Abstract
An essential feature of the brain is its capacity to change. Neuroscientists use the term “plasticity” to describe the malleability of neuronal connectivity and circuitry. How does plasticity work? A review of current data suggests that plasticity encompasses many distinct phenomena, some of which operate across most or all of the lifespan, and others that operate exclusively in early development. This essay surveys some of the key concepts related to neural plasticity, beginning with how current patterns of neural activity (e.g., as you read this essay) come to impact future patterns of activity (e.g., your memory of this essay), and then extending this framework backward into more development-specific mechanisms of plasticity.
Keywords: Plasticity, development, topographic maps, brain
Introduction
An essential feature of the brain is its capacity to change. Neuroscientists use the term “plasticity” to describe the malleability of neuronal connectivity and circuitry. Physical changes at a cellular level manifest as circuit-level changes in patterns of neuronal firing, and it is these circuit-level changes that allow us to learn, to remember, and to adapt to changing conditions of the body and environment. These physical changes in neuronal structure result from a combination of the very thoughts we have (i.e., prior patterns of neural firing), as well as genetic and biochemical influences.
How does plasticity work, precisely, and how much of it continues into adulthood? A review of current data suggests that plasticity encompasses many distinct phenomena, some of which operate across most or all of the lifespan, and others that operate exclusively in early development. This essay will survey some of the key concepts related to neural plasticity, beginning with how patterns of current firing (e.g., as you read this essay) come to impact future patterns of firing (e.g., your memory of this essay), and then extending this framework backward into more development-specific mechanisms of plasticity. For brevity, some oversimplification is necessary; see Kandel et al.1 and the indicated reviews for more detailed accounts of the material in this essay.
To understand how the brain changes, it is first necessary to know a few facts about its structure. The human brain is an immense network of about 90 billion special cells called neurons that are able to receive and transmit electrical activity on the scale of a few milliseconds. Each neuron in this network receives excitatory or inhibitory signals from tens to thousands of other neurons, processes that information (by adding the incoming signals together over brief windows of time), and sends its own impulses to tens to thousands of other neurons. The portions of neurons that receive signals are called dendrites, the portions that send signals are called axons, and the portion between the dendrites and axons is called the cell body (Figure 1). When a neuron connects to another neuron, it does so via a synapse (from the presynaptic cell's axon to the postsynaptic cell's dendrite). Typically, it takes the nearly simultaneous reception of many positive impulses to cause a neuron to fire – a few isolated positive impulses will usually not be sufficient to drive a neuron's response. Further, to fire, the cumulative excitatory signals must outweigh the cumulative inhibitory signals. A neuron's firing is therefore not generally a matter of chance, but instead represents the temporal co-occurrence of many other events; how those incoming events are distributed spatially along the neuron also matters.
Figure 1.
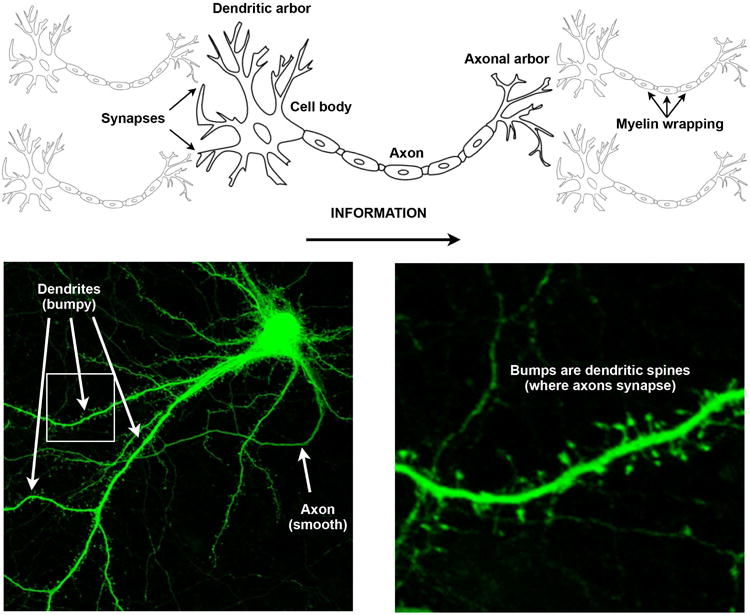
Neurons, their parts, their actual complexity. Top, a schematic view of several neurons, illustrating the major parts of neurons and the direction of information flow. Bottom, microscopic images of a single neuron. This neuron has extensive dendrites with thousands of spines representing possible inputs from other neurons. The neuron also has a prominent cell body, and a smooth axonal process that leads down and left from the cell body. Each dendritic section may receive inhibitory and excitatory signals, and the neuron only fires if excitatory signals, within a small time window, sufficiently outweigh inhibitory signals. Images of neurons courtesy of Austen Milnerwood and the Centre for Applied Neurogenetics at the University of British Columbia. With permission from Austen Milnerwood of the Centre for Applied Neurogenetics, University of British Columbia.
A neuron's firing can represent very specific information, and the coactivity of many neurons provides a rich and detailed description of information. Patterns of activity in the massive network of the entire brain give rise to our experience as humans: our perceptions, our thoughts, our emotions, our actions, and our memories. Plasticity, a general description of how the brain changes, encompasses both the mechanisms that give this network its overarching structure during development and the mechanisms that sculpt the network's architecture during normal experience to enable learning, memory, and proper functioning.
Hebbian Plasticity
Perhaps the most famous form of plasticity is Hebbian plasticity, so named because it was the cornerstone of Donald Hebb's theory of cognition and learning developed in the 1940s2. Hebb proposed that organized activity within a brain circuit could be sustained, and, importantly, re-implemented in the future, if 1) neurons were organized so that a circuit could be self-exciting and therefore sustain its own activity for some period of time and 2) if connections between neurons were selectively strengthened between co-active neurons. This idea is often summarized by the phrase “neurons that fire together wire together.” Hebb's ideas predated the evidence for Hebbian plasticity by decades, but his theories now have extensive empirical support and underpin our current understanding of development, learning, and memory. For example, when an excitatory presynaptic neuron fires milliseconds before a postsynaptic neuron fires, the synapse connecting those neurons is physically enlarged and stocked with the chemicals needed to communicate across that synapse, making the synapse more effective for future communication (Figure 2). Similar mechanisms can even cause the formation of additional synapses between the neurons. Conversely, when a presynaptic neuron fires milliseconds after a postsynaptic neuron, the synapse is weakened. When two neurons fire without relation to each other, synapses between the neurons are neither strengthened nor weakened. Remarkably, even though a neuron may have many thousands of synapses, the adjustment of synaptic strength is synapse-specific. These mechanisms (and others: see Feldman3 for review) tune the strength of trillions of synapses throughout the brain, altering circuit-level patterns of neuronal firing over the network.
Figure 2.
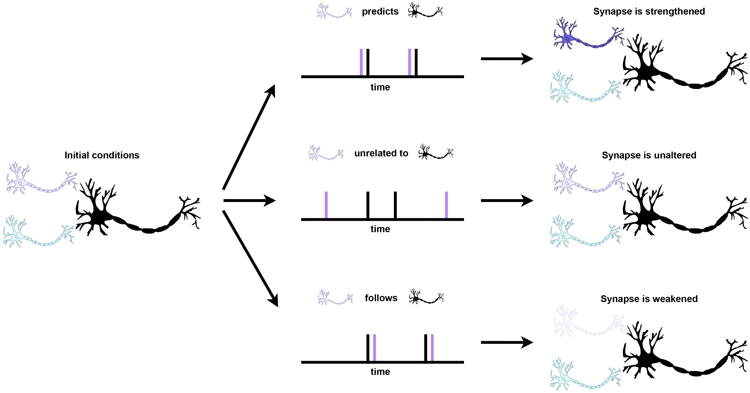
Spike-timing-dependent tuning of synaptic strength. At left, a 3-neuron system in an initial state. If the purple neuron fires milliseconds before the black neuron, the synapse is selectively strengthened. If the purple neuron fires independently of the black neuron, the synapse is unaltered. If the purple neuron fires milliseconds after the black neuron, the synapse is selectively weakened.
To illustrate this plasticity more concretely: As you read these words, ensembles of neurons are activated, and among these neurons some synapses are being strengthened, others weakened. Throughout the rest of the day, and tonight as you sleep, those ensembles will be selectively reactivated (without your awareness), reinforcing, editing, and augmenting the earlier patterns of activity. When you wake up tomorrow, your synapses will be physically changed, and in the future when you recall this information, it will occur in part by reactivating the selective set of neurons you are assembling this very moment to read this essay. In this way, we can see that plasticity occurs over many timescales: Events occurring over milliseconds cause structural changes that become apparent over hours and days, and these structural changes alter brain activity in ways that can be maintained as memories for years or even an entire lifetime.
Changes In Representational Maps
The types of synaptic changes just mentioned, even though they underlie critical functions like learning and memory, may seem like merely fine-tuning connections within an established network. But the brain also exhibits dramatic, larger-scale changes in organization in response to experience.
The brain has several scales of spatial and functional organization. To give just one example: In primary visual cortex, neurons are vertically organized into local circuits called columns (with a lateral scale of dozens of microns; 1 cm = 10,000 microns). These columns are organized into groups of related columns (with a lateral scale of hundreds of microns), and a large number of these columns, each specialized for analyzing particular locations and aspects of the visual world, make up the primary visual cortex (an area spanning centimeters). Such hierarchical organization is found not only in the primary visual area, but also in other parts of the cortex.
One of the larger organizational scales of the brain is that the cortex is organized into areas, much like a quilt has patches. The primary visual cortex just mentioned is one such area. Each of these areas has particular functions. For example, roughly midway between the front and the back of the brain are several strip-like areas dedicated to motor control and to sensation from the skin, muscles, and joints. These areas contain maps of the body, whereby brain tissue is functionally connected to a particular part of the body. Remarkably, these maps are organized such that neurons representing the foot sit next to neurons representing the lower leg, which sit next to neurons representing the upper leg, and so forth. Such topographic organization of these maps exists in many parts of the brain: maps of the body in sensory and motor areas, maps of the retina in visual areas, and maps of frequency in auditory areas, among others. We will focus here on changes in maps of the body.
One of the most important demonstrations of adult plasticity came from experiments performed in monkeys. These experiments showed that maps of the body are readjusted to reflect experience. For example, if sensation is lost in several fingers of a hand due to peripheral nerve damage, within a matter of weeks representation of the intact fingers will take over the cortical space originally occupied by the now-damaged fingers4. Or, if the skin of two fingers is fused, such that the fingers now always share positions and often sensations, distinct sensory representations of each finger are lost in favor of a single representation for the fused fingers5. More benign interventions such as simply stimulating particular digits and not others yields “use-dependent” shifts in the maps, such that portions of digits stimulated more frequently come to occupy larger portions of the cortical representation (Figure 3)6. In humans, a similar experiment was performed using neuroimaging, with similar results: in musicians that play stringed instruments, sensory representations of the left digits (which finger the strings) are larger than the representations found in typical people7. Interestingly, the enlargement was specific to the left hand, was less in the thumb (which is used only to hold the neck of the instrument), and the amount of enlargement was related to the age at which the musician began playing! These experiments (and others, see Buonoman and Merzenich8 for review) highlight the adult brain's capacity to adapt to changes in the body and environment.
Figure 3.
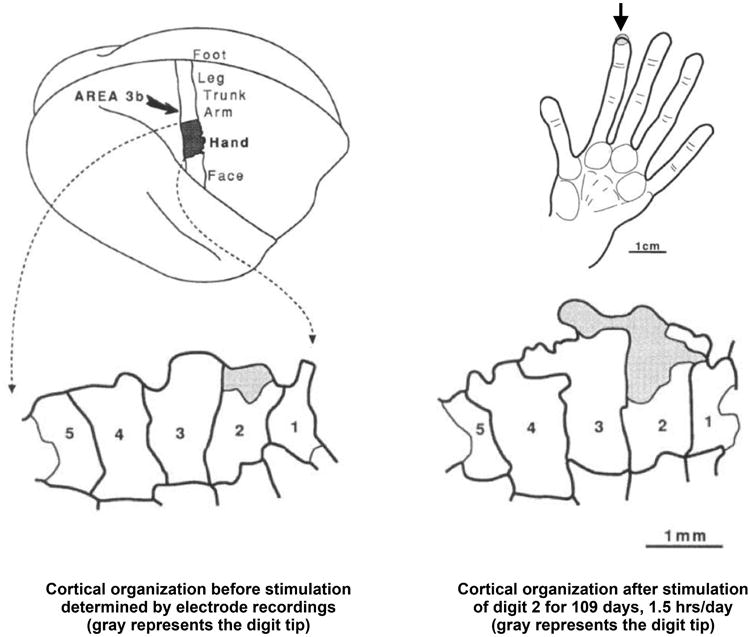
Plasticity in adult cortical finger representations in the monkey. At top, a monkey brain is shown where the strip indicates the primary somatosensory area. This area has a map of the body, part of which is a map of the hand. The representation of the tips of the second digit before and after intense and prolonged stimulation is shown in gray shading. Stimulation selectively enlarges the representation of the manipulated skin. Modified from Jenkins et al6.
Critical (or Sensitive) Periods
Nearly all children learn their native language effortlessly, with appropriate grammar and pronunciation. Others who come to a language later in life have difficulty mastering grammar, pronouncing words, and acquiring vocabulary – the process has become laborious and fundamentally limited. From a neural perspective, this indicates that there is a window of time during which the brain is especially equipped to gain facility with language, after which the window closes and learning a new language becomes very difficult. This window of opportunity is referred to as a critical or sensitive period.
Another well-studied critical period involves the development of vision (see chapter by Maurer and Lewis in Steeves and Harris9). If a child is born with a cataract (a clouded eye lens) that is not corrected within the first postnatal months, the child will suffer degraded or absent vision in that eye, even if the cataract is eventually removed10. In contrast, an adult with normal vision who develops a cataract will enjoy a full restoration of vision when the cataract is removed, no matter how long the adult waits until having the cataract removed10. Thus, there is something special about the initial months of visual stimulation in setting up neural circuitry, and if this stimulation is absent, the lack of early experience cannot be compensated for later in life.
Studies in cats and monkeys have partially revealed the basis for this phenomenon. Neurons in the primary visual area of cortex typically receive input from the left eye, the right eye, or in many cases both eyes. In this primary visual cortex, neurons are spatially segregated in “ocular dominance columns” based on which eye supplies most of their inputs. In a normal animal, each eye makes a roughly equal number of projections to neurons, revealed by ocular dominance columns of roughly equal width and extent (Figure 4, left). In an animal in which one eye is occluded soon after birth, however, the unoccluded eye acquires a disproportionate cortical representation (Figure 4, right) and projects much more widely at the expense of the occluded eye's projections11,12. If this occlusion occurs during the critical period, depending on the duration of occlusion, this change may be fully reversible, partially reversible, or irreversible. If the occlusion occurs after the critical window passes, the ocular dominance columns are unaffected and vision is unimpaired13,14. These animal experiments help to explain the differential effects of congenital and adult-acquired cataracts in humans.
Figure 4.
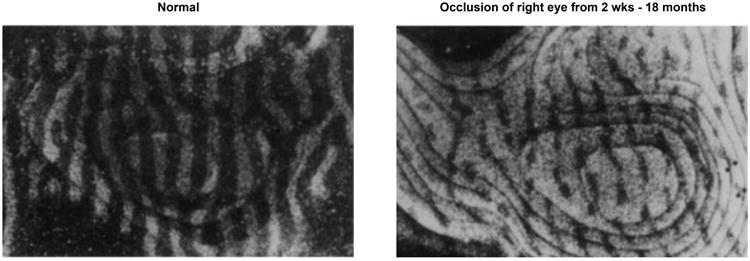
Ocular dominance columns plasticity in macaques. Sections through the visual cortex of macaques. Radiolabeled tracer (white) was injected into one eye prior to sectioning, labeling neurons receiving input from that eye. At left, a normal monkey exhibits ocular dominance columns of equal size. At right, a monkey with the right eye occluded since 2 weeks of age has much larger left eye representations (white, unoccluded) than right eye representations (black, occluded). Modified from Hubel et al.30.
The factors that govern the opening and closing of critical windows are partially understood (see Levelt and Hübener15 or Bavelier et al.16 for review). Functioning inhibitory circuits are needed to open some critical periods17, as are working mechanisms of synaptic tuning such as those mentioned above. The closing of critical periods may involve the physical stabilization of synapses and network structure by myelin (a fatty substance wrapped around the axons of neurons, providing insulation and increasing the speed of neural conduction, see the myelin-wrapped segments in Figure 1) or extracellular structures. It is hoped that a better understanding of the mechanisms governing critical periods will lead to better treatments for brain disorders. For instance, it might be possible to use antidepressants to reopen ocular dominance plasticity to treat visual disorders18.
Establishing The Patterning and Connectivity of The Cortex
We now turn to the earliest stages of brain development and the processes that govern how the brain grows from primitive embryonic structures of a few hundred cells into a richly structured network. In contrast to earlier types of plasticity, which transpired mainly over an “already-wired” network, we now focus on how the network becomes functionally wired in the first place.
For many years neuroscientists debated whether the signals that guided the development of the nervous system were primarily activity-independent (i.e., genetic or molecular) or primarily activity-dependent (influenced by the firing of neurons, especially in response to environmental stimuli). It is now accepted that both types of signals are critical to the developing nervous system, and moreover, that both types of signals can influence each other (see Rakic et al.19 and O'Leary et al.20 for review).
As the nervous system develops from a primitive tube of neural cells into a functionally subdivided system (see Darnell and Gilbert in this series), gradients of molecular markers (e.g., from front to back or top to bottom) trigger gene expression patterns that guide undifferentiated cells into particular differentiation pathways (e.g., into becoming the spinal cord vs. the brain, or the back of the brain vs. the front of the brain). Although the ability of relatively smooth gradients to produce sharp transitions in cellular identity may seem counterintuitive, feedback loops triggered by interactions of signaling molecules and transcription factors are actually adequate to establish step-like changes in cell differentiation fates. Experimentally we now know that loss of these regulatory factors can greatly alter the layout of areas in the cerebral cortex. The top row of Figure 5 shows the gradients of expression of several transcription factors that lead to the establishment of cortical areas such as motor cortex, sensory cortex, auditory cortex, and visual cortex. Genetic deletion of these factors alters the layout of cortical areas, as shown in the bottom row of the figure. These processes are operative very early in development, well before sensory input (i.e., incoming activity from the eyes, ears, etc.) can influence areal patterning.
Figure 5.
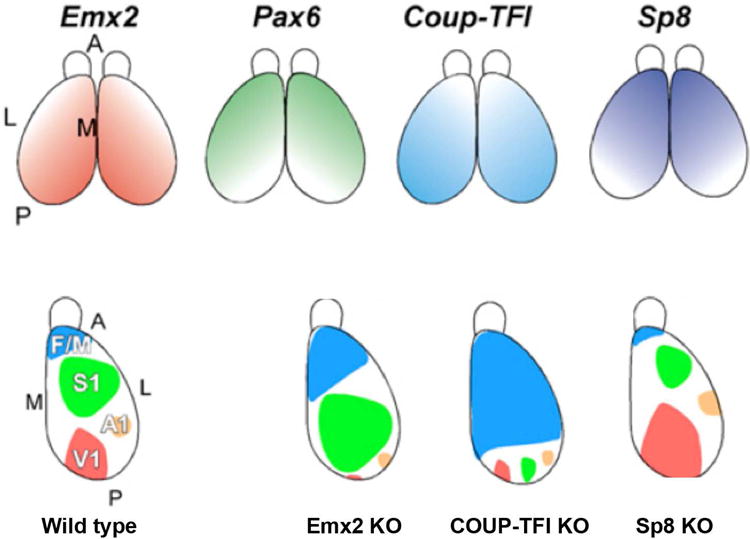
Signal gradients influence expression of transcription factors that regulate cortical area patterning. At top, 4 transcription factors present in gradients that function in establishing cortical patterning in the mouse brain. At bottom left, a diagrammatic illustration of several cortical areas in a normal mouse. At bottom right, the patterning in “knock-out” (KO) mice in which various transcription factors are no longer expressed. Loss of these factors greatly alters the layout of cortical areas in the knock-out mice. V1 denotes visual cortex, A1 auditory cortex, S1 somatosensory cortex, and F/M motor cortex. A denotes anterior, P posterior, L lateral, M medial. Modified from O'Leary et al.20.
At early stages of development, sensory input cannot influence brain development for the simple reason that the wiring is literally not yet present to connect sensory organs to the cortex. And yet, recent evidence indicates that neural activity, even at such early stages of development, is present and necessary for certain aspects of proper neural wiring. This neural activity is organized but spontaneous (i.e., it is intrinsic to the circuitry and does not result from external stimulation, see Blankenship and Feller21 for review). As an example, spontaneous bursts of depolarization in the spinal cord are necessary for correct innervation of muscles by nerves, and altering the frequency of these depolarizations causes axons to innervate the wrong muscles. It is thought in this case that spontaneous activity works through the molecular mechanisms mentioned above to influence axon guidance. Or, as another example, slightly later in development, once the eye is physically wired to the cortex but before eye-opening and patterned visual experience, spontaneous waves of activity pass over the retina, and this spontaneous activity is needed to correctly establish retinotopic maps, ocular dominance columns, and other characteristics of visual cortex (see Ackman and Crair22 for review).
At later stages of development, neural activity strongly influences neural tissue fate. For example, input from facial whiskers is needed for full differentiation of rodent somatosensory cortex23. And, if shortly after birth a piece of visual cortex is transplanted to somatosensory cortex in rodents, the transplanted tissue loses many features of visual cortex and gains features of somatosensory cortex24. More recently it has been found that although molecular gradients specify a broad occipital visual field, that visual stimulation must be targeted to what will become the primary visual area to cause the primary visual area to correctly differentiate from the rest of the occipital visual field (and for the rest of the visual field to correctly become the higher-order visual areas)25. Another important demonstration of how neural activity guides tissue fate comes from experiments in which inputs from the eye are redirected from visual to auditory cortex. This rewiring causes auditory cortex to become responsive to visual stimuli and to develop many of the properties of visual cortex, including very specific characteristics such as orderly orientation tuning (something not normally found in auditory cortex)26. Another example is within the sensorimotor system, where sensory feedback arising from the limb twitches that are characteristic of rapid eye movement (REM) sleep are thought to contribute to the development of sensorimotor maps27. Thus, molecular gradients, spontaneous neural activity, and peripherally driven neural activity are all critical for proper development of the brain.
Conclusion
We have surveyed several ways in which the brain is plastic, beginning with mechanisms that are operating this very second to encode your present experience, tracing back to critical periods that are important in the first months and years of postnatal life, and then mentioning mechanisms that operate very early in development. The history of neural plasticity research is one of surprises. Decades ago, large-scale reorganization of the adult brain was considered impossible. We now know differently. Similarly, it was also long thought that no new neurons were born in an adult brain. We now know that in at least some areas of the human brain, new neurons are born28. Progress in the creation and manipulation of neural stem cells, in combination with advances in molecular biology, may mean that developmental plasticity once thought to be confined to early development may in the future have relevance for adulthood, senescence, and perhaps some disease states (see, e.g., Miller and Hen29).
Acknowledgments
Preparation of this article was made possible in part by a grant from the National Institutes of Health (F30-MH940322) to JDP.
References
- 1.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5th. New York: McGraw-Hill Professional; 2012. [Google Scholar]
- 2.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: John Wiley and Sons, Inc; 1949. [Google Scholar]
- 3.Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 5.Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 8.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 9.Steeves JKE, Harris LR. Plasticity in Sensory Systems. New York: Cambridge University Press; 2012. [Google Scholar]
- 10.Senden MV. Space and Sight: The Perception of Space and Shape in the Congenitally Blind Before and After Operation. Glencoe, Il: Free Press; 1960. [Google Scholar]
- 11.Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- 12.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 13.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 15.Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 16.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 19.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- 24.Schlaggar BL, O'Leary DD. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991;252:1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- 25.Chou SJ, Babot Z, Leingartner A, Studer M, Nakagawa Y, O'Leary DD. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma J, Angelucci A, Sur M. Induction of visual orientation modules in auditory cortex. Nature. 2000;404:841–847. doi: 10.1038/35009043. [DOI] [PubMed] [Google Scholar]
- 27.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 29.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
Further Reading
- Sammons RP, Keck T. Adult plasticity and cortical organization after peripheral lesions. Curr Opin Neurobiol. 2015;35:136–141. doi: 10.1016/j.conb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Sengpiel F. Plasticity of the visual cortex and treatment of amblyopia. Curr Biol. 2014;24:R936–940. doi: 10.1016/j.cub.2014.05.063. [DOI] [PubMed] [Google Scholar]
- Sur M, Nagakura I, Chen N, Sugihara H. Mechanisms of plasticity in the developing and adult visual cortex. Prog Brain Res. 2013;207:243–254. doi: 10.1016/B978-0-444-63327-9.00002-3. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D. Spontaneous network activity and synaptic development. Neuroscientist. 2013;20:272–290. doi: 10.1177/1073858413510044. [DOI] [PMC free article] [PubMed] [Google Scholar]


