Abstract
Light exposure and sleep timing are two factors that influence inter-individual variability in the timing of the human circadian clock. The aim of this study was to quantify the degree to which evening light exposure predicts variance in circadian timing over and above bedtime alone in preschool children. Participants were 21 children ages 4.5–5.0 years (4.7±0.2 years; 9 females). Children followed their typical sleep schedules for 4 days during which time they wore a wrist actigraph to assess sleep timing and a pendant light meter to measure minute-by-minute illuminance levels in lux. On the 5th day, children participated in an in-home dim-light melatonin onset (DLMO) assessment. Light exposure in the 2 h before bedtime was averaged and aggregated across the 4 nights preceding the DLMO assessment. Mean DLMO and bedtime were 19:22±01:04 and 20:07±00:46, respectively. Average evening light exposure was 710.1±1418.2 lux. Children with later bedtimes (lights-off time) had more delayed melatonin onset times (r=0.61, p=0.002). Evening light exposure was not independently associated with DLMO (r=0.32, p=0.08); however, a partial correlation between evening light exposure and DLMO when controlling for bedtime yielded a positive correlation (r=0.46, p=0.02). Bedtime explained 37.3% of the variance in the timing of DLMO, and evening light exposure accounted for an additional 13.3% of the variance. These findings represent an important step in understanding factors that influence circadian phase in preschool-age children and have implications for understanding a modifiable pathway that may underlie late sleep timing and the development of evening settling problems in early childhood.
Keywords: Light, Bedtime, Circadian phase, Melatonin, DLMO, Preschool, Children
1. Introduction
The secretion of the hormone melatonin is under circadian control via a multi-synaptic pathway between the suprachiasmatic nucleus (SCN) and the pineal gland (Kalsbeek et al., 2006, Moore and Klein, 1974). In humans, the rhythm of melatonin is the most robust marker for assessing the timing of the internal biological clock (Klerman et al., 2002, Benloucif et al., 2005). Although understanding of the circadian timing system in adolescents and adults is rapidly increasing (Crowley et al., 2006, Crowley et al., 2014, Kawinska et al., 2005, Gooneratne et al., 2003), basic fundamental knowledge of the circadian system in early childhood remains relatively scarce. Consistent with findings in adolescents and adults, our recent study revealed large inter-individual variability in the circadian timing of children ages 2.5–3.0 years (~3.5 h) (LeBourgeois et al., 2013a). Although light exposure and sleep timing may contribute to this observed variability in circadian phase, little is known about such links in early childhood.
Light is a dominant input into the circadian timing system and can alter many aspects of its physiology including the clock's timing (Khalsa et al., 2003, Czeisler et al., 1981, Duffy and Wright, 2005). Specifically, research in adults demonstrates that light exposure near habitual bedtime induces a phase delay in an intensity-dependent manner (Zeitzer et al., 2000). This non-linear relationship is such that room light (~100 lux) produces half the phase shift achieved with bright light (~9000 lux) (Zeitzer et al., 2000). Data from studies in adults experimentally manipulating the intensity of light in the hours before bedtime further demonstrate the effects of evening indoor light on circadian timing. For example, a field study examining light exposure in the 4 h before bedtime found that adult subjects had later circadian phases after a week of maximizing home lighting (~65 lux) compared to a week of dim light (~3 lux) in the hours before bedtime (Burgess and Molina, 2014). Furthermore, another study in young adults aged 18–30 years found that room lighting (<200 lux) in the 8 h before bedtime suppressed melatonin levels and shortened melatonin duration compared to exposure to dim light (<3 lux) during this sensitive time window (Gooley et al., 2011). Together, these findings demonstrate that even exposure to typical indoor light intensity before bedtime can have profound effects on the circadian timing system of humans. Similar studies examining relationships between evening light exposure and the timing of the circadian clock in preschool-age children are scarce.
Sleep timing in part determines the light/dark cycle experienced by humans, and thus, can indirectly influence circadian phase by ‘gating’ light exposure. Delayed bedtimes may provide the opportunity for light exposure later in the evening, facilitating a delay in the timing of the circadian clock (Khalsa et al., 2003). In a relatively large sample of toddlers, we observed a moderate positive relationship between sleep timing and circadian phase, such that later bedtimes were associated with later circadian timing (LeBourgeois et al., 2013a). This association is consistent with data from adolescents and adults using both observational and experimental approaches (Crowley et al., 2006, Burgess and Eastman, 2004, Burgess and Eastman, 2005, Burgess and Eastman, 2006, Burgess et al., 2003, Martin and Eastman, 2002, Wright et al., 2013). In order to demonstrate the effect of bedtime on circadian timing, one study manipulated the bedtimes of adult participants while keeping fixed wake times. Results indicated a delay in the melatonin rhythm after subjects had late compared to early bedtimes (Burgess and Eastman, 2004). These findings indicate that in addition to evening sleep timing, the intensity of light exposure in the hours before bedtime also impacts the circadian phase of humans.
Considering the wide variability in circadian timing during early childhood and the influence of ambient room light on the circadian system of humans, we examined the degree to which light exposure before bedtime influences the timing of the clock in a field setting. We focus on evening light exposure and bedtime in the present analysis because evening settling problems (i.e., bedtime resistance, delayed sleep onset) are highly prevalent in preschoolers (Lozoff et al., 1985, Bruni et al., 2000, Beltramini and Hertzig, 1983). Both bedtime and light are also modifiable intervention targets that can be altered to shift circadian timing if desired. We hypothesized that evening light exposure would predict variance in circadian phase over and above bedtime alone in our sample of preschool-age children.
2. Materials and methods
2.1. Participants
Twenty-one healthy children (9 females; 20 Caucasian, 1 Multi-Racial) ages 4.5–5.0 years (4.7±0.2 years) participated in the study. Families were recruited from Boulder, CO, USA and immediate surrounding areas via flyers and at community events. Subject screening and exclusionary criteria are described in detail in LeBourgeois et al. (2013a). Briefly, all children were healthy and normally developing and did not regularly use medications that influence sleep or the circadian system. Parents signed an informed consent form approved by the Institutional Review Board at the University of Colorado Boulder. Families received monetary compensation for completing the study.
2.2. Protocol
Children participated in a 5-day protocol, where they followed their typical sleep schedule at home for 4 days. Researchers made in-home visits during this interval to train children on providing saliva samples. On the 5th day of the study, subjects participated in a dim light melatonin onset (DLMO) assessment (described below). Children refrained from the consumption of caffeine and medications throughout the duration of the study, and researchers contacted parents daily to ensure compliance with study protocol. Data were collected between September and May excluding the week following daylight saving time changes. Five children completed the study in the Fall, 11 in the Winter months, and 5 in the Spring.
2.3. Measures
2.3.1. Actigraphy and sleep diary
Subjects wore a wrist actigraph (Actiwatch Spectrum, Phillips Respironics, Pittsburg, PA, USA) on their non-dominant wrist throughout the duration of the study to objectively assess the timing of sleep and wakefulness via motor activity. We used our standard methods for reviewing and scoring actigraphy data, as detailed in our previous publications (LeBourgeois et al., 2013a, Berger et al., 2012). Parents completed a daily sleep diary throughout the study in which they recorded information on their child's sleep times and when the actigraph was not worn (Akacem et al., 2015). We also made daily contact with parents via telephone, text, or email to ensure compliance with study rules.
2.3.2. Ambient light exposure
Participants wore a pendant light meter (Dimesimeter, Lighting Research Center, Troy, NY, USA) around their neck during periods of wakefulness for the 4 days before the circadian phase assessment. The device collects continuous lux levels in 1-minute epochs. Parents placed the pendant light meter over all their child's clothing facing forward. The device was attached upon awakening in the morning and removed during sleep (i.e. naps and at bedtime) and for baths. If the device was not being worn, parents were instructed to place it face up in the same room (e.g. nightstand for periods of sleep, bathroom counter for bath time) as near as possible to the child. Parents completed a daily light meter diary to document whether or not the device was removed during waking hours and if so where the device was during that time. Additionally, researchers confirmed that the child was wearing the device through daily contacts with parents.
Complete light meter diary data were not available for 3 children; however, these subjects were included in the present analysis based upon daily confirmation from parents that the device was worn. According to the light meter diary, 8 children did not remove the device, 6 children removed the device once, and 4 children removed it twice in the 2 h before bedtime during the 4 nights before the DLMO assessment. Light levels did not differ between children who did (n=8) and did not (n=10) remove the device (p=0.83).
2.3.3. Salivary DLMO assessment
On the 5th day of the study, children participated in an in-home salivary DLMO assessment (LeBourgeois et al., 2013a). Researchers created dim light conditions within the home by covering windows with black plastic and using dimmer switches and low wattage bulbs on existing light sources. Children entered dim light conditions of <10 lux 1 h before the first saliva sample. Children provided saliva samples every 30 min for 6 h ending 1 h after the average bedtime of the 4 preceding days. Saliva samples were obtained by having subjects chew on a dental cotton stick (Henry Schein Inc., Denver, PA, USA) for ~2 min to produce at least 2 mL of saliva. Researchers helped children rinse and clean their mouth as needed after eating at least 15 min before obtaining a saliva sample. Children sat still for 5 min before each saliva sample to control for the effects of posture on melatonin levels. Lux levels were measured in the child's angle of gaze at the time of each saliva sample (Extech Instruments, Spring Hill, FL, USA). Saliva samples were centrifuged (LabEssentials Inc., Monroe, GA, USA) and refrigerated in the child's home. Following completion of the assessment, samples were immediately transported to the laboratory where they were frozen (−20 C). Radioimmunoassay was used to quantify the amount of melatonin in each saliva sample (ALPCO Diagnostics, Salem, NH, USA). Melatonin assays were performed at Solid Phase Inc. (Portland, ME, USA) and had a minimum detection of 0.2 pg/mL. The intra-assay coefficient was 4.1% and the interassay coefficient was 6.6%.
2.3.4. Data processing and analysis
DLMO was computed as the time at which salivary melatonin levels surpassed a 4 pg/mL threshold using linear interpolation (LeBourgeois et al., 2013a, Akacem et al., 2015, Simpkin et al., 2014, Carskadon et al., 1997, Deacon and Arendt, 1994). For the present analysis, we averaged light exposure in the 2 h before actigraphically scored bedtime and then aggregated the data across the 4 nights before the circadian phase assessment. Lux levels in the 2 h before bedtime were averaged using MATLAB (MathWorks, Natick, MA, USA) and were log transformed due to a positively skewed distribution.
One-tailed Pearson correlations were used to (a) confirm the relationship between bedtime and DLMO observed in our previous work (LeBourgeois et al., 2013a) and (b) assess associations between evening light exposure and DLMO and bedtime. A partial correlation was performed between evening light exposure and DLMO while controlling for bedtime, and a hierarchical linear regression was used to quantify how much variance in DLMO was predicted by bedtime (step 1) and evening light exposure (step 2). All statistical analyses were performed in SPSS Statistics (IBM Corp., Armonk, NY, USA). All descriptive statistics are presented as M±SD.
3. Results
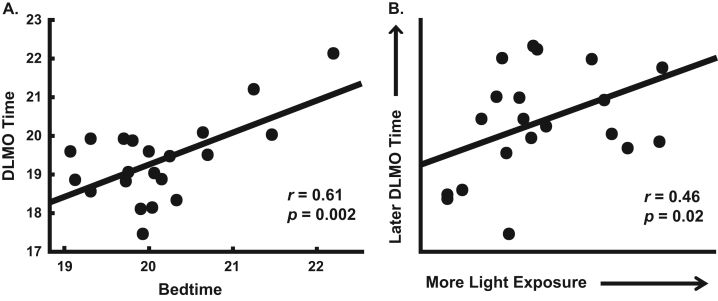
Mean DLMO occurred at 19:22±01:04, bedtime was 20:07±00:46, and light exposure in the 2 h before bedtime across the 4 evenings preceding the circadian phase assessment was 710.1±1418.2 lux. As expected, children with later bedtimes had later circadian phases (r=0.61, p=0.002; Fig. 1A). Light exposure before bedtime was not independently associated with DLMO (r=0.32, p=0.08) or bedtime (r=−0.07, p=0.38); however, a partial correlation between evening light exposure and DLMO while holding bedtime constant yielded a significant positive association (r=0.46, p=0.02; Fig. 1B).
Fig. 1.
Scatterplots of the association between bedtime and DLMO (A) and of evening light exposure and DLMO after controlling for bedtime (B; partial correlation, residuals displayed). Lines show the slope of the regression line.
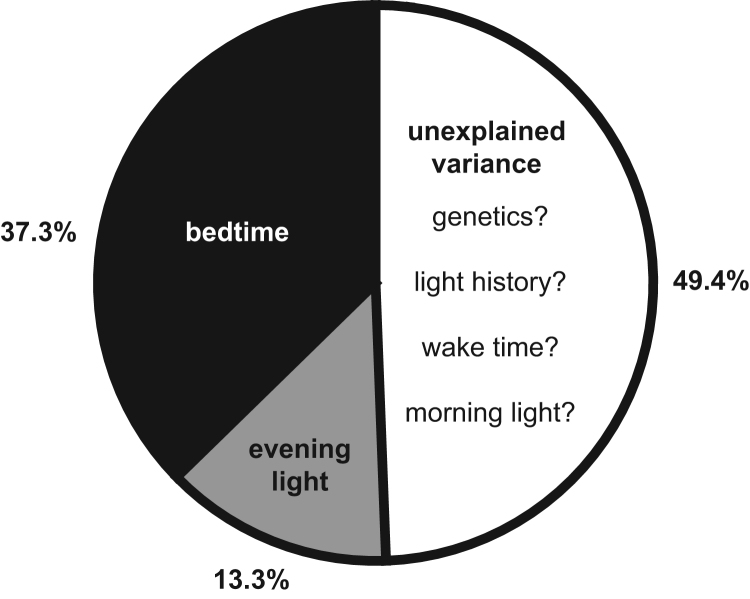
In a hierarchical linear regression, bedtime explained 37.3% of the variance in the timing of DLMO. Adding evening light exposure to the model accounted for 13.3% more variance in DLMO in addition to bedtime (Table 1 and Fig. 2).
Table 1.
Hierarchical linear regression results.
| Step | Factor | R2 | R2Change | F Change | Sig. F Change |
|---|---|---|---|---|---|
| 1 | Bedtime | 0.37 | 0.37 | 11.30 | 0.003 |
| 2 | Evening Light Exposure | 0.51 | 0.13 | 4.85 | 0.041 |
Fig. 2.
Visual depiction of variance in circadian timing predicted by bedtime and evening light exposure (n=21; ages 4.5–5.0 years). Forty-nine percent of the variance in dim light melatonin onset (DLMO) timing was unaccounted for but may be due to additional factors such as genetics, light history, wake time and morning light.
4. Discussion
The results of this field study indicate that the amount of light preschool-age children are exposed to in the 2 h before bedtime predicts variance in circadian phase over and above bedtime alone. Consistent with our previous findings (LeBourgeois et al., 2013a), we observed a positive association between bedtime and DLMO, such that later bedtimes were associated with later circadian phases. After controlling for bedtime, evening light exposure was associated with DLMO. That is, bedtime predicted 37.3% of the variance in DLMO; however, after adding evening light exposure into the model, we accounted for a total of 50.6% (13.3% more) of the variance in circadian timing. Together, these findings demonstrate an important step in understanding relative importance of two modifiable factors – bedtimes and light – that can influence circadian timing in early childhood.
Our findings have implications for understanding how late evening sleep timing may develop and persist during the early childhood years. Evening light exposure and late bedtimes delay the timing of the clock (Khalsa et al., 2003, Zeitzer et al., 2000, Burgess and Eastman, 2004), thus, chronic exposure to light during the evening hours and late bedtimes together may interact to facilitate the development of a late sleep phenotype early in life. This type of sleep pattern is a significant risk factor for a host of negative health outcomes, including bedtime sleep problems, chronic medical conditions (e.g. obesity, diabetes, depression), poor cognitive function, and mood disorders (LeBourgeois et al., 2013b, Miller et al., 2014, Miller et al., 2015, Foster et al., 2013, Genzel et al., 2013, Levandovski et al., 2011, Chan et al., 2014, Roenneberg et al., 2012, Spruyt et al., 2011, Reutrakul et al., 2013). Although early childhood is a sensitive period when poor sleep patterns commonly emerge (Gregory and O'Connor, 2002, Zuckerman et al., 1987), evening light exposure and bedtime are both modifiable factors. Thus, experimental studies that promote an understanding of the effects of evening light exposure and bedtime on circadian timing are a necessary for clinicians to make evidence based recommendations regarding light at night and sleep timing for young children.
Our data suggest that evening light exposure and bedtime are both associated with circadian timing; however, these two factors together accounted for only about half (50.6%) of the variance in melatonin onset time. The remaining variance in circadian timing maybe be influenced by other important variables, including genetics and light history that were not assessed in the present study (Duffy and Wright, 2005, Smith et al., 2004, Takahashi et al., 2008). Thus, additional research in this age group that includes a more comprehensive panel of measures is needed to promote a better and more accurate understanding of the relative importance circadian timing determinants.
In addition to delaying the timing of the clock, evening light exposure also causes an immediate suppression of the hormone melatonin (Lewy et al., 1980). Naturally, melatonin levels begin to rise in the evening hours before bedtime; however, exposure to light in the early biological night prevents the secretion of this hormone. Considering the diverse role of melatonin in physiological processes such as glucose homeostasis (Owino et al., 2016, la Fleur et al., 2001), thermoregulation (Saarela and Reiter, 1994), blood pressure (Simko and Paulis, 2007) and sleep promotion (Zhdanova et al., 1997), chronic suppression of the hormone melatonin likely has negative consequences for overall health and well-being, even in early childhood.
Understanding the effects of evening light on the circadian system in the early years of life is especially important because young children are proposed to be more sensitive to the circadian effects of light than adults. The clear crystalline lens and large pupil size of young children may render them more sensitive to the effects of evening light than adults (Turner and Mainster, 2008, Charman, 2003). One recent study comparing melatonin suppression in response to light at night found that the percentage of melatonin suppression in school-age children was almost twice that of the adults (Higuchi et al., 2014). Furthermore, work from our laboratory has shown that 1 h of bright light exposure (~1000 lux) in the hour before bedtime induces ~90% suppression of melatonin in preschool age children (Akacem et al., 2016). The melatonin levels of these preschoolers remained attenuated for up to 50 min after the light stimulus terminated, thus, demonstrating the robust effects of evening light in young children. Together, these findings are important in the context of our previously published data showing that toddlers with later circadian phases exhibited longer sleep onset latencies and more bedtime resistance (LeBourgeois et al., 2013b). By influencing both the timing and levels of melatonin, we believe that evening light exposure may impair a child's physiological readiness for sleep (i.e. settling down after lights-off) and their ability to fall asleep. The high prevalence (~30%) of evening sleep problems following lights-off time in early childhood (Lozoff et al., 1985, Bruni et al., 2000, Beltramini and Hertzig, 1983) suggests a critical need for evidence-based recommendations on evening light exposure for children.
Light exposure at night is emerging as a serious health concern (Schernhammer and Schulmeister, 2004, Stevens et al., 2013, Cho et al., 2015). Before electrical lighting, the light exposure of humans was limited to the hours between dusk and dawn. With the introduction of electrical lighting and light-emitting electronic devices, humans now "construct" their own light/dark cycle and extend exposure to light far beyond dusk. This pattern of light/dark exposure influences the melatonin rhythm, which in turn communicates photoperiod length to the rest of the body (Wehr, 1998, Wehr et al., 1993). Thus, not only does light exposure at night delay the clock and suppress melatonin, constantly extending light exposure past dusk deceives the body into maintaining a perpetual summer-like photoperiod. These artificial or constructed photoperiods effectively decouple human circadian physiology from the natural 24-h light dark cycle (Czeisler, 2013). A recent study investigating the impact of a constructed light/dark environment on circadian physiology found that subjects had later circadian phases after 1-week of living in a constructed light environment (i.e. freely able to control lights on/lights off times) compared to after 1-week of camping when subjects were strictly exposed to natural light. Additionally, this study found that subjects were exposed to significantly more light after sunset following a week of living in the real world compared to camping (Wright et al., 2013). Studies are needed to understand the effects of living in a chronically extended photoperiod in the early years of life on future health and development.
Certain limitations of the present study are worth noting. First, we studied a relatively small sample size. A larger sample of children would provide the opportunity to more accurately estimate the amount of variance predicted in circadian phase by bedtime and evening light exposure, and studying children longitudinally may provide key insights into developmental changes in evening light sensitivity. Additionally, mean light exposure in the present sample (710.1 lux) is relatively higher than what has been published in adults (e.g. 40–617 lux; (Burgess and Eastman, 2004, Santhi et al., 2012, Scheuermaier et al., 2010). The latter could be due to children going to bed closer to sunset than adults and thus they may have been exposed to sunlight in the 2 h prior to bedtime. Larger scale studies are necessary to determine whether this relatively high average is representative of evening light exposure for this young age group in this geographical location. Furthermore, only healthy preschoolers were included in this study, thus, our findings may not be generalizable to the general population. Lastly, our observed light exposure and sleep behaviors may have been influenced by the fact that parents knew that we were measuring these variables. Future studies should examine light exposure in relationship with circadian timing in a large community or population based sample.
In summary, this study represents an important step in understanding factors that contribute to the observed wide variability in circadian timing during early childhood. Our findings indicate that evening light exposure is important in predicting a significant portion of the observed variability in circadian timing in this age group. Additional studies are needed to determine the degree to which other factors account for the unexplained variance, including genetics, morning light exposure, wake time, and prior light history. Evidence based recommendations for evening light exposure are critical for promoting healthy sleep and development across early childhood and beyond.
Contributions
LDA, MKL, and KPW designed the study; LDA collected the data; LDA analyzed the data; LDA, MKL, and KPW wrote the paper; all authors approved the final manuscript.
Conflicts of interest
MKL: Speaker honorarium from Integrated Listening Systems. KPW: Funding from Philips Inc., Torvec Inc., and PAC-12; Consulting fees or served as a paid member of scientific advisory boards for NIH, Torvec Inc.; Speaker honorarium from American College of Chest Physicians, The Obesity Society, Obesity Medicine Association.
Acknowledgements
We are grateful to the children and their families for participating in this study. We would also like to thank the staff and students of the Sleep and Development Laboratory who collected these data. Many University of Colorado Boulder undergraduate students received support from the Biological Sciences Initiative, the Undergraduate Research Opportunity Program, and the Howard Hughes Medical Institute. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780). Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund. This work was supported by the National Institutes of Health (R01-MH08656).
References
- Akacem L.D. The timing of the circadian clock and sleep differ between napping and non-napping toddlers. PLoS One. 2015;10(4):e0125181. doi: 10.1371/journal.pone.0125181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akacem L.D. Evening bright light suppresses melatonin in preschool age children. Sleep. 2016;39:A49–A50. [Google Scholar]
- Beltramini A.U., Hertzig M.E. Sleep and bedtime behavior in preschool-aged children. Pediatrics. 1983;71(2):153–158. [PubMed] [Google Scholar]
- Benloucif S. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. Rhythm. 2005;20(2):178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- Berger R.H. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J. Sleep. Res. 2012;21(3):235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni O. Daytime behavioral correlates of awakenings and bedtime resistance in preschool children. Suppl. Clin. Neurophysiol. 2000;53:358–361. doi: 10.1016/s1567-424x(09)70181-3. [DOI] [PubMed] [Google Scholar]
- Burgess H.J., Eastman C.I. The dim light melatonin onset following fixed and free sleep schedules. J. Sleep. Res. 2005;14(3):229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.J., Eastman C.I. A late wake time phase delays the human dim light melatonin rhythm. Neurosci. Lett. 2006;395(3):191–195. doi: 10.1016/j.neulet.2005.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.J., Eastman C.I. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci. Lett. 2004;356(2):115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.J., Molina T.A. Home lighting before usual bedtime impacts circadian timing: a field study. Photochem. Photobiol. 2014;90(3):723–726. doi: 10.1111/php.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.J. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav. Sleep. Med. 2003;1(2):102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A. An approach to studying circadian rhythms of adolescent humans. J. Biol. Rhythm. 1997;12(3):278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Chan J.W. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. doi: 10.5665/sleep.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman W.N. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol. Opt. 2003;23(2):181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Cho Y. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015;32(9):1294–1310. doi: 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- Crowley S.J. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29(12):1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- Crowley S.J. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler C.A. Entrainment of human circadian rhythms by light-dark cycles: a reassessment. Photochem. Photobiol. 1981;34(2):239–247. [PubMed] [Google Scholar]
- Czeisler C.A. Perspective: casting light on sleep deficiency. Nature. 2013;497(7450):S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- Deacon S., Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci. Lett. 1994;167(1–2):191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- Duffy J.F., Wright K.P., Jr. Entrainment of the human circadian system by light. J. Biol. Rhythm. 2005;20(4):326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- la Fleur S.E. Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night-time glucose concentrations. J. Neuroendocrinol. 2001;13(12):1025–1032. doi: 10.1046/j.1365-2826.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- Foster R.G. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog. Mol. Biol. Transl. Sci. 2013;119:325–346. doi: 10.1016/B978-0-12-396971-2.00011-7. [DOI] [PubMed] [Google Scholar]
- Genzel L. Sleep timing is more important than sleep length or quality for medical school performance. Chronobiol. Int. 2013;30(6):766–771. doi: 10.3109/07420528.2012.763132. [DOI] [PubMed] [Google Scholar]
- Gooley J.J. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011;96(3):E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne N.S. The validity and feasibility of saliva melatonin assessment in the elderly. J. Pineal Res. 2003;34(2):88–94. doi: 10.1034/j.1600-079x.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- Gregory A.M., O'Connor T.G. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(8):964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Higuchi S. Influence of light at night on melatonin suppression in children. J. Clin. Endocrinol. Metab. 2014;99(9):3298–3303. doi: 10.1210/jc.2014-1629. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythm. 2006;21(6):458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kawinska A. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J. Biol. Rhythm. 2005;20(5):451–460. doi: 10.1177/0748730405280248. [DOI] [PubMed] [Google Scholar]
- Khalsa S.B. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman E.B. Comparisons of the variability of three markers of the human circadian pacemaker. J. Biol. Rhythm. 2002;17(2):181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- LeBourgeois M.K. Circadian phase and its relationship to nighttime sleep in toddlers. J. Biol. Rhythm. 2013;28(5):322–331. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois M.K. Dissonance between parent-selected bedtimes and young children’s circadian physiology influences nighttime settling difficulties. Mind Brain Educ. 2013;7(4):234–242. doi: 10.1111/mbe.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011;28(9):771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Lewy A.J. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lozoff B., Wolf A.W., Davis N.S. Sleep problems seen in pediatric practice. Pediatrics. 1985;75(3):477–483. [PubMed] [Google Scholar]
- Martin S.K., Eastman C.I. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol. Int. 2002;19(4):695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Miller A.L. Sleep timing moderates the concurrent sleep duration-body mass index association in low-income preschool-age children. Acad. Pediatr. 2014;14(2):207–213. doi: 10.1016/j.acap.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Lumeng J.C., LeBourgeois M.K. Sleep patterns and obesity in childhood. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22(1):41–47. doi: 10.1097/MED.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.Y., Klein D.C. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71(1):17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- Owino S. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS One. 2016;11(1):e0148214. doi: 10.1371/journal.pone.0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T. Social jetlag and obesity. Curr. Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Saarela S., Reiter R.J. Function of melatonin in thermoregulatory processes. Life Sci. 1994;54(5):295–311. doi: 10.1016/0024-3205(94)00786-1. [DOI] [PubMed] [Google Scholar]
- Santhi N. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 2012;53(1):47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- Schernhammer E.S., Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br. J. Cancer. 2004;90(5):941–943. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermaier K., Laffan A.M., Duffy J.F. Light exposure patterns in healthy older and young adults. J. Biol. Rhythm. 2010;25(2):113–122. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko F., Paulis L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007;42(4):319–322. doi: 10.1111/j.1600-079X.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Simpkin C.T. Chronotype is associated with the timing of the circadian clock and sleep in toddlers. J Sleep. Res. 2014;23(4):397–405. doi: 10.1111/jsr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.A., Schoen M.W., Czeisler C.A. Adaptation of human pineal melatonin suppression by recent photic history. J. Clin. Endocrinol. Metab. 2004;89(7):3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Spruyt K., Molfese D.L., Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127(2):e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R.G. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am. J Prev. Med. 2013;45(3):343–346. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Takahashi J.S. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P.L., Mainster M.A. Circadian photoreception: ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr T.A. Effect of seasonal changes in daylength on human neuroendocrine function. Horm. Res. 1998;49(3–4):118–124. doi: 10.1159/000023157. [DOI] [PubMed] [Google Scholar]
- Wehr T.A. Conservation of photoperiod-responsive mechanisms in humans. Am. J. Physiol. 1993;265(4 Pt 2):R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wright K.P., Jr. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013;23(16):1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer J.M. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova I.V., Lynch H.J., Wurtman R.J. Melatonin: a sleep-promoting hormone. Sleep. 1997;20(10):899–907. [PubMed] [Google Scholar]
- Zuckerman B., Stevenson J., Bailey V. Sleep problems in early childhood: continuities, predictive factors, and behavioral correlates. Pediatrics. 1987;80(5):664–671. [PubMed] [Google Scholar]