly less than in the MSC and MSC + GLP-1 groups, particularly in the latter. Hence, treatment with MSCs alone or together with GLP-1 significantly mitigated the long-term hyperglycemia-related loss of body weight in NOD mice.
Figure 2.
GLP-1 enhances MSC-mediated glucose tolerance in diabetic NOD mice. Following MSC infusion and GLP-1 treatment, the body weight and FGB levels of individual mice were measured weekly. The AUC and ΔG30 levels in individual mice at the indicated time points post treatment were determined. Data are expressed as the mean ± SEM of each group (n = 6 per group). (A) Body weight. (B) FBG levels. (C) AUC. (D) Plasma ΔG30 levels. *P < 0.05 versus diabetic controls. †P < 0.05 versus mice treated with MSCs alone. AUC: values of the area under the curve for the levels of blood glucose over the IPGTT period. ΔG30: net increase in the levels of blood glucose at 30 min post glucose challenge.
Second, measurements of FBG showed that while the healthy control mice remained euglycemic, all diabetic mice exhibited hyperglycemia, regardless of the treatment (Figure 2B). However, FBG levels in the diabetic control mice were significantly higher than in the MSC and MSC + GLP-1 groups. Apparently, treatment with GLP-1 did not alter the effect of MSCs on FBG levels in NOD mice with severe diabetes. Further IPGTT tests indicated that AUC values in the MSC and MSC + GLP-1 mice were significantly less than in the diabetic controls at 2 and 4 wks post treatment (P < 0.05 for both, Figure 2C). Notably, ΔG30 values in the mice treated with MSC + GLP-1 were significantly less than in the mice treated with MSCs alone at 4 and 6 wks post treatment (all P < 0.05, Figure 2D). These data indicate that MSC treatment significantly improved glucose tolerance and GLP-1 treatment enhanced the antihyperglycemic effect of MSCs by improving the acute glucose response rate in severely diabetic mice.
GLP-1 Enhances the Effects of MSCs on Preserving Islet β Cell Function in Severely Diabetic Mice
GLP-1 can promote insulin secretion, and MSC treatment can also preserve the function of islet β cells. To determine the impact of GLP-1 treatment on the role of MSCs in preserving islet β cell function, the levels of plasma insulin and the content of islet insulin were analyzed by enzyme-linked immunosorbent assay and immunohistochemistry, respectively. As shown in Figure 3A, plasma insulin in the diabetic control mice gradually declined to extremely low levels at the end of the observation period, reflecting a progressive loss of islet β cells. In contrast, plasma insulin levels were elevated in the mice treated with MSCs 2 wks post treatment and slightly reduced at later time points. A similar pattern of plasma insulin was detected in the mice treated with both MSCs and GLP-1. Importantly, plasma insulin levels in the MSC-treated mice were significantly higher than in the diabetic controls, but significantly lower than in the mice treated with MSC + GLP-1 at all time points post treatment (P < 0.05). Similar patterns of insulin content were detected in the pancreatic islets of the different groups of mice throughout the observation period (Figures 3B and 3C). Collectively, GLP-1 treatment significantly enhanced the role of MSCs in preserving the function of islet β cells in severely diabetic mice.
Figure 3.
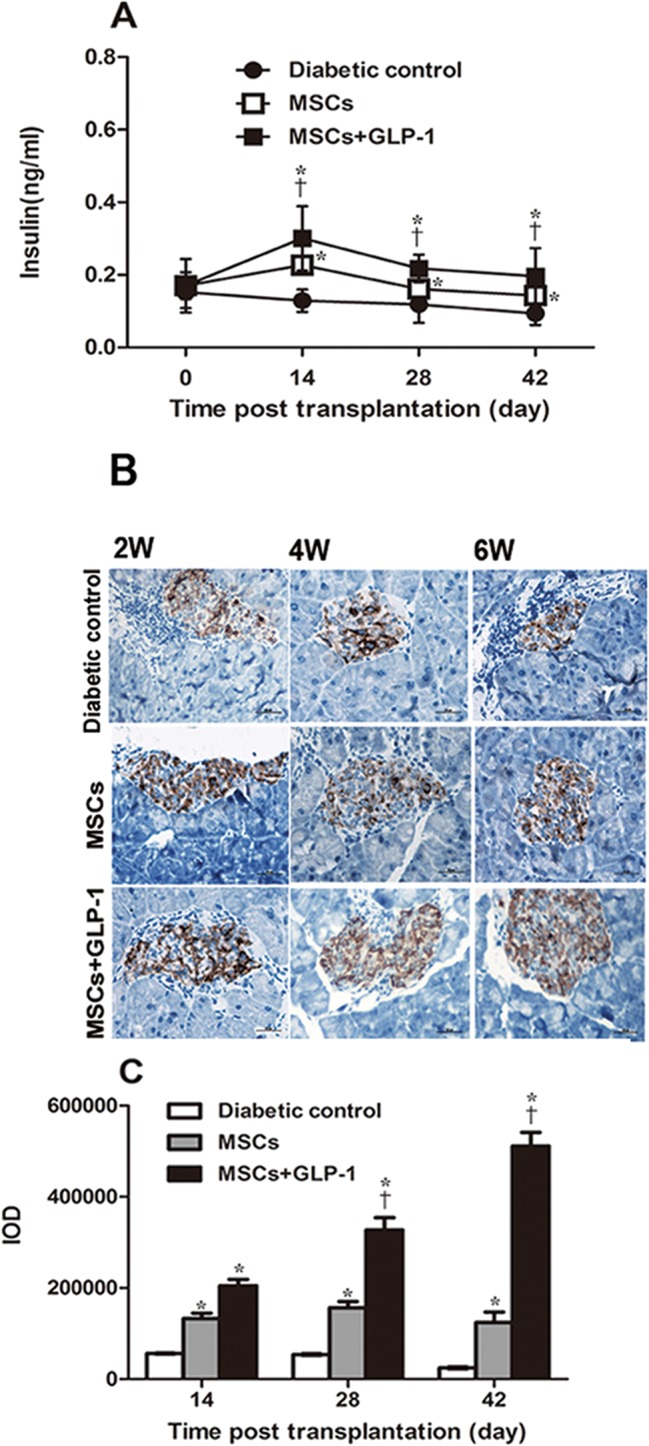
GLP-1 enhances the effect of MSCs on preserving islet β cell function in NOD mice. Following treatment with MSCs and GLP-1 or MSCs alone, the levels of fasting insulin in individual mice at the indicated time points post treatment were measured and the content of insulin in the pancreatic islets was determined by immunohistochemistry. Data are representative images (magnification × 400) or expressed as the mean ± SEM of each group of mice (n = 6 per group per time point) from three separate experiments. (A) Fasting plasma insulin levels. (B) Immunohistochemistry analysis of insulin content in the pancreatic islets. (C) Quantitative analysis of insulin levels. *P < 0.05 versus diabetic controls. †P < 0.05 versus mice treated with MSCs alone.
Treatment with MSCs or with MSC + GLP-1 Modulates Inflammation in Severely Diabetic Mice
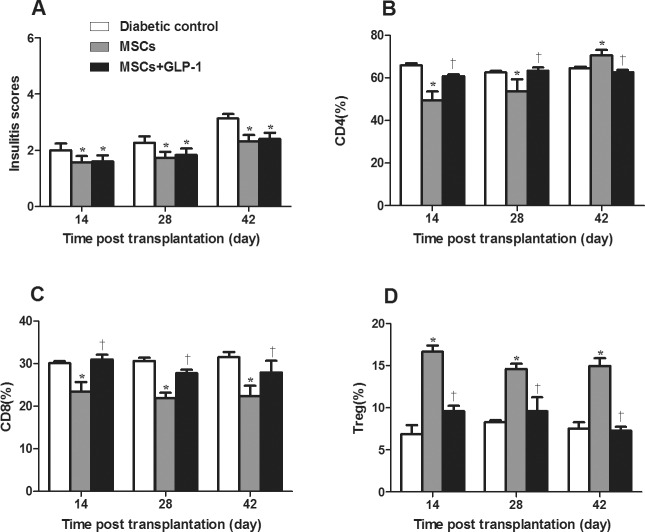
To understand the potential mechanisms underlying the therapeutic efficacy, the degree of insulitis in the different groups of mice was measured longitudinally. The insulitis scores gradually increased with time (Figure 4A). However, the insulitis scores in the MSC and MSC + GLP-1 groups were significantly less than in the diabetic control group at any time point tested (P < 0.05 for all), and there was no significant difference in the scores between the MSC and MSC + GLP-1 groups. These data indicate that treatment with MSCs significantly reduced the content of inflammatory infiltrates in the pancreatic islets of severely diabetic mice.
Figure 4.
Treatment with MSCs and GLP-1 modulates inflammation in NOD mice. Following treatment with MSCs and GLP-1 or MSCs alone, some mice from the groups of diabetic mice were euthanized at the indicated time points post treatment and the degree of inflammatory infiltrates in the pancreatic islets was examined. The frequency of splenic CD4+ and CD8+ T cells and CD4+CD25+Foxp3+ Tregs was determined by flow cytometry. Data are expressed as the mean ± SEM of individual group of mice (n = 6 per group per time point) from three separate experiments. (A) Insulitis levels. (B) Percentages of splenic CD4+ T cells. (C) Percentages of splenic CD8+ T cells. (D) Percentages of splenic Tregs. *P < 0.05 versus diabetic controls. †P < 0.05 versus mice treated with MSCs alone.
Analysis of splenic mononuclear cells indicated that treatment with MSCs alone significantly reduced the frequency of CD4+ and CD8+ T cells but increased the percentage of CD4+CD25+Foxp3+ Tregs in diabetic mice over the follow-up period, except at 42 d post treatment, when the frequency of CD4+ significantly increased compared to the diabetic controls (P < 0.05 for all, Figure 4B). However, treatment with both MSCs and GLP-1 did not significantly alter the frequency of splenic CD4+ and CD8+ T cells and Tregs relative to the diabetic controls.
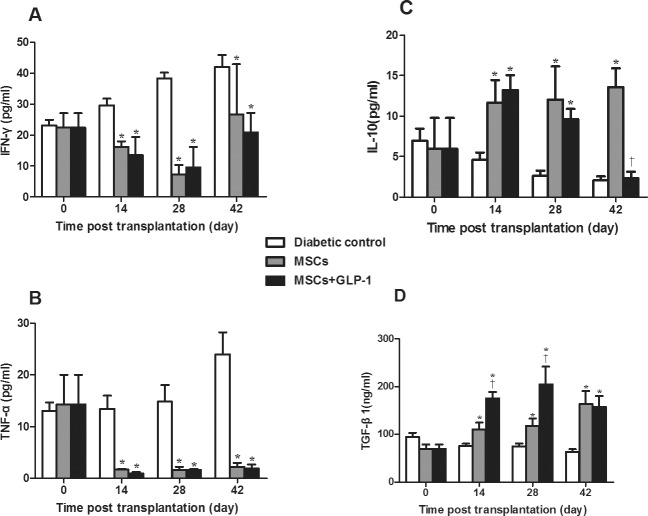
Further analysis of plasma cytokines revealed that treatment with MSCs or MSCs + GLP-1 significantly reduced plasma proinflammatory IFN-γ and TNFα levels but increased plasma antiinflammatory IL-10 and TGF-β1 levels at all time points post treatment, except for the IL-10 level in the MSC + GLP-1 group at 6 wks post infusion, compared with the diabetic controls (P < 0.05, Figure 5). Interestingly, the plasma TGF-β1 levels in the MSC + GLP-1 group were significantly higher than in the MSC group at 2 and 4 wks post treatment. Apparently, while treatment with GLP-1 mitigated the MSC-mediated change in the frequency of splenic CD4+ and CD8+ T cells and Tregs and plasma IL-10 levels, GLP-1 significantly increased plasma TGF-β1 levels in severely diabetic mice.
Figure 5.
Treatment with MSCs and GLP-1 alters plasma cytokine levels in NOD mice. Following treatment with MSCs and GLP-1, plasma IFN-γ, TNF-α, IL-10 and TGF-β1 levels in individual mice were measured. Data are expressed as the mean ± SEM of individual group of mice (n = 6 per group per time point) from three separate experiments. (A) Plasma IFN-γ levels. (B) Plasma TNF-α levels. (C) Plasma IL-10 levels. (D) Plasma TGF-β1 levels. *P < 0.05 versus diabetic controls. †P < 0.05 versus mice treated with MSCs alone.
DISCUSSION
In this study, we evaluated the therapeutic efficacy of a combination of MSCs and GLP-1 on glucose tolerance, preserving islet β cell function and modulating inflammation in NOD mice with severe type 1 diabetes. We found that treatment with MSCs and GLP-1 significantly improved glucose tolerance, preserved islet β cell function and increased plasma antiinflammatory TGF-β1 levels in severely diabetic mice compared to mice treated with MSCs alone. These novel data extend previous findings (15–21) and suggest that a combination of MSCs and GLP-1 could benefit patients with severe diabetes. Given that there is no effective therapy for severe type 1 diabetes, our findings might aid in the design of new therapeutic strategies for this condition.
It is well known that many patients with type 1 diabetes are not diagnosed until the severe stage, even with ketoacidosis, particularly in developing countries such as China. Many patients with severe type 1 diabetes usually have few islet β cells and display severely impaired glucose tolerance. Hence, controlling hyperglycemia and improving glucose tolerance as well preserving islet β cell function are crucial for prevention of long-term hyperglycemia-related complications, which are major factors in the morbidity and mortality of diabetic patients. In this study, we found that treatment with either MSCs or MSCs and GLP-1 mitigated long-term hyperglycemia-related loss of body weight and reduced the levels of FBG and AUC in severely diabetic mice. More importantly, treatment with MSCs and GLP-1 significantly reduced ΔG30 levels and increased fasting plasma insulin levels and the content of pancreatic islet insulin in NOD mice with severe type 1 diabetes compared with mice treated with MSCs alone. These results demonstrate that treatment with GLP-1 significantly enhanced the efficacy of MSCs in preserving islet β cell function in severely diabetic mice. The increased islet β cell function could stem from MSC-mediated inhibition of β cell destruction and MSC-promoting progenitor cell differentiation into insulin-producing cells (24,25). Alternatively, the outcome might come from GLP-1–mediated islet β cell replication in NOD mice (14). Hence, we suppose that treatment with both MSCs and GLP-1 can have a synergistic effect on preservation of islet β cells, or GLP-1 can bring an additional effect to MSC-based therapy for severe type 1 diabetes. Furthermore, it is also possible that GLP-1 and MSCs can collectively modulate the microenvironment of islet β cells, promoting the survival of islet β cells in diabetic mice. We are interested in further investigating the mechanisms underlying the action of MSCs or GLP-1 alone and in combination in preserving islet β cells in severe diabetic conditions.
A previous study showed that MSCs can inhibit pathogenic Th1 response and induce Treg response (10). Treatment with GLP-1 also promotes Treg response in NOD mice (15–18). In this study, we found that treatment with either MSCs alone or in combination with GLP-1 significantly reduced insulitis in severely diabetic mice. Moreover, treatment with either MSCs alone or in combination with GLP-1 significantly reduced plasma proinflammatory IFN-γ and TNFα levels but increased plasma IL-10 and TGF-β1 levels in NOD mice. Strikingly, treatment with MSCs alone significantly decreased the percentages of splenic CD4+ and CD8+ T cells but increased the frequency of splenic Tregs. Treatment with both MSCs and GLP-1 significantly mitigated the changes in the frequency of splenic CD4+, CD8+ and Tregs in NOD mice. However, the plasma TGF-β1 levels in the mice treated with both MSCs and GLP-1 were significantly higher than in the mice treated with MSCs alone at 14 and 28 d post treatment, indicating that treatment with GLP-1 enhanced TGF-β1 response in NOD mice. Given that TGF-β1 is an important inhibitory cytokine that can attenuate pathogenic Th1 response in NOD mice (26,27), the higher TGF-β1 response may contribute to preservation of islet β cells in these mice. Indeed, enhanced efficacy in the improvement of islet β cell function was observed in mice treated with MSC + GLP-1 compared with mice treated with MSCs alone. High levels of TGF-β1 responses are usually associated with potent Treg responses in NOD mice (26). However, we observed that treatment with both MSCs and GLP-1 significantly reduced the frequency of splenic Tregs compared with the mice treated with MSCs alone, which was in disagreement with previous reports (15–18). Because inducible Tregs can migrate into pancreatic islets and create benign insulitis (28,29), the significantly reduced frequency of Tregs could reflect the migration of antiinflammatory Tregs into the pancreatic islets of NOD mice.
It is well known that infused MSCs preferably migrate to inflammatory organs (30), where they exert a direct effect on local inflammation or tissue regeneration (31–33). We wondered whether the enhanced preservation of islet β cells in the MSC + GLP-1 mice was associated with increased numbers of MSCs migrating to injured pancreas in severely diabetic mice. We found that the infused MSCs migrated and accumulated in the pancreas, intestine, liver, lung and kidney and peaked in most organs at 28 d post infusion in NOD mice regardless of GLP-1 treatment. Furthermore, our data demonstrate that the infused MSCs migrated and accumulated into the pancreatic tissues, consistent with previous reports (30,34), and treatment with GLP-1 did not interfere with the migration and accumulation of infused MSCs in NOD mice. Hence, significantly enhanced preservation of islet β cells in the GLP-1 + MSC mice may stem from the role of GLP-1 in promoting islet β cell replication. Furthermore, we found that infused MSCs in the pancreatic tissues and other organs, except for the kidney, were dramatically reduced in NOD mice at 6 wks post infusion. These reduced numbers may reflect the destruction of infused MSCs by the host immune system. Given that decreased numbers of infused MSCs appeared to be associated with less effect on controlling hyperglycemia and modulating Th1 and Treg responses (9,10), we are interested in further investigating whether frequent infusion with MSCs can prolong the effect of MSCs and GLP-1 on reversing hyperglycemia and attenuating autoimmune responses in severely diabetic mice.
CONCLUSION
We found that liraglutide did not affect the distribution and accumulation of infused MSCs, but significantly enhanced the function of MSCs to preserve islet β cells in severely diabetic mice. Furthermore, liraglutide significantly increased plasma TGF-β1 levels in NOD mice, which might attenuate pathogenic Th1 response and contribute to preservation of islet β cells in NOD mice. Therefore, our findings suggest that liraglutide might enhance the therapeutic efficacy of MSCs in the treatment of severe type 1 diabetes.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Natural Science Foundation of China (81500638), the Project of Nanjing Science and Technology Development (201402031) and the Key Project of Nanjing Medical Science and Technology Development (ZKX14016).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Li L-r, et al. (2016) Liraglutide enhances the efficacy of human mesenchymal stem cells in preserving islet β-cell function in severe non-obese diabetic mice. Mol. Med. 22:800–808.
REFERENCES
- Wallberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013;34:583–91. doi: 10.1016/j.it.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. Int Immunol. 2012;24:339–46. doi: 10.1093/intimm/dxs049. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Sowers JR. Implications for glucose measures in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes. 2014;63:45–47. doi: 10.2337/db13-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock GL, et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008;86:1762–66. doi: 10.1097/TP.0b013e318190b052. [DOI] [PubMed] [Google Scholar]
- Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–67. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal Stem Cells: Rising Concerns over Their Application in Treatment of Type One Diabetes Mellitus. J Diabetes Res. 2015;2015:675103. doi: 10.1155/2015/675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillaro T, Peluso G, Galderisi U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–48. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103:129–37. doi: 10.1007/s12185-015-1918-6. [DOI] [PubMed] [Google Scholar]
- Mareschi K, et al. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp Hematol. 2016;44:138–50. doi: 10.1016/j.exphem.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ding G, Xu X. Immunomodulatory functions of mesenchymal stem cells and possible mechanisms. Histol Histopathol. 2016;2:11750. doi: 10.14670/HH-11-750. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65–76. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katuchova J, et al. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr Pathol. 2015;26:95–103. doi: 10.1007/s12022-015-9362-y. [DOI] [PubMed] [Google Scholar]
- Pais R, Gribble FM, Reimann F. Stimulation of incretin secreting cells. Ther Adv Endocrinol Metab. 2016;7:24–42. doi: 10.1177/2042018815618177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon S, Gautier JF. An Update on the Effect of Incretin-Based Therapies on β-Cell Function and Mass. Diabetes Metab J. 2016;40:99–114. doi: 10.4093/dmj.2016.40.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, et al. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87:198–206. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- Tian L, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151:3049–60. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- Cabrera SM, et al. Effects of combination therapy with dipeptidyl peptidase-IV and histone deacetylase inhibitors in the non-obese diabetic mouse model of type 1 diabetes. Clin Exp Immunol. 2013;172:375–82. doi: 10.1111/cei.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, et al. Combining MK626, a novel DPP-4 inhibitor, and low-dose monoclonal CD3 antibody for stable remission of new-onset diabetes in mice. PLoS One. 2014;9:e107935. doi: 10.1371/journal.pone.0107935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008;149: 1338–49. doi: 10.1210/en.2007-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, et al. Exendin-4 treatment of nonobese diabetic mice increases beta-cell proliferation and fractional insulin reactive area. J Diabetes Complications. 2010;24:163–67. doi: 10.1016/j.jdiacomp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Xue S, et al. Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency. Ann NY Acad Sci. 2008;150:152–56. doi: 10.1196/annals.1447.049. [DOI] [PubMed] [Google Scholar]
- Hadjiyanni I1, Drucker DJ. Glucagon-like peptide 1 and type 1 diabetes: NOD ready for prime time? Endocrinology. 2007;148:5133–35. doi: 10.1210/en.2007-1112. [DOI] [PubMed] [Google Scholar]
- Mareschi K, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–54. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–92. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- Li L, et al. Transplantation of mesenchymal stem cells improves type 1 diabetes mellitus. Cell Tissue Res. 2016;364:345–55. doi: 10.1007/s00441-015-2330-5. [DOI] [PubMed] [Google Scholar]
- Kikodze N, et al. Cytokines and T regulatory cells in the pathogenesis of type 1 diabetes. Georgian Med News. 2013;222:29–35. [PubMed] [Google Scholar]
- Ishigame H, et al. Excessive Th1 responses due to the absence of TGF-β signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proc Natl Acad Sci USA. 2013;110:6961–66. doi: 10.1073/pnas.1304498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberi-Douraki M, Pietropaolo M, Khadra A. Continuum model of T-cell avidity: Understanding autoreactive and regulatory T-cell responses in type 1 diabetes. J Theor Biol. 2015;383:93–105. doi: 10.1016/j.jtbi.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ. Control of Regulatory T Cell Migration, Function, and Homeostasis. J Immunol. 2015;195:2507–13. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen AS, Maijenburg MW, Nolte MA, Voermans C. Organ-specific migration of mesenchymal stromal cells: Who, when, where and why? Immunol Lett. 2015;168:159–69. doi: 10.1016/j.imlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Danieli P, Malpasso G, Ciuffreda MC, Gnecchi M. Testing the Paracrine Properties of Human Mesenchymal Stem Cells Using Conditioned Medium. Methods Mol Biol. 2016;1416:445–56. doi: 10.1007/978-1-4939-3584-0_26. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123–46. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- Madec AM, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–99. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- Najafi R, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013;13:959–72. doi: 10.1517/14712598.2013.782390. [DOI] [PubMed] [Google Scholar]