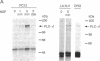Abstract
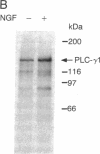
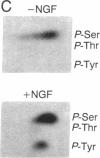
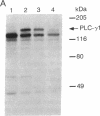
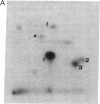
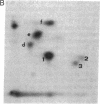
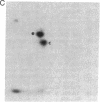
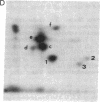
Nerve growth factor (NGF) promotes the survival and differentiation of specific populations of neurons. The molecular mechanisms by which cells respond to NGF are poorly understood, but two clues have emerged recently. First, NGF rapidly stimulates tyrosine phosphorylation of several unidentified proteins in the NGF-responsive pheochromocytoma cell line PC12 [Maher, P. (1988) Proc. Natl. Acad. Sci. USA 85, 6788-6791]. Second, the protein-tyrosine kinase encoded by the protooncogene trk (p140trk), a member of the receptor class of tyrosine kinases, becomes activated and phosphorylated on tyrosine after NGF treatment of PC12 cells [Kaplan, D. R., Martin-Zanca, D. & Parada, L. F. (1991) Nature (London) 350, 158-160]. We now report that NGF rapidly induces tyrosine phosphorylation of phospholipase C-gamma 1 (PLC-gamma 1), and we present evidence that the responsible tyrosine kinase is either p140trk or a closely associated protein. Treatment of responsive cells with NGF elicited phosphorylation of PLC-gamma 1 on tyrosine and serine. PLC-gamma 1 immunoprecipitated from NGF-stimulated cells was phosphorylated in vitro by coprecipitating protein kinase activity, and the phosphorylations occurred principally on tyrosine. The responsible kinase could be depleted from cellular lysates by antibodies specific for p140trk. This procedure also depleted a 140-kDa protein that normally coprecipitated with PLC-gamma 1 and became phosphorylated on tyrosine in vivo in response to NGF. Analysis of tryptic peptides from PLC-gamma 1 indicated that the residues phosphorylated in vitro by p140trk-associated kinase activity were largely congruent with those phosphorylated in vivo after NGF treatment. Our findings identify PLC-gamma 1 as a likely substrate for the trk-encoded tyrosine kinase, and they provide a link between NGF-dependent activation of p140trk and the stimulation of intracellular second messenger pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Contreras M. L., Guroff G. Calcium-dependent nerve growth factor-stimulated hydrolysis of phosphoinositides in PC12 cells. J Neurochem. 1987 May;48(5):1466–1472. doi: 10.1111/j.1471-4159.1987.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Cremins J., Wagner J. A., Halegoua S. Nerve growth factor action is mediated by cyclic AMP- and Ca+2/phospholipid-dependent protein kinases. J Cell Biol. 1986 Sep;103(3):887–893. doi: 10.1083/jcb.103.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Lazarovici P., Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989 Mar;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama T., Huang K. P., Guroff G. Protein kinase C as a component of a nerve growth factor-sensitive phosphorylation system in PC12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2353–2357. doi: 10.1073/pnas.83.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Huff K., End D., Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol. 1981 Jan;88(1):189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kim U. H., Fink D., Jr, Kim H. S., Park D. J., Contreras M. L., Guroff G., Rhee S. G. Nerve growth factor stimulates phosphorylation of phospholipase C-gamma in PC12 cells. J Biol Chem. 1991 Jan 25;266(3):1359–1362. [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Nerve growth factor effects and receptors in cultured human neuroblastoma cell lines. J Neurosci Res. 1982;8(2-3):375–391. doi: 10.1002/jnr.490080226. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Stemple D. L., Mahanthappa N. K., Anderson D. J. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: a sequence of events in sympathetic development. Neuron. 1988 Aug;1(6):517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Choi W. C., Lee K. Y., Rhee S. G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988 Oct 5;263(28):14497–14504. [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Moon K. H., Suh H. W., Rhee S. G. Inositol phospholipid-specific phospholipase C: complete cDNA and protein sequences and sequence homology to tyrosine kinase-related oncogene products. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5419–5423. doi: 10.1073/pnas.85.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R. The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Damsky C. H., Reichardt L. F. Purification and characterization of mammalian integrins expressed by a rat neuronal cell line (PC12): evidence that they function as alpha/beta heterodimeric receptors for laminin and type IV collagen. J Cell Biol. 1988 Sep;107(3):1241–1252. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor A. E., Schubert D., Allen W. R. Alterations of lipid metabolism in response to nerve growth factor. J Neurochem. 1982 Dec;39(6):1677–1683. doi: 10.1111/j.1471-4159.1982.tb08002.x. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]