Abstract
Synaptic loss, particularly related to the forebrain cholinergic system, is considered to be an early event that leads to Alzheimer’s disease (AD) and has led to the development of acetylcholinesterase inhibitors (AChE-Is) as the mainstay of treatment for several degenerative disorders that culminate in dementia. The primary dose-limiting toxicities of all clinically available AChE-Is are, similar to useful actions on cognition, cholinergically mediated and they ultimately limit the value of this drug class in achieving anything but symptomatic improvements. In addition, AChE levels in brain areas associated with AD decline with disease progression, which likely ultimately limits the therapeutic utility of this drug class. New research indicates that selective inhibition of butyrylcholinesterase (BuChE), a closely related enzyme that is markedly elevated in AD brain, increases acetylcholine (ACh) and augments cognition in rodents free of the characteristic undesirable actions of AChE-Is. BuChE inhibition hence represents an innovative treatment approach for AD, and agents are currently being synthesized to optimally achieve this. The novel compound, tetrahydrofurobenzofuran cymserine (THFBFC), is derived from our effort to produce a potent and BuChE-selective inhibitor as a candidate to test the hypothesis that BuChE-Is would be efficacious and better tolerated than AChE-Is in AD. Herein, we applied innovative enzyme kinetic analyses to characterize the quantitative interaction of THFBFC with human BuChE. These provided values for the agent’s IC50, together with specific new kinetic constants, such as KT50, KT1/2, RI, oKRT, oPmax, KPT and PT1/2, to aid define target concentrations for clinical translation. Additional classical kinetic parameters, including Ki, Km or Ks, kcat or Vmax and Vmi were also determined. THFBFC proved to be a potent competitive inhibitor of human BuChE and, like its isomer dihydrobenzodioxepine cymserine, is a potentially interesting AD drug candidate.
Keywords: Acetylcholinesterase, Alzheimer’s disease, Anticholinesterase, Bisnorcymserine, Butyrylcholinestrase, Cymserine, Enzyme inhibition kinetics, Tetrahydrofurobenzofuran
Introduction
Alzheimer’s disease (AD), a progressive degenerative disorder that is the most common cause of dementia in the elderly, is typified by an increasing impairment of memory that is associated with behavioural problems (Cummings 2004; Farlow and Cummings 2007). These psychiatric disturbances largely arise from dysfunction and death of neurons in critical brain regions important in cognition and mood, including the basal forebrain, hippocampus and frontal, parietal and entorhinal cortex. From a neuropathological standpoint, AD is epitomize by the presence of amyloid plaques that primarily comprise of amyloid-β peptide (Aβ), neurofibrillary tangles (NFTs) and a loss of both synaptic processes and presynaptic markers of the cholinergic system in the noted brain areas (Sambamurti et al. 2002; Selkoe 2005). Most AD is sporadic in aetiology, evolving from unknown causes, with age and apolipoprotein E status predominating incidence (Sambamurti et al. 2002, 2006; Hardy 2006). A far smaller fraction of AD, particularly early onset, is familial AD (FAD). Mutations in one of several genes, exemplified by presenilins (PS1, PS2) and amyloid-β precursor protein (APP), result in FAD (Sisodia and St George-Hyslop 2002; Selkoe 2005). A shared result of many of these mutations is that they raise Aβ, particularly the hydrophobic Aβ42 (Sambamurti et al. 2002; Selkoe 2005), aggregates of which target synapses and impair memory (Lacor 2004; Lesne et al. 2006).
Up to now, acetylcholinesterase inhibitors (AChE-Is) represent the principal strategy to treat mild to moderate AD, to which the NMDA antagonist, memantine, is occasionally added (Cummings 2004; Farlow and Cummings 2007). Approaches are, however, being developed to lower Aβ, by lowering its production through inhibition of the secretase activities (β and γ) that cleave it from APP and by increasing its clearance, as well as to reduce tau phosphorylation, oxidative stress and neuroinflammation (Lahiri et al. 2004; Doraiswamy and Xiong 2006). Recent studies suggest that current clinically available AChE-Is (donepezil, rivastigmine and galantamine), which primarily act by reducing the rate of hydrolysis of ACh in brain to amplify its cholinergic activity, may secondarily impact these targets (Standridge 2004; Small 2005). Although this may, indeed, be true in cell culture and animal models, whether or not this is achieved clinically remains unknown (Small 2005). They, regrettably, appear to have little impact on the underlying pathological process that leads to AD to offset disease progression. Their symptomatic actions are generally modest and recent clinical trials have triggered debate as to the true benefit of this drug class (Courtney 2004; Lopez 2005). Current AChE-Is are dose-limited by their own primary actions that result in the common cholinergically-mediated adverse effects of nausea, vomiting, diarrhoea and dizziness (Farlow and Cummings 2007), which likely occur prior to initiating any potentially favourable secondary activities. Recent research indicates, however, that centrally mediated inhibition of a homologous cholinesterase (ChE) form, butyrylcholinesterase (BuChE), may achieve the cognitive improvements associated with current AChE-Is without the classic dose-limiting actions (Greig et al. 2005c).
Butyrylcholinesterase efficiently catalyses the hydrolysis of numerous endogenous substances, including choline esters (Silver 1974; Soreq and Zakut 1993; Darvesh et al. 2003; Giacobini 2003) and, although predominantly associated with glial cells in human brain, it is additionally expressed in neuronal somata and their proximal dendrites in brain areas affected by AD, such as in amygdala, hippocampus and thalamus (Darvesh et al. 1998, 2003; Darvesh and Hopkins 2003). The survival and ‘normality’ of the brain of AChE knockout (AChE−/−) versus normal (AChE+/+) mice, together with the distinct and overlapping distribution patterns of AChE- and BuChE-positive neurons, supports the opinion that BuChE is involved in neural function (Mesulam et al. 2002; Rice et al. 2007), for example co-regulation of cholinergic and non-cholinergic neurotransmission (Greig et al. 2002; Giacobini 2003; Darvesh et al. 2003). This viewpoint is fortified by animal studies indicating that selective BuChE-Is, cymserine analogues, elevate brain levels of ACh in AChE+/+ and −/− animals, augment LTP in brain slice preparations and improve cognitive performance of aged rodents without adverse actions (Greig et al. 2005c; Hartmann et al. 2007). As a secondary action, these same compounds reduced Aβ levels in the brain of transgenic mice expressing mutant human APP and PS1 (Greig et al. 2005c).
Whether or not these actions would translate to humans and, in particular to AD, remains to be determined. To assess this, our studies have focused on the design and development of novel ‘drug-like’ compounds based on the pharmacophore of the classic short-acting anticholinesterase, (−)-physostigmine, from which we originally derived long-acting (−)-cymserine and its BuChE selective analogues (Greig et al. 1995, 2005b; Yu et al. 1999) (Fig. 1). Our previous efforts culminated in the synthesis of the novel compound, tetrahydrofurobenzofuran cymserine (THFBFC) (Luo et al. 2005a, b, 2006), which was loosely based on the backbone of the short-acting anticholinesterase, (−)-physovenine (Fig. 1), a congener of (−)-physostigmine that similarly is a natural alkaloid deriving from the Calabar bean, Physostigma venenosum (Greig et al. 1995). Herein, we characterized the mode and kinetic binding constants of THFBFC through innovative enzyme interaction analyses to assess its suitability as a drug candidate to elucidate the therapeutic potential of BuChE inhibition in AD, as well as to define target concentrations in humans to aid clinical translation.
Fig. 1.
Chemical structures of the selective BuChE-I, (±)-tetrahydrofurobenzofuran cymserine (THFBFC), and its isomer, (±)-dihydrobenzodioxepine cymserine (adapted from Luo et al. 2005a, b, 2006; Kamal et al. 2007). As well as of the two natural alkaloids, (−)-physostigmine and (−)-physosvenine, and of the long-acting physostigmine analogues (−)-phenserine (AChE-selective), (−)-cymserine (semi-BuChE-selective) and (−)-bisnorcymserine (BuChE-selective)
Materials and methods
Materials
Butyrylthiocholine iodide (BuSCh, was used as a substrate), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB as a colouring reagent), and 1,5-bis(4-allyldimethyl-ammoniumphenyl) pentan-3one dibromide (BW284C51) were obtained from Sigma (St Louis, MO, USA) while human serum BuChE was obtained from a local blood bank (unit no 8599114; ARCBS Sydney ORhD positive). THFBFC was synthesized as reported earlier (Luo et al. 2005b), and was prepared as a racemate [(±)-THFBC] (Fig. 1).
Assay of BuChE activity
Quantification of BuChE activity was assessed by the Ellman technique (1961). The assay mixture contained BuSCh, 0.25 mM DTNB, 0.05 mM BW284C51 (a classic selective, potent and irreversible inhibitor of AChE) and 50 mM sodium phosphate buffer, pH 7.2. The rate of substrate hydrolysis by BuChE was determined by measuring the absorbance of the reaction-product at 405 nm λ. Concentrations of substrate and THFBFC were determined from preliminary studies to insure optimal correlations.
Vmax and Vmi determinations
Apparent maximal activity (Vmax) and Vmi represent two different subtypes of maximum activity. The former is based on calculation of the apparent maximum activity of BuChE at a particular concentration of THFBFC along with varied concentrations of the substrate. The latter expresses the apparent maximum activity at a particular concentration of BuSCh along with varied concentrations of THFBFC.
Protein estimation
Total protein concentration of human serum was estimated by Bradford method (Bradford 1976) using Coomassie G-250 Dye. An optical density of reaction product was measured at 595 nm λ.
Graphics
Graphs were plotted using GOSA (Bio-Log, Toulouse, France) and Prism (version 4, GraphPad, San Diego, CA, USA) software and values of the correlation coefficients and specific kinetic constants were obtained by the regression analysis (linear and several different non-linear forms) computed within these packages.
Results
The reflective binding activity index of THFBFC for human serum BuChE at 0.6 mM BuSCh (substrate) concentration (Fig. 2a) demonstrated the interaction of THFBFC with the passing of reaction time (RT). The time required to achieve half Vmax (KT1/2) increased smoothly over all tested concentrations of THFBFC, while the pseudo maximum product (Pmax) remained largely unaltered by the same conditions (Fig. 2b). New kinetic constants (NKCs) defining the interaction of the inhibitor and enzyme are presented in Table 1, and are derived from secondary replots of the primary main plot of Fig. 2a. Figure 2c illustrates the relationship between the percentage activity of BuChE versus the THFBFC concentration to allow calculation of an IC50 value (the concentration required to inhibit 50% of enzymatic activity). Its secondary plot expresses the relationship of IC50 versus reaction time in this study (Fig. 2d). Determined from the analysis depicted in this figure, the IC50 value of THFBFC for BuChE was calculated as 25.0 ± 2.2 nM.
Fig. 2.
Reflective binding activity of BuChE versus reaction time at different concentrations of THFBFC. b A secondary replot of primary main plot of a. c Demonstration of % activity of BuChE versus THFBFC concentration to calculate IC50 at each reaction time. d Demonstration of IC50 versus reaction time
Table 1.
Comparative values of new kinetic constants (NKC) at dual concentrations of BuSCh in the case of human BuChE inhibition by THFBFC (current study) and its isomer dihydrobenzodioxepine cymserine (DHBDPC) [adapted from Kamal et al. 2007)
| NKC | BuSCh (0.6 mM) | BuSCh (0.1 mM) | ||
|---|---|---|---|---|
| KT50 (nM) | 60.53 | 13.28 | 2.035a | 0.966 |
| PPC (nM) | 2,782 | 21.37 | 2.231a | 1.13 |
| RI (nM/min) | 0.76 ± 0.3 | 0.41 ± 0.031 | 1.14 ± 0.08 | 0.149 ± 0.02 |
Bold font differentiates the current data from prior data with a close analogue
PPC concentration of inhibitor to double pseudo Pmax, KT50 concentration of inhibitor to double KT1/2, RI rate of IC50
Value determined after excluding the value of the 50-nM point as it proved to be too high and could not be accommodated in the equation
The inhibition style of human serum BuChE by THFBFC at dual concentration of BuSCh before (Fig. 3a) and after completion of the reaction (Fig. 3b) demonstrates recovery of inhibition over passing time. Figure 4 allows characterization of the physiological nature of the binding of THFBFC to enzyme. Specific NKCs of physiological relevance, oKRT (the overall rate of inhibition constant) and oPmax (the overall maximum product concentration), were determined at dual substrate concentrations (0.05 and 0.40 mM BuSCh), at various preincubation (0–60 min) and reaction times (6–180 min; Fig. 4a, b). The values of oKRT were determined as 27 and 46 min, and oPmax as 14 ± 0.2 and 110 ± 1.2 at 0.05 and 0.40 mM BuSCh, respectively (inset of Fig. 4a, b). The plot (Fig. 4c) for activity of BuChE versus preincubation time (PT) demonstrates a slow binding pattern of the affect of THFBFC over BuChE along with range of preincubation times, and allowed the determination of two further new constants: (1) KPT (the preincubation time constant) with a value of 0.079 and 0.097, and (2) the PT1/2 (preincubation time required for half ν) with a value of 8.78 and 7.14 min, for 0.05 and 0.40 mM BuSCh, respectively (Fig. 4c). A plot of percent activity and percent inhibition of BuChE by THFBFC versus preincubation time is shown in Fig. 4d.
Fig. 3.
Inhibition of human serum BuChE by THFBFC at 0.05 mM BuSCh and 0.4 mM BuSCh before (a) and after completion of reaction (b)
Fig. 4.
Preincubation data presentation at 0.05 mM BuSCh (a) and 0.4 mM BuSCh (b), where open square, dark filled triangle, open inverted triangle, dark filled diamond, open circle stands for control, 0, 5, 30 and 60 min preincubation of THFBFC with BuChE, respectively, while R2 in the case of a was as follows: 0.911, 0.964, 0.975, 0.974, 0.969 and for b as follows: 0.941, 0.976, 0.976, 0.980 and 0.980 for control and at 0, 5, 30 and 60 min data experimental plots, respectively. Inset is a secondary replot of KRT and Pmax versus preincubation time. c Plot for activity of BuChE versus preincubation time. d Plot for % activity and % inhibition of BuChE by THFBFC versus preincubation time
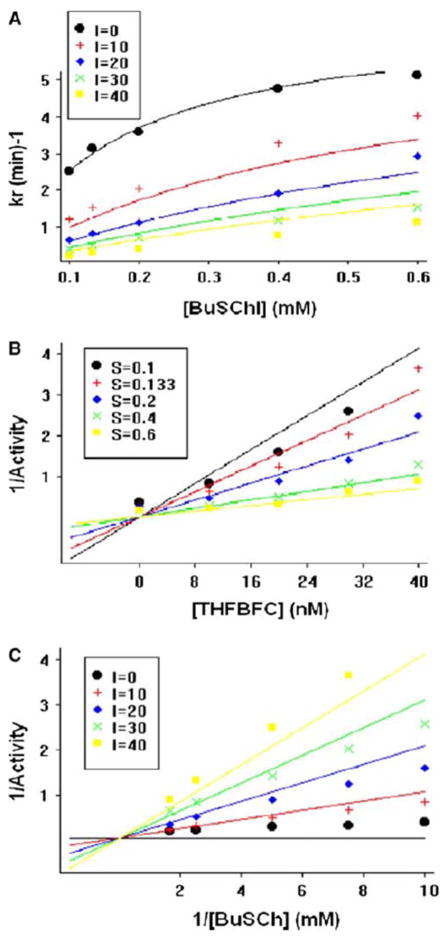
The classical kinetic parameters of Ki (inhibition constant), Ks (substrate constant), Km (Michaelis–Menten constant), kcat (catalytic constant), Vmax (apparent maximal activity) and Vmi (Vmaxi apparent) were, additionally, evaluated (Figs. 5, 6). The values of these were determined from appropriate equations related to different potential types of inhibition by use of GOSA, and are shown in Table 2. The difference between the general equation and competitive inhibition model is not statistically significant but the latter model is superior (with a higher number of degrees of freedom), so this analysis depicts a competitive inhibition system for BuChE inhibition by THFBFC. Overall, this kinetic study of BuChE inhibition by THFBFC is illustrated in Scheme 1. Secondary replots of the primary plot of Dixon (Fig. 6a) and the Lineweaver–Burk plot (Fig. 6b) of BuChE inhibition by THFBFC were constructed to differentiate between the most plausible modes of inhibitions.
Fig. 5.
Kinetic study of BuChE: a BuChE net activity versus its substrate at different concentrations of THFBFC, as described in the legend box. b Dixon plot for BuChE reciprocal activity versus THFBFC concentration at different substrate concentrations, as described in the legend box. c Lineweaver–Burk plot of BuChE reciprocal activity versus its reciprocal substrate concentration at different concentrations of THFBFC, as detailed in the legend box
Fig. 6.
Secondary replots of primary plot of Dixon (a) and Lineweaver–Burk (b) plot of BuChE inhibition by THFBFC
Table 2.
Comparative results for the most likely potential modes of inhibition of BuChE by THFBFC
| Parameters | GI | CI | MI | PCI |
|---|---|---|---|---|
| Km (mM) | - | - | - | 0.181 ± 0.091 |
| Ks (mM) | 0.167 ± 0.08 | 0.165 ± 0.075 | 0.181 ± 0.09 | - |
| kcat (au) | 6.86 ± 1.30 | 6.83 ± 1.16 | 7.09 ± 1.35 | 7.1 ± 1.39 |
| Ki (nm) | 3.55 ± 8.3e+7 | 3.91 ± 1.33 | - | - |
| ki1 (nm) | - | - | 5.28 ± 3.26 | 5.28 ± 3.34 |
| ki2 (nm) | - | - | 40 ± 65 | 40 ± 73.4 |
| SSE | 2.003 | 2.011 | 2.11 | 2.11 |
C competitive, G general, I inhibition, M mixed, P partial, SSE sum of squared errors
Scheme 1.
Diagram depicting the major kinetic interactions between (i) the BuChE-I, THFBFC, (ii) the enzyme, BuChE, and (iii) the substrate, BuSCh, that underpin the mode of inhibition of BuChE by THFBFC
Discussion
An increasing body of evidence supports a role for BuChE as a co-regulator of the brain cholinergic system. The recent development of AChE−/− mice that readily survive with normal levels and localization of BuChE (Mesulam et al. 2002) and no neuronal, dendritic, astrocytic, synaptic, microglial or endothelial differences (Rice et al. 2007), together with the development of entirely selective BuChE-Is, such as (−)-N1-phenethylcymserine (PEC) (Yu et al. 1999), that elevate brain ACh levels in normal and AChE−/− mice (Greig et al. 2005c; Hartmann et al. 2007), indicates that brain BuChE has a physiological function. The relevance of this can be assessed from recent studies by Cerbai et al. (2007), where donepezil (AChE selective), rivastigmine (AChE/BuChE unselective) and PEC (BuChE selective) were administered systemically to normal healthy rats to achieve similar twofold to threefold elevations in brain extracellular ACh levels, as determined by in vivo microdialysis and HPLC. To achieve this, donepezil induced a 27% AChE inhibition, rivastigmine a 40% AChE and 25% BuChE inhibition and PEC a 39% BuChE inhibition in the brain of rats, in which total ChE levels comprise >90% AChE and <10% BuChE (Cerbai et al. 2007). By corollary, the super-sensitivity of BuChE−/− mice to selective AChE-Is, at concentrations that are well tolerated in BuChE+/+ animals (Duysen et al. 2007), reiterates the physiological relevance of BuChE in neurotransmission.
Within the human nervous system AChE, likewise, predominates over BuChE (Giacobini 2003; Darvesh et al. 2003). In the temporal cortex of normal human brain, for example AChE accounts for some 90% of ChE activity, with BuChE accounting for the remainder (Perry et al. 1978). However, whereas AChE is localized mainly to neurons, BuChE is associated primarily with glial cells as well as neurons and, in specific brain areas, nuclei of BuChE-rich neurons and neural tracts that utilize BuChE alone appear to exist (Darvesh and Hopkins 2003; Darvesh et al. 2003). The normal relationship between AChE and BuChE changes during disease. In AD, AChE activity can routinely decline by some 45% in specific brain areas during disease progression, accompanying the archetypal loss of presynaptic ACh. By contrast, BuChE activity concurrently is elevated by up to twofold (Perry 1986; Arendt et al. 1984, 1992), likely consequent to the increased number of glial cells found in AD brain, and some of this co-localizes with the hallmark cortical and neocortical amyloid-rich neuritic plaques and NFTs (Guillozet et al. 1997). A disparity, thereby, develops between reduced ACh synthesis/availability and one of its main hydrolysing enzymes—an elevated BuChE.
Selective BuChE inhibition, that appears to be unassociated with classic AChE-I adverse actions in animal models (Greig et al. 2005c), may therefore be useful in ameliorating a cholinergic deficit, which likely worsens in AD due to increased activity of BuChE (Greig et al. 2002; Giacobini 2003; Darvesh et al. 2003). Our recent synthesis of novel carbamate ChEIs, such as on the skeletons of tetrahydrofurobenzofurans and dihydromethanobenzodioxepines, was undertaken to test this hypothesis (Luo et al. 2005a, b, 2006). The assessment of the apparent binding activity index of THFBFC for human serum BuChE at 0.6 mM BuSCh concentration provides a measure of enzyme–inhibitor interaction in a concentration-dependent manner (Fig. 2). The foundation of the Ellman assay (Ellman et al. 1961) that underlies our study is that units of active BuChE with an open active site are able to bind with substrate, BuSCh, to catalyse it into butyrate and thiocholine. Released thiocholine then reacts with DTNB to generate a colored reaction-product in a concentration- and time-dependent manner. The time required to achieve half Vmax (KT1/2) was increased smoothly by increasing the concentration of THFBFC, while the pseudo maximum product (Pmax) remained unaltered (Fig. 2b). By contrast, in our previous study of the isomer, dihydrobenzodioxepine cymserine (Fig. 1), KT1/2 and Pmax were both increased dramatically around 20 nM (Kamal et al. 2007), which indicates a different enzyme binding interaction pattern of these two closely related compounds. The major difference in the values of KT50 and PPC, reflected in Table 1, also supports this conclusion.
For our initial description of the synthesis of novel anticholinesterases based on tetrahydrofurobenzofuran (Luo et al. 2005a), we performed a preliminary enzyme kinetic analysis by characterizing the inhibitor–enzyme interaction at half-log inhibitor concentrations increments between 0.3 nM and 10 μM utilizing a single substrate concentration (0.5 mM) and reaction time (30 min). This permitted calculation of simple comparative IC50 values of human BuChE by the synthesized compounds. THFBFC, compound 12 with an IC50 of 27 ± 4 nM (Luo et al. 2005a), was chosen for further study, herein, due to a combination of its potency for BuChE versus AChE (IC50 2,650 ± 400 nM) and selectivity (<100-fold), together with the knowledge that cymserine analogues are long acting (Greig et al. 1995, 2005c). Our more in depth analysis over a concentration range (6–50 nM) that precisely skirts the original IC50 estimate provides a slightly lower value (Fig. 2c). In addition, a non-significant relationship between IC50 versus reaction time (Fig. 2d) predicts a complex nature of reversible inhibition of BuChE by THFBFC, as compared to a significant relationship found in our prior analysis of inhibition of BuChE by the isomer, dihydrobenzodioxepine cymserine (Kamal et al. 2007). Clearly, whereas these two isomers have closely related structures (Fig. 2) and both bind within and potently inhibit BuChE, the three-dimensional change of their conformity in their tricyclic rings (Luo et al. 2005a, 2006) provides them slightly different enzyme binding kinetic characteristics.
Assessment of the inhibition of human BuChE by THFBFC at dual concentrations of BuSCh (0.05 and 0.40 mM) before (Fig. 3a) and after completion of reaction (Fig. 3b) demonstrates initial slow binding of the inhibitor in the absence of the substrate, comparable to a pseudo-irreversible type of inhibition. With the addition of and corresponding competition of BuSCh, THFBFC interaction with BuChE appears to be more of a reversible nature with the passage of reaction time, and is clearer in Fig. 3b. Analysis of the data presented in Fig. 4 allowed calculation of new binding constants for human BuChE inhibition, focused to aid clinical translation and that may prove of wide applicability. These can be compared to other closely related cymserine analogues that we have recently studied as AD drug candidates.
Specifically, the Kiapp and a predicted Ki values for the selective BuChE-I, (−)-bisnorcymserine, have recently been reported as 0.7 and 0.131 nM, respectively (Kamal et al. 2006b), which compares favourably with those of our initial BuChE-I, (−)-cymserine, with values of 115 and 38 nM, respectively (Kamal et al. 2006a). By contrast, the calculated Ki value of dihydrobenzodioxepine cymserine was reported as 2.22 nM, according to a competitive inhibition equation, or 3.24 and 7.91 nM for Ki1 and Ki2, respectively, in line with a partial mixed type of competitive inhibition equation (Kamal et al. 2007). Then again, the estimated Ki value for THFBFC, studied herein, is 3.91 nM according to the competitive inhibition equation, or 5.28 and 40 nM for Ki1 and Ki2, respectively, in line with the partial competitive as well as mixed type of inhibition equation (Table 2). The interaction of THFBFC with BuChE can be understood through Scheme 1. In either case, the closely related isomers, THFBFC and dihydrobenzodioxepine cymserine, synthesized to block potential metabolic interactions with the indole moieties of the tricyclic backbone of cymserine analogues, are less potent than (−)-bisnorcymserine but more than (−)-cymserine. All the same, it is likely that the estimated Ki values for THFBFC and dihydrobenzodioxepine cymserine fall within a clinically achievable range, as plasma levels of the close analogues, (−)-phenserine and (+)-phenserine (Fig. 1), reached 5.79 nM and in excess of 300 nM at a well tolerated doses of 10 and 80 mg in humans, respectively (Greig et al. 2005a, b).
To more fully characterize the nature of inhibition by THFBFC, secondary slope replots were generated of the primary plot of Dixon in Fig. 6a. This bears close resemblance to a traditional plot for competitive inhibition in which the replot line goes approximately through the origin (0.055 and −0.0006 X- and Y-axes, respectively). To further identify the potential mode of action, secondary replots [slope, reciprocal Kmapp (1/Kma) and Vmaxapp (1/Vmax) of the primary Lineweaver–Burk plot of BuChE inhibition by THFBFC] were undertaken (Fig. 6b) to differentiate between competitive inhibition and a mixed type of inhibition. The parameter, 1/Vmax, should significantly increase in the latter case; however, it was found to be close to flat (slope value: 0.001), again reflecting that inhibition was more of a competitive type in the current study. However, the described slight divergence regarding the 1/Vmi plot (slope value: −0.022 ± 0.0011; Fig. 6a) suggests the potential of a minor mixed component. Hence, as illustrated in Scheme 1, the portion that belongs to a mixed type of competitive inhibition is, accordingly, shaded.
In summary, THFBFC, similar to dihydrobenzodioxepine cymserine (Kamal et al. 2007), represents an interesting and potent BuChE-I that possesses IC50 and Ki values, together with associated kinetic parameters, that are likely attainable in AD subjects. Of note, the replacement of both indole nitrogen groups (the N1 and N8 groups shown in Fig. 1) in the tricyclic backbone common to (−)-physostigmine and the AD experimental AD drugs, (−)-phenserine, (+)-phenserine (Lahiri et al. 2007) and (−)-bisnorcymserine, by oxygen moieties provides drug-like, potent BuChE-Is that are competitive rather than non-competitive oriented types of inhibitors (Kamal et al. 2006a, b, 2007). For THFBFC, this additionally results in a potentially interesting slow onset of inhibition (Fig. 3), as the rapid elevation of brain ACh levels has been suggested to underpin the centrally mediated nausea common to AChE-Is. Up to now, a selective BuChE-I has yet to reach the clinic. Hence, whether or not their potential translates to AD and related dementias to impact symptoms and/or disease progression remains to be determined. Insight may derive from BuChE mutations in humans, that are not uncommon (Souza et al. 2005), and several result in a loss of BuChE activity (akin to a steady-state level of inhibition). The BuChE-K variant (Ala539Thr) is present in some 33% of Caucasian and Asian individuals, and those possessing two alleles for BuChE-K (homozygous) show an approximate 30% reduced BuChE activity (Bartels et al. 1992; Ballard et al. 2005). In an increasing number of studies where AD subjects were genotyped for BuChE genetic variants and their disease progression measured (O’Brien et al. 2003; Holmes et al. 2005; Ballard et al. 2005; Déniz-Naranjo et al. 2007), those with BuChE deficiency had a delayed onset of AD and a slower cognitive decline compared to patients with wild-type BuChE levels. The choice of which cymserine analogue to finally translate to clinical assessment will ultimately depend on the cost of bulk synthesis and tolerability in cellular and animal models.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AChE-Is
Acetylcholinesterase inhibitors
- AD
Alzheimer’s disease
- APP
Amyloid-β precursor protein
- Aβ
Amyloid-β peptide
- BuChE
Butyrylcholinesterase
- BuSCh
Butyrylthiocholine iodide
- BuChE-Is
Butyrylcholinesterase inhibitors
- ChEs
Cholinesterases
- ChE-Is
Cholinesterase inhibitors
- CNS
Central nervous system
- FAD
Familial Alzheimer’s disease
- THFBFC
Tetrahydrofurobenzofuran cymserine
- KPT
Preincubation time constant
- KT50
Concentration of inhibitor doubles KT1/2
- oKRT
Overall rate of inhibition constant
- PPC
Concentration of inhibitor doubles pseudo Pmax
- oPmax
Overall maximum product concentration
- KT1/2
Time required for half Vmax
- PT1/2
Preincubation time required for half ν
- RI
Rate of IC50
- RβA
Reflective binding activity
- Ki
Inhibition constant
- Ks
Substrate constant
- Km
Michaelis-Menten constant
- Kcat
Catalytic constant
- Vmax
Apparent maximal activity
- Vmi
Vmaxi
References
- Arendt T, Bigl V, Walther F, Sonntag M. Decreased ratio of CSF acetylcholinesterase to butyrylcholinesterase activity in Alzheimer’s disease. Lancet. 1984;1:173. doi: 10.1016/s0140-6736(84)90116-8. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bruckner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development—a study of molecular forms. Neurochem Int. 1992;21:381–396. doi: 10.1016/0197-0186(92)90189-x. [DOI] [PubMed] [Google Scholar]
- Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res. 2005;2:307–318. doi: 10.2174/1567205054367838. [DOI] [PubMed] [Google Scholar]
- Bartels CF, Jensen FS, Lockridge O. DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet. 1992;50:1086–1103. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cerbai F, Giovannini MG, Melani C, Enz A, Pepeu G. N1phenethyl-norcymserine, a selective butyrylcholinesterase inhibitor, increases acetylcholine release in rat cerebral cortex: a comparison with donepezil and rivastigmine. Eur J Pharmacol. 2007;572:142–150. doi: 10.1016/j.ejphar.2007.06.053. [DOI] [PubMed] [Google Scholar]
- Courtney C. AD2000 collaborative group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Hopkins DA. Differential distribution of butyrylcholinesterase and acetylcholinesterase in the human thalamus. J Comp Neurol. 2003;463:25–43. doi: 10.1002/cne.10751. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Grantham DL, Hopkins DA. Butyrylcholinesterase in normal human amygdala and hippocampal formation. J Comp Neurol. 1998;393:374–390. [PubMed] [Google Scholar]
- Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- Déniz-Naranjo MC, Muñoz-Fernández C, Alemany-Rodríguez MJ, del Pérez-Vieitez MC, Aladro-Benito Y, Irurita-Latasa J, Sánchez-García F. Butyrylcholinesterase, ApoE and Alzheimer’s disease in a population from the Canary Islands (Spain) Neurosci Lett. 2007;427:34–38. doi: 10.1016/j.neulet.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Xiong GL. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert Opin Pharmacother. 2006;7:1–10. doi: 10.1517/14656566.7.1.1. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Darvesh S, Lockridge O. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer’s disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology. 2007;233:60–69. doi: 10.1016/j.tox.2006.11.069. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer’s disease. Am J Med. 2007;120:388–97. doi: 10.1016/j.amjmed.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Butyrlcholinesterase: its function and inhibitors. Martin Dunitz; London and New York: 2003. [Google Scholar]
- Greig NH, Pei X-F, Soncrant T, Ingram DK, Brossi A. Phenserine and ring-C hetero-analogues: drug candidates for the treatment of Alzheimer’s disease. Med Chem Rev. 1995;15:3–31. doi: 10.1002/med.2610150103. [DOI] [PubMed] [Google Scholar]
- Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr. 2002;14:77–91. doi: 10.1017/s1041610203008676. [DOI] [PubMed] [Google Scholar]
- Greig NH, Ruckle J, Comer P, Brownell L, Holloway HK, Flanagan DR, Jr, Canfield CJ, Burford RG. Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects. Curr Alzheimer Res. 2005a;2:483–492. doi: 10.2174/156720505774330564. [DOI] [PubMed] [Google Scholar]
- Greig NH, Sambamurti K, Yu Q-S, Brossi A, Bruinsma G, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005b;2:281–291. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005c;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet AL, Smiley JF, Mash DC, Mesulam M. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol. 1997;42:909–18. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3:71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J. Excessive levels of hippocampal acetylcholine in acetylcholinesterase-knockout mice are moderated by butyrylcholinesterase activity. J Neurochem. 2007;100:1421–1428. doi: 10.1111/j.1471-4159.2006.04347.x. [DOI] [PubMed] [Google Scholar]
- Holmes C, Ballard CG, Lehmann D, David Smith A, Beaumont H, Day IN, Nadeem Khan M, Lovestone S, McCulley M, Morris CM, Munzo DG, Russ C, Del Ser T, Warden D. Rate of progression of cognitive decline in Alzheimer’s disease: effect of butyrylcholinesterase K gene variation. J Neurol Neurosurg Psychiatry. 2005;76:640–643. doi: 10.1136/jnnp.2004.039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MA, Al-Jafari AA, Yu Q-S, Greig NH. Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine. Biochim Biophys Acta. 2006a;1760:200–206. doi: 10.1016/j.bbagen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Yu Q-S, Holloway HW, Tweedie D, Klein P, Greig NH. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J Alzheimers Dis. 2006b;10:43–51. doi: 10.3233/jad-2006-10108. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Klein P, Yu Q-S, Holloway HW, Tweedie D, Greig NH. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem Res. 2007 doi: 10.1007/s11064-007-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Rogers JT, Greig NH, Sambamurti K. Rationale for the development of cholinesterase inhibitors as anti-Alzheimer agents. Curr Pharm Des. 2004;10:3111–3119. doi: 10.2174/1381612043383331. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer’s disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320:386–396. doi: 10.1124/jpet.106.112102. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lopez OL. Alteration of a clinically meaningful outcome in the natural history of Alzheimer’s disease by cholinesterase inhibition. J Am Geriatr Soc. 2005;53:83–87. doi: 10.1111/j.1532-5415.2005.53015.x. [DOI] [PubMed] [Google Scholar]
- Luo W, Yu QS, Holloway HW, Greig NH, Brossi A. Syntheses of tetrahydrofurobenzofurans and dihydro-methanobenzodioxepines from 5-hydroxy-3-methyl-3H-benzofuran-2-one. Re-arrangement and ring expansion under reductive conditions on treatment with hydrides. J Org Chem. 2005a;70:6171–6176. doi: 10.1021/jo0503052. [DOI] [PubMed] [Google Scholar]
- Luo X, Yu QS, Zhan M, Parrish D, Deschamps JR, Kulkarni SS, Holloway HW, Alley GM, Lahiri DK, Brossi A, Greig NH. Novel anticholinesterases based on the molecular skeletons of furobenzofuran and benzodioxepine. J Med Chem. 2005b;48:986–994. doi: 10.1021/jm049309+. [DOI] [PubMed] [Google Scholar]
- Luo W, Yu QS, Kulkarni SS, Holloway HW, Parrish D, Tweedie D, Lahiri DK, Brossi A, Greig NH. (−) And (+)-o-carbamoyl phenols of pyrroloindole, furoindole, furobenzofuran and benzodioxepine: enantiomeric syntheses and structure/activity relationship for human acetyl- and butyrylcholinesterase inhibitory action. J Med Chem. 2006;49:2174–2185. doi: 10.1021/jm050578p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–639. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- O’Brien KK, Saxby BK, Ballard CG, Grace J, Harrington F, Ford GA, O’Brien GT, Swan AG, Fairbairn AF, Wesnes W, Del Ser T, Edwardson JA, Morris CM, McKeith IG. Regulation of attention and response to therapy in dementia by butyrylcholinesterase. Pharmacogenetics. 2003;13:231–239. doi: 10.1097/00008571-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Perry EK. The cholinergic hypothesis—ten years on. Br Med Bull. 1986;42:63–69. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterase in senile dementia of Alzheimer’s type. Neuropathol Appl Neurobiol. 1978;4:273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Rice SG, Nowak L, Duysen EG, Lockridge O, Lahiri DK, Reyes PF. Neuropathological and immunochemical studies of brain parenchyma in acetylcholinesterase knockout mice: implications in Alzheimer’s disease. J Alzheimers Dis. 2007;11:481–489. doi: 10.3233/jad-2007-11410. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2002;1:1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Anitha S, Venugopal C, Prakasam A, Zhou Y, Lahiri DK, Greig NH. A partial failure of membrane protein turnover may cause Alzheimer’s disease: a new hypothesis. Curr Alzheimer Res. 2006;3:81–90. doi: 10.2174/156720506775697142. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Silver A. The biology of cholinesterases. Elsevier; Amsterdam: 1974. [Google Scholar]
- Sisodia SS, St George-Hyslop PH. gGamma-secretase, notch, Abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Small DH. Acetylcholinesterase inhibitors for the treatment of dementia in Alzheimer’s disease: do we need new inhibitors? Expert Opin Emerg Drugs. 2005;10:817–825. doi: 10.1517/14728214.10.4.817. [DOI] [PubMed] [Google Scholar]
- Soreq H, Zakut H. Human cholinesterases and anticholinesterases. Academic; New York: 1993. [Google Scholar]
- Souza RL, Mikami LR, Maegawa RO, Chautard-Freire-Maia EI. Four new mutations in the BCHE gene of human butyrylcholinesterase in a Brazilian blood donor sample. Mol Genet Metab. 2005;84:349–353. doi: 10.1016/j.ymgme.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Standridge JB. Pharmacotherapeutic approaches to the treatment of Alzheimer’s disease. Clin Ther. 2004;26:615–630. doi: 10.1016/s0149-2918(04)90064-1. [DOI] [PubMed] [Google Scholar]
- Yu QS, Holloway HW, Utsuki T, Brossi A, Greig NH. Phenserine-based synthesis of novel selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J Med Chem. 1999;42:1855–1861. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]