Abstract
In general, proteins can only execute their various biological functions when they are appropriately folded. Their amino acid sequence encodes the relevant information required for correct three-dimensional folding, with or without the assistance of chaperones. The challenge associated with understanding protein folding is currently one of the most important aspects of the biological sciences. Misfolded protein intermediates form large polymers of unwanted aggregates and are involved in the pathogenesis of many human diseases, including Alzheimer’s disease (AD) and Type 2 diabetes mellitus (T2DM). AD is one of the most prevalent neurological disorders and has worldwide impact; whereas T2DM is considered a metabolic disease that detrementally influences numerous organs, afflicts some 8% of the adult population, and shares many risk factors with AD. Research data indicates that there is a widespread conformational change in the proteins involved in AD and T2DM that form β-sheets like motifs. Although conformation of these β-sheets is common to many functional proteins, the transition from α-helix to β-sheet is a typical characteristic of amyloid deposits. Any abnormality in this transition results in protein aggregation and generation of insoluble fibrils. The abnormal and toxic proteins can interact with other native proteins and consequently catalyze their transition into the toxic state. Both AD and T2DM are prevalent in the aged population. AD is characterized by the accumulation of amyloid-β (Aβ) in brain, while T2DM is characterized by the deposition of islet amyloid polypeptide (IAPP, also known as amylin) within beta-cells of the pancreas. T2DM increases pathological angiogenesis and immature vascularisation. This also leads to chronic cerebral hypoperfusion, which results in dysfunction and degeneration of neuroglial cells. With an abundance of common mechanisms underpinning both disorders, a significant question that can be posed is whether T2DM leads to AD in aged individuals and the associations between other protein misfolding diseases.
Keywords: Protein folding, Misfolding, Proteostasis, Alzheimer’s disease, Type 2 Diabetes Mellitus, Amyloid Precursor Protein, Neurofibrillary tangles, Tau, α-synuclein, tauopathy, Parkinson’s disease
INTRODUCTION
The problem of protein folding is one of the many unsolved paradigms in biochemistry. Protein folding has been recognized as one of the most critical puzzles to understand in the current century, especially because the complex protein structure makes it difficult to predict the correct folding patterns [1]. With the identification of several diseases as protein folding disorders, the explosion of genome information and the need for efficient ways to predict protein structure, protein folding has been projected as a fundamental issue in molecular sciences research [2]. The simplest way of initiating protein folding is to unfold the protein in high concentration of a chemical denaturant and then to dilute the solution so rapidly such that the denaturant concentration falls below the level at which the native state is thermodynamically unstable [3]. Another strategy involves the use of a battery of complementary stopped-flow and quenched-flow techniques, each of which is capable of monitoring a specific aspect of the formation of native like protein structure [4]. In addition, other novel methods to initiate refolding reactions are being introduced, which include the use of temperature jumps under conditions where cold denaturation takes place and an increase in temperature leads to refolding [5]. In some cases, the rapid change of oxidation state of a metalloprotein can also trigger the onset of the folding reaction [6]. With such approaches, folding events on the micro- and submicrosecond time scale are becoming accessible to investigate [7].
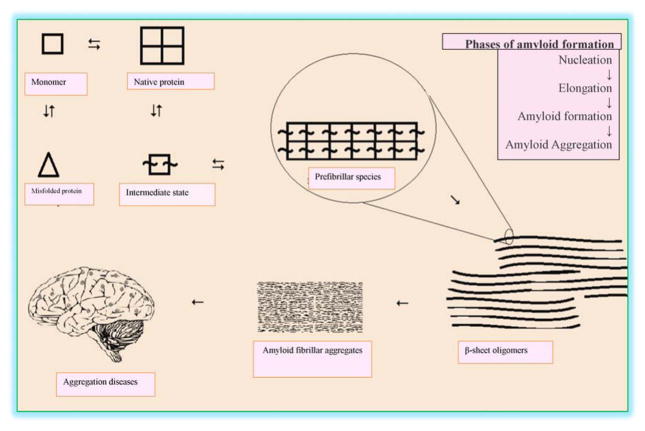
Protein folding disorders have been broadly categorized in two groups. The first involves disorders known as amyloidosis, characterized by accumulation of wrongly folded proteins as unfolded proteins; Alzheimer’s disease (AD) is the best known example of this group [8]. The second group of protein folding disorders is characterized by incomplete protein folding due to minor error in the genetic blueprint, affecting its physiological function [8]. AD is a progressive neurological disease of the brain characterized by presence of protein aggregates and irreversible loss of intellectual abilities, like reasoning and memory; thereby detrementally affecting the normal social and occupational lifestyle of the individual [9]. The aggregates, known as neuritic plaques, chiefly comprise of amyloid-β peptide (Aβ), which is formed by the proteolytic cleavage of amyloid precursor protein (APP) [10], a large type I integral membrane protein of 695–770 aa that is expressed in many tissues but particularly concentrated within the synapse of neurons. Cleavage of APP by two different enzymatic activities, sequentially by BACE-1 (rather than normal processing by α-secretase) followed by γ-secretase, releases 40 or 42 aa long Aβ peptide fragments [11]. These fragments can then combine into oligomers, which can further aggregate to ultimately form insoluble Aβ clumps surrounding neurons, and these oligomers and insoluble Aβ forms have been reported to possess detrimental toxic effects (Fig. 1) [11]. The Aβ containing aggregates having a β-sheet structure that is a characteristic of amyloid [12]. AD is therefore distinguishing by the accumulation of insoluble Aβ plaques within key regions of the brain. The now famous amyloid cascade hypothesis, which has now dominated this field for many years [13], proposes that brain Aβ generation is the primary influence driving AD pathogenesis. The rest of the disease process, including formation of neurofibrillary tangles containing tau protein, synaptic and neuronal loss, and neuroinflammation is projected to result from an imbalance between Aβ production and its clearance. However, an uncertainty over Aβ plaques being a cause or a consequence of AD remains, although current evidence favours the former being the case [8]. This uncertainty nevertheless remains, particularly in the light of the numerous AD drug failures, the majority of which were aimed to target key pathways critical within the amyloid cascade hypothesis [14]. Like AD, Type 2 Diabetes Mellitus (T2DM) is also considered as a degenerative metabolic disease that occurs in later age, and is associated with the gradual loss of insulin secreting pancreatic beta-cells [15]. It is becoming increasingly believed that islet amyloidosis is the progeny of many diseases, including T2DM. The production of islet amyloid polypeptide (IAPP) oligomers that results in amyloid deposition is considered as a major contributor in pathogenesis and progression of T2DM [16]. This has been found present in 96% of T2DM patients and is recognised as a hallmark for diagnosis of this disease [17].
Figure 1.
Protein folding and unfolding in aggregation diseases
During the past decade, research and elucidation of protein folding and aggregation in AD and T2DM have become focal points in pharmaceutical research; thereby enhancing the probability of developing both new therapeutic approaches and pharmacological tools to understand mechanisms.
MISFOLDED PROTEINS AND NEURODEGENERATIVE DISEASES
In a diseased condition, most of the time, target proteins attain toxicity following their transition from a α-helix to a β-sheet form [18]. Although numerous functional native proteins possess β-sheet conformations within them, the transition from an α-helix to a β-sheet is characteristic of amyloid deposits [19], and often associated with the change of a physiological function to a pathological one. Such abnormal conformational transition exposes hydrophobic amino acid residues and promotes protein aggregation [18, 20]. The toxic proteins often interact with other native proteins and may catalyze their transition into a toxic sate, and hence they are called infective conformations [18]. The newly formed toxic proteins can repeat this cycle to intiate a self-sustaining loop; thereby amplifying the toxicity to generate a catastrophic effect, beyond homeostatic reparative mechanisms, to eventually impair cellular function or induce cellular demise [21].
Proteins function properly when their constituent amino acids fold correctly [22]. On the other hand, misfolded proteins assemble into insoluble aggregates with other proteins and can be toxic for the cells [18, 20]. Ataxin-1 is highly prone to misfolding due to inherited gene defects that cause neurodegenerative diseases (NDDs), which is mainly due the repetition of glutamine within its amino acid chain; the toxicity of this protein being directly proportional to the number of glutamines [23]. There are 21 proteins that mainly interact with ataxin-1 and influence its folding or misfolding, 12 of which increase the toxicity of ataxin-1 for nerve cells, while 9 of the identified proteins reduce its toxicity [23]. Ataxin-1 resembles a double twisted spiral or helix and has a special structure, termed a “coiled coil domain”, that promotes aggregation. Proteins which possess “coiled coil domain” and interact with ataxin-1 have been reported to enhance promotion of ataxin-1 aggregation and toxic effects [24].
The gradual accumulation of misfolded proteins in the absence of their appropriate clearance can cause amyloid disease, the most prevalent one being AD. Parkinson’s disease and Huntington’s disease have similar amyloid origins [25]. These diseases can be sporadic or familial and their incidence increases dramatically with age. The mechanistic explanation for this correlation is that as we age (and are subjected to increasing numbers of mutations and/or oxidative stress causing changes to protein structure, etc.), the delicate balance of the synthesis, folding, and degradation of proteins is disturbed, ensuing in the production, accumulation and aggregation of misfolded proteins [26].
Among the environmental factors that increase the risk of degenerative diseases, a particularly important factor is contact with substances affecting mitochondrial function; thus resulting in an increase of oxidative damage to proteins [27]. However, it is clear that no single environmental factor is wholly responsible. In addition, there are genetic factors accountable too. For example, in the simplest forms of familial Parkinson’s disease, mutations are associated with dominant forms of the disease. In the case of AD, and for other less common NDDs, the genetics can be even more complicated, since different mutations of the same gene and combination of these mutations may affect the disease risk differently [28].
MISFOLDING IN NEUROFIBRIL TANGLES
The neurofilament is a linear 9–10 nm microfilament found within the neuronal cell body, the axon and the dendrites. It provides a major component of the neuronal cytoskeleton by providing structural support for axons and regulates their diameter. The neurofilament has a poorly defined lumen with protruded short “side arms” and appears to be composed of globular subunits of polypeptide chains. Unlike neurotubules, neurofilaments are stable and can be readily isolated by subcellular fractionation. Neurofibrillary tangles (NFTs) are prominent feature that occur within the pyramidal cells of the hippocampus and neurons of the cerebral cortex of AD. Albeit, similar neuronal changes likewise occur in elderly Down’s syndrome subjects and to a lesser degree in intellectually normal aged people [29], NFTs are a primary hallmark of AD. These tangles are composed of bundles of paired helically twisted filaments (PHF), which may be either neurofilaments or microtubules and are formed by intracellular hyperphosphorylation and by the c-terminal truncation of tau protein. These intracellular conformationally twisted fibres of tau protein impair axonal transport between the cell body and numerous synapses, which is crucial to neuronal function and survival [30–32]. Concomitantly, nitric oxide dependent oxidative stress leads to mitochondrial energy deprivation in AD [33].
The formation of amyloid plaques and NFTs are considered to underpin the dysfunction and demise of neurons within brain, instigating neuroinflammation and subsequent symptoms of AD [34]. The traditional understanding is that tau protein binds to microtubules and assists with their formation and stabilization. Simplistically, when tau is hyperphosphorylated it is unable to bind to microtubules that become unstable. The unbound tau clumps to generate NFT formations [35]. With time, these lesions develop into filamentous NFTs that interfere with numerous essential intracellular functions. A correlation has been found between the quantitative neuropathological markers of AD (neuronal loss, and neuritic plaque and NFTs load) in the hippocampus and frequent neuropsychiatric behaviors, typified by aggression in AD patients. An increase in hippocamal NFTs load, rather than the other variables, was associated with severity of aggression and chronic aggression in AD patients [36]; providing a pathogenic link between neurofibrillary tangle load and neuropsychiatric behaviors in dementia subjects.
Several observations in patients with Down’s syndrome and familial forms of AD (FAD) signify that genetic factors resulting in changes in Aβ are sufficient to cause AD, including tangles and other neurofibrillary changes [37]. Even though FAD only accounts for a relatively small fraction of AD cases (approximately 5%), mutations on APP, as well on presenilin1 (PS1), presenilin2 (PS2) and Adamalysin10 (ADAM10) foster the accumulation of Aβ and induce the entire range of AD pathology starting with senile plaques of accumulated Aβ, passing through NFT formation and leading to synaptic dysfunction, neuronal loss, brain atrophy and dementia. This lays the foundation of the amyloid cascade hypothesis of AD, is supported by the neurotoxicity of Aβ oligomers in cellular and animal model systems, and proposes that Aβ acts as the initiator to induce neurofibrillary changes that, following a silent asymptomatic period of up to two decades, results in a syndrome known as amnestic mild cognitive impairment (a likely prodrome to dementia) that can develop into AD. Interestingly, it appears the development of detectable entorhinal NFTs, rather than amyloid senile plaques (that can occur in the brain of the healthy elderly), correlates to the development of mild cognitive impairment and is considered to herald incipient AD [38].
BACKGROUND OF AD
AD represents a critical geriatric health issue, and primarily accounts for dementia cases reported after the age of 65 years. The global prevalence of dementia within the elderly population has increased drastically during recent times. Accounting for some 35.6 million cases worldwide in 2010, it is predicted to double over the next 20 years and expected to reach up to 65.7 million by 2030, unless countermeasures can be found and successfully applied [39]. This rising trend has been forecasted not only across industrialized nations, but also in lower and developing economies. In the US alone, 5.4 million individuals were afflicted with AD in 2012, making it the 6th leading cause of death [40].
AD is characterized by late onset neuropsychiatric symptoms, which starts with a subtle decline in cognition (accompanied with behavioral sequellae and a loss of independence in activities of daily living), leading to a substantial loss of higher brain (exceutive) function and, ultimately, to losing motor control; eventually resulting in total incapacitation and development of critical respiratory problems as a major cause of death [9]. The pathological impact on the brain makes AD an irreversible neurodegenerative condition, in which progressive loss of brain neuronal tissue occurs, initially through loss of synapses and dendritic arborization followed by neuronal cell body gray matter loss [9]. At the neuropathological level, AD is characterized by the cellular build-up of misfolded proteins, like as Aβ and cerebral amyloid angiopathy (CAA) in brain, intracellular aggregates of tau in the NFTs and extracellular aggregates of Aβ in the senile plaques [41]. Extracellular accumulation of Aβ, as discussed, appears to represent the first lesion (with declines in CSF levels of Aβ) that forms years before clinical signs; while the induction of NFTs, CAA, neuroinflammation and synaptic loss initiates self-sustaining cycles that drives disease progression towards development of the clinical condition [42].
As Aβ and phosphorylated-tau neuropathology appears to be well established at least a decade or more before the diagnosis of AD, it is perhaps readily understandable why short-term prevention trials to overcome already long-established Aβ, phosphorylated-tau, or possibly other neuropathology, have thus far failed [43]. The association of clinical AD with other disease conditions, exemplified by CAA and other cerebrovascular pathology, suggest that accompanying conditions and not inevitably Aβ and phosphorylated-tau neuropathologies may actually launch many patients into clinically apparent AD. Indeed, AD neuropathology may be a necessary but not an entirely sufficient cause for the onset or expression of clinical dementia in sporadic AD. Age, co-morbidity, vascular pathology, genetic, environmental, a not yet identified biochemical trigger, or individual factors such as a lack of cognitive reserve might be necessary for clinical expression of dementia [43]. Currently, there is a lack of specific knowledge as to the precise timing of the events that lead to MCI and AD, making it difficult to define a therapeutic window for successful preventive efforts. Interestingly, phosphorylated-tau provides an example of the complexity of the AD neuropathological process that results in clinical dementia. Chronic traumatic encephalopathy (CTE) dementia, sometimes termed dementia pugilistica and presenting most often in contact sport athletes (boxers, wrestlers and American Football players) but more recently in military personnel following blast injury, occurs in the absence of significant or in some cases any Aβ amyloid deposition [44]. In this scenario, once initiated P-tau associated neurofibrillary pathology alone appears sufficient to cause clinical dementia; reiterating the problem of finding the correct window of opportunity for drug treatment, as targeting the amyloid cascade after tau-phosphorylation has been initiated likely would not off-set progression to clinical dementia [43].
Anatomical analyses in AD show pathological changes spread throughout the cortex and surrounding structures, particularly the hippocampus where memory formation and consolidation occur, to result in a generalized loss of cortical gray matter and, in particular, an early loss of basal forebrain cholinergic neurons [45]; albeit all neurotransmitter systems are impacted during disease progression. Longitudinal studies in autosomal dominant AD in which there is a predictable age of onset provides an opportunity to define the sequence and degree of pathological changes that give rise to symptomatic disease [46], with the caveat that sporadic AD and FAD are not necessarily alike.
Concentrations of Aβ within CSF appear to gradually fall some 25 years prior to expected symptom onset, with Aβ deposition gradually occurring in brain some 15 years before anticipated symptom onset. Elevated levels of tau and phosphorylated-tau appear in CSF and increasing brain atrophy appears detectable at this same time too: 15 years prior to expected symptom onset. A decline in cerebral metabolism and initial impairments in episodic memory appear evident some 10 years before anticipated symptom onset. Finally, global cognitive impairments appear evident some 5 years prior to expected symptom onset, with patients meeting full diagnostic criteria for dementia by some 3 years following expected symptom onset [46]. This time-dependent progression of neuropathological changes merges with the initial occurrence and then increasing neuropsychiatric changes: troubled or incapacitated skills in learning, short-term memories, abstract thinking and personality changes that then extend to losses in lingual, visuo-spatial capacities and ultimately impairment of motor skills and increasing helplessness.
AD has a multifactorial etiology, with age and familial background as key risk factors. A number of epidemiological studies point towards a role of metal ions (iron homeostasis and copper), prior head injuries, a low educational status and a functional association of the AD cascade has been noted with select chronic disorders, like T2DM, hyperthyroidism and familial Down’s syndrome [9]. As discussed earlier, genetic predisposition additionally impacts AD risk, in which early onset FAD mutations/duplications associated with the APP gene at chromosome 21 [47] as well as select mutations within the PS1 and PS2 lead with certainty to AD associated clinical dementia. Additionally, a relatively infrequent but not rare allele of ApoE, specifically ε4, appears to be highly augmented amongst typical late onset AD patients making it too a key risk factor. In general, ApoE maintains cholesterol and triglyceride homeostasis within the brain periphery, and exists as a combination of three different isoforms, ε2, ε3, and ε4. The less common ApOE ε4/ε4 correlates to the highest AD risk, with the ε2 allele providing some protection against AD disease risk [48]. The mechanisms responsible for ApoE actions in AD remain an area of significant interest and include a possible malfunction in CNS cholesterol homeostasis to induce plaque formation by chaperoning Aβ deposition. Additionally, ApoE may assist Aβ degradation, a process that is less competently undertaken by the ApoE-ε4 allele [48,49].
AD Aβ PEPTIDE GENERATION
As discussed, significant literature supports the awry processing and clearance of Aβ, the major component of amyloid plaques [10, 50–55], in brain as a key initiator of the process that ultimately leads to AD [20]. The length of Aβ varies from 38–43 residues, which is generated from APP via sequential proteolytic cleavage [51, 55, 56]. This APP exists as three common isoforms (APP695, APP751, and APP770), with the former being most abundant in neuronal cells [51, 56]. Resulting APP cleavage can generate Aβ forms that are either non-amyloidogenic or amyloidogenic (disease-related) depending on the protease responsible [56, 57]. In the latter process, β-secretase (β-site APP cleaving enzyme, BACE1) cleaves APP close to its N terminus between residues M671 and D672. Thereafter, a γ-secretase complex, which comprises PS1 or PS2, nicastrin, anterior pharynx-defective-1 (APH-1), and presenilin enhancer-2 (PEN-2), cleaves the remaining fragment of membrane bound APP at the C-terminus resulting in the generation of monomeric Aβ peptides of varying lengths [25, 51, 56].
OLIGOMERIZATION OF Aβ PEPTIDE IN AD
Aβ senile plaques are extracellular depositions of Aβ that are largely 40 or 42 aa in length (Aβ40 and Aβ42) [10, 50–55]. The Aβ40 form is the more common of the two, whereas Aβ42, consequent to the additional two hydrophobic C-terminus amino acids (isoleucine and alanine), is the more fibrillogenic and hence associated with AD. The previously described FAD mutations appear to increase the relative production of Aβ42 (relative to Aβ40) and understanding receptor mediated mechanisms along with molecular chaperons and other proteasomes and homeostatic mechanisms to clear Aβ is hence fundamental [49]. In AD, such housekeeping mechanisms are clearly perturbed [49], leading to the fibrilization of Aβ into amyloid plaques. Indeed, a number of pathways via which Aβ forms are normally cleared from brain have been reviewed [49] and involve the candidate enzymes, insulin-degrading enzyme (IDE), neprilysin (NEP), endothelin-converting enzyme (ECE), angiotensin converting enzyme (ACE), plasmin and matrix metalloproteinases (MMPs) whose actions continue to be evaluated in cell culture and animal models. The pre-fibrillar aggregates of amyloid across a number of studies appear to be more toxic than the mature amyloids, as the former can generate reactive species on neuronal membranes subjecting them to oxidative stress or calcium induced apoptosis [31, 58].
Aβ peptide clearance clearly involves mechanisms supporting its eflux out of brain across the blood-brain barrier. In aged brains, failue of this system supports Aβ accumulation within brain arteries, replacing the smooth muscle tissues and leading to CAA. CAA may subsequently increase the risk for intra-cerebral haemorrhage that, in turn, may give rise to vascular dementia [47, 59].
Aβ can exist in various aggregation states, including monomers, oligomers (sometimes termed Aβ-derived diffusible ligands (ADDLs)) and eventually insoluble fibrils. This general term ‘oligomers’ includes diverse types of assemblies, epitomized by dimers, trimers, protofibrils, ADDLs and annular or pore-like oligomers [60, 61]. Cerf and colleagues have suggested that oligomers may be classified into pre-fibrillar or fibrillar oligomers, as they appears to have different aggregation pathways [60]. In general, Aβ oligomers are considerd to be small, soluble oligomers that can include up to five or six monomeric units or ADDLs, and protofibrils [62–67]. It is considered that Aβ oligomers might represent intermediates with the developmental pathway of amyloid fibril, rather than an essential factor in fibril formation [60, 68].
In the last few years, it has been becoming increasingly evident that rather than the plaques, the Aβ oligomers are the principle pathogenic agents in AD [62, 69]. Indeed, scientific literature, supporting the notion that oligomeric intermediates are comparatively more toxic and better correlate with Alzheimer’s symptomology than does final insoluble Aβ aggregates, is growing [25, 60]. Mice genetically engineered to express oligomers but not plaques (APP(E693Q)) developed the disease. Additionally, mice that are further engineered to transform oligomers into plaques (APP(E693Q) x PS1ΔE9) appeared to be no more cognitively impaired than oligomer alone mice [64]. Amongst the numerous differences between oligomers of different sizes and insoluble brain Aβ deposits is the smaller diameter of Aβ oligomers that increases their likely ability to more readily diffuse into synaptic clefts and induce neuronal and synaptic dysfunction [25].
The process of amyloid plaque formation starts with an increased concentration of total Aβ and a higher Aβ42/Aβ40 ratio that gradually leads to oligomerization and, ultimately, formation of Aβ deposits that results in inflammatory responses, astrocytic activation, synaptic spine loss and neuritic dystrophy [25]. Whereas the general process via which Aβ monmers can, via intermediates (oligomers, ADDLs), eventually generate senile plaques is agreed upon, the potential via which reversal might occur (i.e., whether soluble Aβ could derive from a plaque) remains unknown. It has been reported that the structure of Aβ, rather than its sequence, plays the principle role in Aβ induced toxicity [70]. The β-sheet have been reported as the dominating structure in Aβ oligomers [60]. Indeed, Cerf and colleagues [60] have reported a specific anti-parallel β-sheet structure in Aβ-(1–42) oligomers. They reported spectral similarities between Aβ oligomers and pore-forming porins, and advocated that the ability of Aβ oligomers to develop a porin-like structure might be linked to their toxicity in AD.
A further key pathologic feature of AD, tauopathy, has likewise been reported to be promoted by oligomerization. Tau oligomers have been reported as pathological structures associated with AD progression in mouse models that play a prime function in behavioral impairments and resulting neurodegeneration [71–73]. There are increasing reports of the higher toxicity of tau oligomers compared with tau filaments, and they have been proposed as precursors of tau filaments [71, 74, 75] that can potentially drive the disease process [73].
Furthermore, small oligomeric forms of α-synuclein, the protein associated with Parkinson’s disease, have likewise been reported as a key factor implicated in neuronal death [73, 76]. The potent toxicity of α-synuclein oligomers and their ability to induce membrane dysfunction and breakdown have been described both in cellular and animal models [70, 77–80]. The conformational organization of oligomeric α-synuclein contains β-sheet structural elements [70, 81]. Indded, increasing evidence supports a basis for multi-pathway aggregation of α-synuclein and should considered in studies focused towards elucidating the molecular mechanisms of its fibrillation [82].
ROLE OF Aβ STRUCTURE IN AD
Select alterations within specific proteins or peptides from their native conformations and their subsequent aggregation into insoluble fibrils has been proposed as a prime initiator and cause of several neurodegeneratice conditions, including AD [70]. These conformational changes in proteins can both define and differentiate various diseases [83]. As described for AD, growing evidence points towards monomeric Aβ peptides as building blocks for amyloid fibers via a range of intermediate structures [62, 84]. A conformational shift from α-helix to β-sheet within the protein structure has been reported during the aggregation of amyloid fibrils associated with AD [85]. Although, a universal structure for Aβ fibrils does not exist, several antiparallel β-sheets structural models have been proposed in earlier studies [86–90]. Native Aβ peptide in AD is α-helix rich and polymorphic at the molecular structural level [85, 91].
The process of Aβ fibrillation involves a conformational shift which ultimately leads to the formation of extended β-sheets [19]. Involvement of an oligomeric α-helix containing intermediate has been proposed as a key step in Aβ fibrillogenesis [19]. Aβ peptide 1–40 fibril polymorphs share a common, parallel β-sheet organization and take on similar peptide conformations but diverge in their overall symmetry and in other structural features. In a study on the disease-associated mutant, (D23N)Aβ40, the researchers reported stabilized parallel and antiparallel β-sheets within the amyloid fibrils [92]. The role of antiparallel β-sheet structures are suggested for fibrils that are formed by short peptides with one β-strand segment only. Certain structural models for Aβ fibrils include a β-hairpin with intramolecular backbone hydrogen bonding between the β-strand segments on either side of a β-turn present between Val-24 and Asn-27 [90], Gly-25 and Lys-28 [87, 88], or Ile32 and Gly-37 [89]. Hence there appears to be key regions within the structure of Aβ that favor the adoption of β-strand generation, residues 12 to 24 as well as 30 to 40, with the presence of a turn occuring primarily between these two regions, residues 25 to 30 [15, 50, 93, 94]. This turn is stabilized by electrostatic interactions and supports the two separate β-strands forming two parallel β-sheets in close contact via their side chains. In accord with this, key mutations leading to early AD are mostly localized at amino acids residues 21 or 22 of Aβ (within Aβ: Flemish mutation (Ala-21-Gly), Dutch (Glu-22-Gln), Italian (Glu-22-Lys), Arctic (Glu-22-Gly), Iowa (Asp-23-Asn)) [95–99]. Indeed, consequent to the early misfolding and protein-protein interaction regions associated with Aβ, the sequence in the region of amino acids 16 to 21 is widely recognized as the self-recognition element underpinning self-aggregation, and has provided the basis for the development of rationally-designed peptide-based aggregation inhibitors with the potential to competitively interact with this key region and thereby inhibit/interfere with polymerization [50, 93, 94].
Although study of the molecular mechanism underpinning of Aβ fibrillogenesis has been the focus of numerous studies [94], the precise process of protein misfolding and aggregation still remains to be fully elucidated. A critical event in the aggregation cascade appears to be the development of small oligomers that provide a core/nucleus to then catalyze protein misfolding via a process identified as the nucleation-dependent polymerization model [100]. This model has been proposed for a series of diseases involving protein misfolding, in addition to accounting for Aβ aggregation [50, 94]. The model envisions two phases: a lag phase followed by an elongation phase [15, 94, 100]. In the initial lag phase, Aβ self-associates into small oligomeric species when a critical concentration is exceeded within the local environment to provide an ordered nucleus to catalyze the further growth of the polymer by providing a template for fibril growth during the more rapid elongation phase. Hence the lag (nucleation) phase is the rate-limiting nucleus formation step as it involves a thermodynamically and kinetically unfavorable process since noncovalent interactions between monomers are relatively weak. However, once the nucleus has developed, it provides a seed for exponential fibril growth. This growth phase persists until a steady-state is achieved between available Aβ aggregates and monomers. With enlargement, depending on the conformational stability of the growing amyloid structure, the fibril can ultimately break either naturally or as a result of an active cellular process. The potential generation and dispersion of new amyloid seeds can thereby result. The presence of such pre-formed nuclei can then serve as a template to seed further fibril growth, with amyloid formation becoming self-propagating. Such a seeding process can reduce or eliminate the initial nucleation phase [93, 101], and both changes in transition metal content (i.e., Cu and Zn, and potentially Fe) [15] as well as the presence of other proteins that can modify aggregation rate – either accelerating or lowering it (i.e., acetylcholinesterase and butyrylcholinesterase, respectively [102, 103]), as reviewed by DeToma and colleagues [15].
Elegant studies in cell culture and transgenic AD mice by Jucker and colleagues [104–106] have demonstrated that injection of Aβ seeds, whether small or large, soluble or insoluble, protease-sensitive or resistant can generate amyloid deposits both locally within the brain and eventually in axonally linked regions. Indeed, with sufficient time, extensive brain areas can become compromised, including neocortical and subcortical regions as occurs in affected AD subjects. Interestingly, Aβ deposition in brain occurred even after peritoneal cavity administration of Aβ extract. These studies support the proposition that Aβ aggregation and deposition can potentially seed by a process that has parallels to the molecular templating of prion proteins [50,94,106].
AGGREGATED TAU AND α-SYNUCLEIN
Increasing evidence from cellular and animal studies suggests that the prion-like seeding process may additionally pertain to other pathological proteins, several of which generate intracellular amyloid-like inclusions. As discussed, a further pathological hallmark of AD is the presence of hyperphosphorylated forms of the microtubule associated protein tau within key brain regions that aggregate to form NFTs, which bind Congo red and exhibit a cross β-sheet conformation. The human tau gene yeilds six isoforms of between 50 and 70 kDa size that normally have minimal distinct structure and are considered unfolded [107, 108]. Alterations in the amount and/or the structure of tau protein can impact its role as a microtubule stabilizer [109], as it interacts with microtubules via binding domains localized both in a proline-rich sequence within the N-terminal and via some 4 regions in the the C-terminal half of the molecule. Those in the C-terminal are comprised of highly conserved amino acid binding elements (18 aa length) that are separated by less conserved inter-repeat sequences (13 to 14 aa length) [73, 110, 111]. Microtubule binding activity is regulated through phosphorylation/dephosphorylation of select residues at multiple sites, together with intra- and intermolecular interactions, in a complex manner [110, 111]. Several studies have described abnormal tau posttranslational modifications that include hyperphosphorylation, acetylation, glycation, nitration, truncation, and others that modify the structure of tau in AD [109–115]. Kolarova and colleagues [109] proposed that both the number of NFTs and the state of proteolysis at the C-terminus of tau (associated with conformational changes and microtubule interaction) are vaulable to define AD progression [109]. In addition to reported β-sheet conformations, studies have described a role of paired α-helical filament conformations in tau pathogenesis [116] that can occur in a wide number of tauothies in addition to AD [73, 110, 111].
In a manner similar to that described for Aβ, the intracerebral administration of brain extracts containing aggregated tau [117,118] or even purified tau [119, 120] to tau transgenic as well as normal wild-type mice appear to induce tau lesions that then have the potential to spread to axonally linked brain areas. Tau seeding has likewise been demonstrated in cellular studies [121], and appears to occur across different seed sizes, and even soluble forms [106, 120, 122].
The phospholipid-binding protein α-synuclein that is expressed highly in presynaptic terminals where it functions as a molecular chaperone to promote SNARE complex formation to regulate synaptic vesicle formation and synaptic function, likewise, undergoes aggregation. Misfolded α-synuclein assemblies develop into intracellular fibrillar inclusions, termed Lewy bodies and Lewy neurites in α-synucleinopathies exemplified by Parkinson’s disease and dementia with Lewy bodies [123, 124]; albeit Lewy bodies are found across a number of neurodegenerative conditions, including AD, indicating that overlapping pathologies exist amongst the neurodegenerative disorders. Like tau, α-synuclein normally exists in an unfolded state as a soluble monomer (approximately 14 kDa size) that lacks a distinct secondary or tertiary structure [125, 126] and that clearly undergoes conformational changes to generate an insoluble neurotoxic form. It has been reported to attain an α-helical secondary structure upon its interaction with and binding to the lipid wall of vesicles, and to natively form α-helically folded tetramers (58 kDa) when isolated under non-denaturing conditions [127, 128]. Such tetramers are considered its physiologically relevant form that appears to be aggregation resistant [128]. By contrast, recombinantly expressed monomers of α-synuclein have been reported to readily aggregate into amyloid-like fibrils in vitro [127]; suggesting that helically folded tetramer formation may be a favored mechanism for this protein to minimize aggregation, and that mechanisms leading to the destabilization or reduction in native tetramer formation may support aberrant monomer aggregation [127, 128], as soluble oligomers have been reported elevated within the cortical tissue of subjects with idiopathic Parkinson’s diseases and dementia with Lewy bodies [128]. Disease generating missense mutations and multiplications of the α-synuclein gene [129] in addition to oxidative stress [130] and post-translational modifications such as phosphorylation [131, 132] have similarly been described to impact the aggregation rate of α-synuclein, as have truncation [133], the presence of fatty acids and metal ions, and loss of chaperones [128, 134].
In vitro studies by Aperti and colleagues have shown that oligomers appear to be globular in form and of variable size during early stages, but following incubation they become elongated and generate protofilaments. As α-synuclein oligomers change configuration their α-helical secondary structure declines (reducing from 47% in globular spheroidal oligomers to some 37% in protofilaments). Simultaneously, as protofilaments form, their β-sheet structure rises (to 54% from some 29% present in spheroidal oligomers) so that β-sheets comprise the major conformation (approximately 66%) within the final formed fibrils [135, 136]. In a manner parallel to that described for tau and Aβ, intracerebral injections of synthetic (whether human or mouse) α-synuclein fibrils [137, 138], or an autopsy-derived brain extract from a subject with dementia with Lewy bodies [139], induced neuronal dysfunction and neurodegeneration involving a Lewy-body-like pathology in wild-type host mice with cell-to-cell transmission into anatomically interconnected regions. By contrast, similar intracerebral inject of soluble α-synuclein was not accompanied by neuronal dysfunction or pathology [139]. Interestingly, Guo and colleagues [124] reported that α-synuclein fibril brain inoculation can not only seed further α-synuclein aggregation and pathology but also, depending on its conformation, the promotion of tau cross-seeding; again underpinning the tremendous heterogeneity evident in the pathology associated with neurodegenerative disorders and particularly of synucleinopathies.
BACKGROUND OF T2DM
Diabetes mellitus comprises a group of metabolic disorders characterized by prolonged high blood glucose levels and a loss of glucose homeostasis. Whereas type I diabetes mellitus is due to a lack of insulin production, in T2DM insulin demand from pancreatic β-cells is insufficient consequent to elevated requirement in the context of insulin resistance [140]. T2DM accounts for up to 90% of total diabetes incidence and has become a major health problem globally [141]. Approximately 285 million people suffer from T2DM worldwide [142], which associates with a serious complications leading to increased risks of vascular dysfunction (alterations in blood pressue), loss in vision (diabetic retinopathy), impairments of cognitive function (dementia) and deficiences in kidney function (requirement of dialysis) [143, 144]. In recent years, prediabetes and T2DM appear to be reaching epidemic proportions particularly in industrailized nations, with significant associated socio-economic impact, loss in productivity and increased long-term health care cost.
Several classes of medications have proved effective in managing T2DM including Metformin – of the biguanide drug class, sulfonylureas such as Gluburide, dipeptidyl peptidase 4 inhibitors epitomized by Sitagliptin, thiazolidinediones such as Rosi- and Pioglitazone, and incretin mimetics epitomized by exenatide, and are widely used [145]. These, particularly when combined with the management of lifestyle and complimentary therapeutic approaches, can prove to be yet more effective and can additionally potentially mitigate T2MD related conditions [146–149]. For example, exercise, a low calorie balanced diet, and a reduction in body weight appear to be effective therapies that aid in the upregulation of endogenous protective factors and resetting of homeostatic metabolic processes [150–153]; albeit further elucidation of underlying mechanism is needed [153].
PROTEIN FOLDING AND MISFOLDING IN T2DM
Like NDDs, T2DM is considered as a degenerative metabolic disorder that occurs with greatest prevelance in late age [15]. Although the early underlying biochemical defects that lead to T2DM have yet to be fully clarified, the presence of proteinaceous plaques that primarily comprise IAPP is found in the majority of patients (approximately 90%), is largely absent in healthy subjects [154], is considered a significant contributor in T2DM pathogenesis and progression [16] and recognized as a hallmark for diagnosis of this disease [17, 155].
IAPP is part of the calcitonin-like family of polypeptides and is present across animal species. Although, it does not form amyloid in all species (for example, it does not in wild-type rats and mice), it is extremely amyloidogenic in humans [155, 156]. IAPP derives from an 89 aa pre-prohormone. The elimination of its 22 residue signal sequence results in 67 aa pro-IAPP. This is then further processed within the Golgi and insulin secretory granule of pancreatic β-cells to yield the mature hormone IAPP of 37 aa length [157]. Important post-translational modifications comprise an intramolecular disulfide bridge (formed between residues 2 and 7) together with amidation of the C-terminal to provide a physiologically relevant peptide, which is amassed within the insulin secretory granule (in a ratio of between 1:50 and 1:100 relative to insulin) and is co-secreted with insulin [156,157]. The primary physiological actions of IAPP are receptor mediated, not fully elucidated in humans, but appear to involve a key role in glucose homeostasis by enhancing the effects of insulin. This is largely achieved by suppressing the release of glucagon from pancreatic α-cells to thereby prevent glucose release from the liver, by reducing gastric emptying, and by stimulating the satiety center within the brain [158]. IAPP, however, is predisposed to concentration-dependent amyloidosis that causes cellular dysfunction consequent to membrane disruption, channel formation and toxicity.
A key difference between amyloidogenic human IAPP and the non-amyloidogenic rat polypeptide is amino acid changes at 6 positions. In particular, there appear to be conserved regions within the two terminal parts of the polypeptides (specifically residue 1–16 and residue 30–37) that appear to be conserved in all calcitonin family peptides and hence likely play key biological roles. The former region is considered involved in activating the receptor and the latter in binding in an antagonistic manner [159]. The middle domain between these two conserved regions largely differentiates the different IAPP forms (residues 18–29) and appears responsible for the different aggregation propensities. In rat IAPP there are three prolines (positions 25, 28 and 29) and these are believed responsible for its inability to form amyloid. Much attention has been focused on the sequence within the 20–29 regions and the role it plays in controlling amyloid formation. Prolines and N-methyl amino acids are known to disrupt β-sheet formation [50, 93, 94]. The relevance of this mid region in aggregation is underlined by a single point mutation, a Ser20Gly substitution, which enhances amyloid formation, leads to β-cell death and an early onset familial form of T2DM found in Japan [160]. Of note, mutations outside of this mid region can eliminate amyloid formation, indicating that one cannot rule out contributions from other regions impacting IAPP’s amyloidogenicity [161, 162].
Studies by Wu and Shea [159] suggest that IAPP does not form a single well-defined three-dimensional structure, but can interconvert between several co-existing conformations. Interestingly, rat IAPP has been found to possess only α-helix-coil conformations, whereas human IAPP can assume two discrete conformers encompassing helix-coil conformations and β-rich conformations that include a helix-hairpin and an extended β-hairpin [159]. This β-hairpin structure, that is present only in the amyloidogenic IAPP forms, appears to be the likely candidate to generate an amyloid-capable structure, as β-hairpin structures are found across a number of amyloidogenic peptides. Additionally, the β-hairpin structure within IAPP appears to be less soluble and flexible than helix-coil structures, and thus represents a hot-spot to facilitate nucleation and ensuing fibril growth [159, 163]. In support of this, the higher aggregation rate associated with the described Ser20Gly IAPP mutation [164] can be justified by an increased predilection to generate a β-turn, as glycines are considered turn inducers and IAPP residue 20 sits directly within the turn region of our hairpin [159, 163].
Other factors that likely also influence the rate of aggregation of human IAPP and include the pH of the microenvironment of the polypeptide, impacting its solubility, with an acidic pH inhibiting fibrillization. Likewise, interaction with metal ions, particularly Zn2+ and Cu2+, as well as with other proteins, principally insulin and C-peptide, have been described to both interact with human IAPP and modify its homeostasis and oligomerization [15]. The presence of such factors is of considerable physiological relevance as, in contrast to Aβ for which processing by different endogenous secretase activities results in the generation of discrete peptides with different amyloidogenic propensity [37, 93, 97–99], the very same human IAPP of 37 aa length is generated and secreted in health and T2DM [15, 154–156]. Hence in healthy individuals regulatory mechanisms must be present to limit its tendency to aggregate into cytotoxic oligomers or efficient mechanisms must exist to clear them before they induce adverse actions. Interestingly, unlike humans, non-human primates, dogs and cats that have IAPPs with high aggregation propensities and that all have the potential to develop T2DM, other species that are renowned for their ability endure excessive food consumption without apparent health complications, such as pigs and rodents, have IAPPs with low aggregation propensities and do not normally develop T2DM [159, 163]. In line with this, a sedentary lifestyle in combination with excessive high calorie food consumption, as compared to our ancestors, appears to be driving human T2DM globally [164].
In individuals with a long-term constantly raised blood sugar level (chronic hyperglycemia) their β-cells would be expected to secrete compensatory elevated levels of insulin together IAPP and, with a shift in lipid metabolism towards conversion of excessive glucose into lipids, would chronically stress this complex homeostatic system [165]. The generation of excess free radicals provides the potential to further disrupt the charge and conformation of the native proteins and proficiency of clearance mechanism, eventually impacting their folding and modifying their physiological role into a pathological one [165–167].
LINKAGE BETWEEN AD AND T2DM
AD and T2DM are both prevalent in the aged population. Whereas the cerebral accumulation of Aβ is a major pathological hallmark of AD [168], the deposition of a very different polypeptide, amylin, that likewise succumbs to β-sheet formation and self-aggregation occurs within the pancreas, especially in β-cells, in T2DM [169]. While T2DM leads to pathological angiogenesis and immature vascularization [170], it also results in chronic cerebral hypoperfusion leading to neuro-glial dysfunction and degeneration [171]. Individuals with T2DM are at increased risk of suffering transient ischemic attacks that, although often silent, can leads to severe consequences in the long-term, including dementia [172]. In part, such dementia is associated vascular dysfunction, better termed as vascular cognitive impairment [173]. It is now widely recognized that Aβ is generated in the normal brain in an excitation-dependent manner, and may have physiological function(s) [174, 175]; however, a healthy vascular system aids in its proper and timely clearance. This is critical as once oligomerization and deposition of Aβ starts, the processes of seeding and spread together with neuroinflammation and other events are initiated, and neuronal dysfuction and toxicity ensue [176]. With the occurrence of defective insulin signaling, insulin resistance, and mitochondrial dysfuction within the brain [177–179], common denominators are clearly apparent between T2DM and AD; posing the question as to whether one leads to the other in aged individuals.
CONCLUSION
By far the majority of cellular functions throughout the body, whether occuring within the pancreas or brain, are perform by involvement of different types of proteins. As a consquence, cells possess a complex system of mechanisms to maintain protein homeostasis (proteostasis) and efficiently regulate protein synthesis and assembly to provide a functionally active state that is optimally balanced to the cell’s changing microenviromental needs [180]. Deficiencies in proteostasis are becoming increasingly recognized as an overarching mechanism through which chronic protein misfolding and self-aggregation, often accompanied by impairments in clearance mechanisms [49], leads to cellular dysfunction and apoptosis [180]. The resulting protein aggregates lack their functionally active conformation and not only impart local stress and toxic effects but can impose global detrimental properties. This can instigate self-propagating destructive cycles, such as chronic inflammation, that can further drive proteome imbalance and result in a number of different age-dependent degenerative diseases. As such processes occur over a very long and ‘clinically silent’ time, defining drug targets for treatment and engaging them during the window of opportunity of when they are relevant to impact disease progression has proved extraordinarily difficult [43] and, together with other factors, has substantially contributed to drug development failures in NDDs [181, 182]. Elucidating protein-misfolding has consequently become a hot spring for modern scientific investigation as it impacts the pathogenesis of wide array of diseases, including AD and T2DM.
Although significant advancements have been accomplished in this field, our understanding of proteostasis mechanisms and how to rebalance and to effectively and safely modulate them as a viable strategy for therapeutic intervention remains limited. Likely consequent to the organizational principles that regulate proteostasis mechanims across diverse cells within the body, numerous studies have revealed that there is a strong correlation between aggregate-deposition diseases, albeit a different misfolded protein may be involved. An agedependent decline in proteostasis capacity and deregulation in homeostatic mechansism may account for age representing a major risk factor for such diseases, and underpin the vulnerability of individuals presenting with multiple pathologies and succumbing to more than one disorder. A greater in-depth knowledge of the cascades that regulate and modify proteostasis may provide new insights into the development of novel drug targets and potential drugs that might help in the better management and effective treatment of diseases associated with protein misfolding, exemplified by AD and T2DM.
Acknowledgments
The authors are grateful to (i) the Deanship of Scientific Research (DSR), (ii) King Fahd Medical Research Center (KFMRC), King Abdulaziz University (Jeddah, Saudi Arabia), and (iii) The Intramural Research Program of the National Inbstitute on Aging, National Institutes of Health for support. Thanks are additionally due to Mohammad S Gazdar (Librarian, KFMRC) for providing assistance in searching the scientific literature.
Abbreviations
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- CAA
Cerebral amyloid angiopathy
- ER
Endoplasmic reticulum
- NDDs
Neurodegenerative diseases
- NFTs
Neurofibrillary tangles
- T2DM
Type 2 Diabetes Mellitus
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Chen W, Liu X, Huang Y, Jiang Y, Zou Q, Lin C. Improved method for predicting protein fold patterns with ensemble classifiers. Genet Mol Res. 2012;11(1):174–181. doi: 10.4238/2012.January.27.4. [DOI] [PubMed] [Google Scholar]
- 2.Brito RMM, Dubitzky W, Rodrigues JR. Protein folding and unfolding simulations: a new challenge for data mining. OMICS. 2004;8(2):153–166. doi: 10.1089/1536231041388311. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Nussinov R. Protein Folding Protocols. Springer; 2007. [Google Scholar]
- 4.Dobson CM, Evans PA, Radford SE. Understanding how proteins fold: the lysozyme story so far. Trends Biochem Sci. 1994;19(1):31–37. doi: 10.1016/0968-0004(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 5.Ballew RM, Sabelko J, Gruebele M. Direct observation of fast protein folding: the initial collapse of apomyoglobin. Proc Natl Acad Sci USA. 1996;93(12):5759–5764. doi: 10.1073/pnas.93.12.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascher T, Chesick JP, Winkler JR, Gray HB. Protein folding triggered by electron transfer. Science. 1996;271(5255):1558–1560. doi: 10.1126/science.271.5255.1558. [DOI] [PubMed] [Google Scholar]
- 7.Dobson CM, Šali A, Karplus M. Protein Folding: A Perspective from Theory and Experiment. Angewandte Chemie International Edition. 1998;37(7):868–893. doi: 10.1002/(SICI)1521-3773(19980420)37:7<868::AID-ANIE868>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Pietzsch J. Protein Folding diseases. http://www.nature.com/horizon/proteinfolding/background/disease.html.
- 9.Mirza Z, Ali A, Ashraf GM, Kamal MA, Abuzenadah AM, Aga C, Damanhouri GA, Sheikh IA. Proteomics Approaches to Understand Linkage Between Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS & neurological disorders drug targets. 2013 doi: 10.2174/18715273113126660144. [DOI] [PubMed] [Google Scholar]
- 10.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 11.Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harbor perspectives in medicine. 2012;2(5) doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serpell LC, Smith JM. Direct visualisation of the β-sheet structure of synthetic Alzheimer’s amyloid. J Mol Biol. 2000;299(1):225–231. doi: 10.1006/jmbi.2000.3650. [DOI] [PubMed] [Google Scholar]
- 13.Aliev G, Ashraf GM, Kaminsky YG, Sheikh IA, Sudakov SK, Yakhno NN, Benberin VV, Bachurin SO. Implication of the nutritional and nonnutritional factors in the context of preservation of cognitive performance in patients with dementia/depression and Alzheimer disease. Am J Alzheimers Dis Other Demen. 2013;28(7):660–670. doi: 10.1177/1533317513504614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminsky YG, Reddy VP, Ashraf GM, Ahmad A, Benberin VV, Kosenko EA, Aliev G. Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia. Aging and disease. 2013;4(5):244–255. doi: 10.14336/AD.2013.0400244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chemical Society reviews. 2012;41(2):608–621. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52(9):2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 17.Kapurniotu A. Amyloidogenicity and cytotoxicity of islet amyloid polypeptide. Biopolymers. 2001;60(6):438–459. doi: 10.1002/1097-0282(2001)60:6<438::AID-BIP10182>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Dobson CM. Protein-misfolding diseases: Getting out of shape. Nature. 2002;418(6899):729–730. doi: 10.1038/418729a. [DOI] [PubMed] [Google Scholar]
- 19.Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J Mol Biol. 2001;312(5):1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 20.Dobson CM. Protein misfolding, evolution and disease. Trends in biochemical sciences. 1999;24(9):329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 21.Reynaud E. Protein misfolding and degenerative diseases. Nature Education. 2010;3(9) Article-28. [Google Scholar]
- 22.Cooper GM. Protein Folding and Processing. http://www.ncbi.nlm.nih.gov/books/NBK9843/
- 23.La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet. 2010;11(4):247–258. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda H, Jafar-Nejad H, Patel AJ, Sun Y, Chen H-K, Rose MF, Venken KJT, Botas J, Orr HT, Bellen HJ, Zoghbi HY. The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins. Cell. 2005;122(4):633–644. doi: 10.1016/j.cell.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 26.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 27.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47(6 Suppl 3):S161–170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 28.Bertram L, Tanzi RE. Alzheimer’s disease: one disorder, too many genes? Hum Mol Genet. 2004;13(Spec No 1):R135–141. doi: 10.1093/hmg/ddh077. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal K, Wisniewski HM, Grundke-Iqbal I, Korthals JK, Terry RD. Chemical pathology of neurofibrils. Neurofibrillary tangles of Alzheimer’s presenile-senile dementia. J Histochem Cytochem. 1975;23(7):563–569. doi: 10.1177/23.7.1141687. [DOI] [PubMed] [Google Scholar]
- 30.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging. 2005;26(7):1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81(11):678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 32.Kamal MA, Shahida K, Gan SW, Firoz CK, Khan A, Abuzenadah A, Kamal W, Aliev G, Reale M. Alzheimer’s Disease and Type 2 Diabetes Mellitus: The Link to Tyrosine Hydroxylase and Probable Nutritional Strategies. CNS Neurol Disord Drug Targets. 2013 doi: 10.2174/18715273113126660153. [DOI] [PubMed] [Google Scholar]
- 33.Aliev G, Obrenovich ME, Tabrez S, Jabir NR, Reddy VP, Li Y, Burnstock G, Cacabelos R, Kamal MA. Link between cancer and Alzheimer disease via oxidative stress induced by nitric oxide-dependent mitochondrial DNA overproliferation and deletion. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/962984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 35.Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32(3):150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Lai MK, Chen CP, Hope T, Esiri MM. Hippocampal neurofibrillary tangle changes and aggressive behaviour in dementia. Neuroreport. 2010;21(17):1111–1115. doi: 10.1097/WNR.0b013e3283407204. [DOI] [PubMed] [Google Scholar]
- 37.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 38.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115(5):1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 42.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1) doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker RE, Greig NH, Giacobini E, Schneider LS, Ferrucci L. A new roadmap for drug development for Alzheimer’s disease. Nat Rev Drug Discov. 2014;13(2):156. doi: 10.1038/nrd3842-c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther. 2014;6(1):4. doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C-K, Thal L, Pizzo D, Hansen L, Masliah E, Geula C. Apoptotic signals within the basal forebrain cholinergic neurons in Alzheimer’s disease. Exp Neurol. 2005;195(2):484–496. doi: 10.1016/j.expneurol.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38(1):24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 48.Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994;53:429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Baranello RJ, Bharani K, Padmaraju V, Chopra N, Lahiri DK, Greig NH, Pappolla MA, Sambamurti K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr Alzheimer Res. 2014 doi: 10.2174/1567205012666141218140953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 51.Jakob-Roetne R, Jacobsen H. Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl. 2009;48(17):3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 52.Scott LE, Orvig C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem Rev. 2009;109(10):4885–4910. doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 53.Bonda DJ, Lee H-g, Blair JA, Zhu X, Perry G, Smith MA. Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics. 2011;3(3):267–270. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauk A. The chemistry of Alzheimer’s disease. Chem Soc Rev. 2009;38(9):2698–2715. doi: 10.1039/b807980n. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenthaler SF, Haass C, Steiner H. Regulated intramembrane proteolysis--lessons from amyloid precursor protein processing. J Neurochem. 2011;117(5):779–796. doi: 10.1111/j.1471-4159.2011.07248.x. [DOI] [PubMed] [Google Scholar]
- 57.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chemical reviews. 2006;106(6):1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 58.Stefani M. Generic cell dysfunction in neurodegenerative disorders: role of surfaces in early protein misfolding, aggregation, and aggregate cytotoxicity. Neuroscientist. 2007;13(5):519–531. doi: 10.1177/1073858407303428. [DOI] [PubMed] [Google Scholar]
- 59.Weller RO, Nicoll JA. Cerebral amyloid angiopathy: pathogenesis and effects on the ageing and Alzheimer brain. Neurol Res. 2003;25(6):611–616. doi: 10.1179/016164103101202057. [DOI] [PubMed] [Google Scholar]
- 60.Cerf E, Sarroukh R, Tamamizu-Kato S, Breydo L, Derclaye S, Dufrêne YF, Narayanaswami V, Goormaghtigh E, Ruysschaert J-M, Raussens V. Antiparallel beta-sheet: a signature structure of the oligomeric amyloid beta-peptide. Biochem J. 2009;421(3):415–423. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- 61.Bitan G, Fradinger EA, Spring SM, Teplow DB. Neurotoxic protein oligomers--what you see is not always what you get. Amyloid. 2005;12(2):88–95. doi: 10.1080/13506120500106958. [DOI] [PubMed] [Google Scholar]
- 62.Baumketner A, Bernstein SL, Wyttenbach T, Bitan G, Teplow DB, Bowers MT, Shea J-E. Amyloid beta-protein monomer structure: a computational and experimental study. Protein Sci. 2006;15(3):420–428. doi: 10.1110/ps.051762406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, Rozovsky I, Stine WB, Snyder SW, Holzman TF. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neurol. 1995;136(1):22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 64.Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, Levey AI, Krafft GA, Levy E, Checler F, Glabe C, Bilker WB, Abel T, Schmeidler J, Ehrlich ME. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Ann Neurol. 2010;68:220–30. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158(2):328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 67.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24(4):219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 68.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282(14):10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 69.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277(35):32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 70.Celej MS, Sarroukh R, Goormaghtigh E, Fidelio GD, Ruysschaert J-M, Raussens V. Toxic prefibrillar α-synuclein amyloid oligomers adopt a distinctive antiparallel β-sheet structure. Biochem J. 2012;443(3):719–726. doi: 10.1042/BJ20111924. [DOI] [PubMed] [Google Scholar]
- 71.Davidowitz EJ, Chatterjee I, Moe GS. Targeting tau oligomers for therapeutic development for Alzheimer’s disease and tauopathies. Current Topics in Biotechnology. 2008;4:47–64. [Google Scholar]
- 72.Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of Pathological Tau Species and Memory Loss in a Conditional Model of Tauopathy. J Neurosci. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626–36. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gómez-Ramos A, Díaz-Hernández M, Cuadros R, Hernández F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Letters. 2006;580(20):4842–4850. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 75.Gómez-Ramos A, Díaz-Hernández M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci. 2008;37(4):673–681. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39(10):2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 77.Kim H-Y, Cho M-K, Kumar A, Maier E, Siebenhaar C, Becker S, Fernandez CO, Lashuel HA, Benz R, Lange A, Zweckstetter M. Structural properties of pore-forming oligomers of alpha-synuclein. J Am Chem Soc. 2009;131(47):17482–17489. doi: 10.1021/ja9077599. [DOI] [PubMed] [Google Scholar]
- 78.van Rooijen BD, Claessens MMAE, Subramaniam V. Membrane binding of oligomeric α-synuclein depends on bilayer charge and packing. FEBS Letters. 2008;582(27):3788–3792. doi: 10.1016/j.febslet.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 80.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279(45):46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 81.van Rooijen BD, Claessens MMAE, Subramaniam V. Lipid bilayer disruption by oligomeric alpha-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim Biophys Acta. 2009;1788(6):1271–1278. doi: 10.1016/j.bbamem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Hong D-P, Han S, Fink AL, Uversky VN. Characterization of the non-fibrillar α-synuclein oligomers. Protein Pept Lett. 2011;18(3):230–240. doi: 10.2174/092986611794578332. [DOI] [PubMed] [Google Scholar]
- 83.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6(1):11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 84.De Anda-Hernndez MA, Lira-De Len KI, Mena R, Campos-Pea VAM. In: Neuroscience - Dealing With Frontiers. Contreras CM, editor. InTech; 2012. [Google Scholar]
- 85.Ding F, Borreguero JM, Buldyrey SV, Stanley HE, Dokholyan NV. Mechanism for the alpha-helix to beta-hairpin transition. Proteins. 2003;53(2):220–228. doi: 10.1002/prot.10468. [DOI] [PubMed] [Google Scholar]
- 86.Chaney MO, Webster SD, Kuo YM, Roher AE. Molecular modeling of the Abeta1-42 peptide from Alzheimer’s disease. Protein Eng. 1998;11(9):761–767. doi: 10.1093/protein/11.9.761. [DOI] [PubMed] [Google Scholar]
- 87.George AR, Howlett DR. Computationally derived structural models of the beta-amyloid found in Alzheimer’s disease plaques and the interaction with possible aggregation inhibitors. Biopolymers. 1999;50(7):733–741. doi: 10.1002/(SICI)1097-0282(199912)50:7<733::AID-BIP6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 88.Li L, Darden TA, Bartolotti L, Kominos D, Pedersen LG. An atomic model for the pleated beta-sheet structure of Abeta amyloid protofilaments. Biophys J. 1999;76(6):2871–2878. doi: 10.1016/S0006-3495(99)77442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tjernberg LO, Callaway DJ, Tjernberg A, Hahne S, Lilliehöök C, Terenius L, Thyberg J, Nordstedt C. A molecular model of Alzheimer amyloid beta-peptide fibril formation. J Biol Chem. 1999;274(18):12619–12625. doi: 10.1074/jbc.274.18.12619. [DOI] [PubMed] [Google Scholar]
- 90.Lazo ND, Downing DT. Amyloid fibrils may be assembled from beta-helical protofibrils. Biochemistry. 1998;37(7):1731–1735. doi: 10.1021/bi971016d. [DOI] [PubMed] [Google Scholar]
- 91.Tycko R. In: Proteopathic Seeds and Neurodegenerative Diseases. Jucker M, Christen Y, editors. Springer; Berlin Heidelberg: 2013. pp. 19–31. [Google Scholar]
- 92.Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJW, McFarlane HT, Madsen AØ, Riekel C, Eisenberg D. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 93.Estrada LD, Soto C. Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr Top Med Chem. 2007;7:115–26. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- 94.Cuanalo-Contreras K, Mukherjee A, Soto C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:638083. doi: 10.1155/2013/638083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2002;1:1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- 96.Betts V, Leissring MA, Dolios G, Wang R, Selkoe DJ, Walsh DM. Aggregation and catabolism of disease-associated intra-Abeta mutations: reduced proteolysis of AbetaA21G by neprilysin. Neurobiol Dis. 2008;31:442–50. doi: 10.1016/j.nbd.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venugopal C, Demos CM, Rao KS, Pappolla MA, Sambamurti K. Beta-secretase: structure, function, and evolution. CNS Neurol Disord Drug Targets. 2008;7:278–94. doi: 10.2174/187152708784936626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3:71–3. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 99.Sambamurti K, Suram A, Venugopal C, Prakasam A, Zhou Y, Lahiri DK, Greig NH. A partial failure of membrane protein turnover may cause Alzheimer’s disease: a new hypothesis. Curr Alzheimer Res. 2006;3:81–90. doi: 10.2174/156720506775697142. [DOI] [PubMed] [Google Scholar]
- 100.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 101.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–10. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jean L, Lee CF, Shaw M, Vaux DJ. Structural elements regulating amyloidogenesis: a cholinesterase model system. PLoS One. 2008;3(3):e1834. doi: 10.1371/journal.pone.0001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diamant S, Podoly E, Friedler A, Ligumsky H, Livnah O, Soreq H. Butyrylcholinesterase attenuates amyloid fibril formation in vitro. Proc Natl Acad Sci U S A. 2006;103(23):8628–33. doi: 10.1073/pnas.0602922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330(6006):980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. The presence of Aβ seeds, and not age per se, is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol. 2012;123(1):31–7. doi: 10.1007/s00401-011-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welzel AT, Walsh DM. Aberrant protein structure and diseases of the brain. Ir J Med Sci. 2011;180:15–22. doi: 10.1007/s11845-010-0606-z. [DOI] [PubMed] [Google Scholar]
- 108.Vandrovcova J, Anaya F, Kay V, Lees A, Hardy J, de Silva R. Disentangling the role of the tau gene locus in sporadic tauopathies. Curr Alzheimer Res. 2010;7:726–34. doi: 10.2174/156720510793611619. [DOI] [PubMed] [Google Scholar]
- 109.Kolarova M, García-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012 doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 111.Ballatore C, Brunden KR, Huryn DM, Trojanowski JQ, Lee VM, Smith AB., 3rd Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J Med Chem. 2012;55(21):8979–96. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mondragón-Rodríguez S, Basurto-Islas G, Binder LI, García-Sierra F. Conformational changes and cleavage; are these responsible for the tau aggregation in Alzheimer’s disease? Future Neurology. 2009;4(1):39–53. [Google Scholar]
- 113.Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta. 2005;1739(2–3):216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Kuhla B, Haase C, Flach K, Lüth H-J, Arendt T, Münch G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem. 2007;282(10):6984–6991. doi: 10.1074/jbc.M609521200. [DOI] [PubMed] [Google Scholar]
- 115.Carrell RW, Gooptu B. Conformational changes and disease--serpins, prions and Alzheimer’s. Curr Opin Struct Biol. 1998;8(6):799–809. doi: 10.1016/s0959-440x(98)80101-2. [DOI] [PubMed] [Google Scholar]
- 116.Sadqi M, Hernández F, Pan U, Pérez M, Schaeberle MD, Avila J, Muñoz V. Alpha-helix structure in Alzheimer’s disease aggregates of tau-protein. Biochemistry. 2002;41(22):7150–7155. doi: 10.1021/bi025777e. [DOI] [PubMed] [Google Scholar]
- 117.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A. 2013;110(23):9535–40. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lasagna-Reeves CA, Castillo-Carranza DL, Jackson GR, Kayed R. Tau oligomers as potential targets for immunotherapy for Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2011;8(6):659–65. doi: 10.2174/156720511796717177. [DOI] [PubMed] [Google Scholar]
- 120.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013;587(6):717–23. doi: 10.1016/j.febslet.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20(2):130–8. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 124.Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154(1):103–17. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 126.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 127.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ebrahimi-Fakhari D, Saidi LJ, Wahlster L. Molecular chaperones and protein folding as therapeutic targets in Parkinson’s disease and other synucleinopathies. Acta Neuropathol Commun. 2013;1(1):79. doi: 10.1186/2051-5960-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 131.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 132.Tenreiro S, Eckermann K, Outeiro TF. Protein phosphorylation in neurodegeneration: friend or foe? Front Mol Neurosci. 2014;7:42. doi: 10.3389/fnmol.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 134.Olivares D, Huang X, Branden L, Greig NH, Rogers JT. Physiological and pathological role of alpha-synuclein in Parkinson’s disease through iron mediated oxidative stress; the role of a putative iron-responsive element. Int J Mol Sci. 2009;10(3):1226–60. doi: 10.3390/ijms10031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE. Secondary structure of alpha-synuclein oligomers: characterization by raman and atomic force microscopy. J Mol Biol. 2006;355(1):63–71. doi: 10.1016/j.jmb.2005.10.071. [DOI] [PubMed] [Google Scholar]
- 136.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40(26):7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 137.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luk KC, Kehm V, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209(5):975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(Pt 4):1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9(3):193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 141.WHO. Diabetes. http://www.who.int/mediacentre/factsheets/fs312/en/
- 142.Diabetes and Impaired Glucose Tolerance. http://www.idf.org/diabetesatlas/diabetes-and-impaired-glucose-tolerance.
- 143.Sue Kirkman M, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS, Older A Consensus Development Conference on D. Diabetes in older adults: a consensus report. Journal of the American Geriatrics Society. 2012;60(12):2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Effects of Intensive Glucose Lowering in Type 2 Diabetes. New England Journal of Medicine. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gupta V, Kalra S. Choosing a Gliptin. Indian Journal of Endocrinology and Metabolism. 2011;15(4):298–308. doi: 10.4103/2230-8210.85583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014 doi: 10.1111/dom.12354. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 147.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586–99. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer’s disease. Curr Alzheimer Res. 2005;2(3):377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- 149.Mikhail N. Effects of incretin-based therapy in patients with heart failure and myocardial infarction. Endocrine. 2014 doi: 10.1007/s12020-014-0175-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 150.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Buresh R. Exercise and glucose control. J Sports Med Phys Fitness. 2014;54(4):373–82. [PubMed] [Google Scholar]
- 152.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014 doi: 10.1016/j.trsl.2014.05.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 153.Matiello R, Fukui RT, Silva MER, Rocha DM, Wajchenberg BL, Azhar S, Santos RF. Differential regulation of PGC-1α expression in rat liver and skeletal muscle in response to voluntary running. Nutrition & Metabolism. 2010;7(1) doi: 10.1186/1743-7075-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Höppener JW, Ahrén B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343(6):411–9. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 155.Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587(8):1119–27. doi: 10.1016/j.febslet.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pillay K, Govender P. Amylin uncovered: a review on the polypeptide responsible for type II diabetes. Biomed Res Int. 2013;2013:826706. doi: 10.1155/2013/826706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marzban L, Trigo-Gonzalez G, Verchere CB. Processing of pro-islet amyloid polypeptide in the constitutive and regulated secretory pathways of beta cells. Mol Endocrinol. 2005;19:2154–2163. doi: 10.1210/me.2004-0407. [DOI] [PubMed] [Google Scholar]
- 158.Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci. 2012;69:1947–1965. doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu C, Shea JE. Structural similarities and differences between amyloidogenic and non-amyloidogenic islet amyloid polypeptide (IAPP) sequences and implications for the dual physiological and pathological activities of these peptides. PLoS Comput Biol. 2013;9(8):e1003211. doi: 10.1371/journal.pcbi.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao P, Tu LH, Abedini A, Levsh O, Akter R, Patsalo V, Schmidt AM, Raleigh DP. Sensitivity of Amyloid Formation by Human Islet Amyloid Polypeptide to Mutations at Residue 20. J Mol Biol. 2012;421:282–295. doi: 10.1016/j.jmb.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abedini A, Raleigh DP. Destabilization of human IAPP amyloid fibrils by proline mutations outside of the putative amyloidogenic domain: is there a critical amyloidogenic domain in human IAPP? J Mol Biol. 2006;355:274–281. doi: 10.1016/j.jmb.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 162.Fox A, Snollaerts T, Errecart Casanova C, Calciano A, Nogaj LA, Moffet DA. Selection for nonamyloidogenic mutants of islet amyloid polypeptide (IAPP) identifies an extended region for amyloidogenicity. Biochemistry. 2010;49:7783–7789. doi: 10.1021/bi100337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dupuis NF, Wu C, Shea JE, Bowers MT. Human islet amyloid polypeptide monomers form ordered β-hairpins: a possible direct amyloidogenic precursor. J Am Chem Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hayden MR. Islet amyloid, metabolic syndrome, and the natural progressive history of type 2 diabetes mellitus. JOP: Journal of the pancreas. 2002;3(5):126–138. [PubMed] [Google Scholar]
- 166.De Lorenzi E, Giorgetti S, Grossi S, Merlini G, Caccialanza G, Bellotti V. Pharmaceutical strategies against amyloidosis: old and new drugs in targeting a “protein misfolding disease”. Current medicinal chemistry. 2004;11(8):1065–1084. doi: 10.2174/0929867043455549. [DOI] [PubMed] [Google Scholar]
- 167.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. The Lancet. 1996;347(8999):444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 168.Lemere CA, Oh J, Stanish HA, Peng Y, Pepivani I, Fagan AM, Yamaguchi H, Westmoreland SV, Mansfield KG. Cerebral amyloid-beta protein accumulation with aging in cotton-top tamarins: a model of early Alzheimer’s disease? Rejuvenation research. 2008;11(2):321–332. doi: 10.1089/rej.2008.0677. [DOI] [PubMed] [Google Scholar]
- 169.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 170.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive Cerebral Neovascularization in a Model of Type 2 Diabetes. Diabetes. 2010;59(1):228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.ElAli A, Theriault P, Prefontaine P, Rivest S. Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral beta-amyloid brain entry and aggregation. Acta Neuropathologica Communications. 2013;1 doi: 10.1186/2051-5960-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mukherjee E, Carroll R, Matfin G. Endocrine and Metabolic Emergencies: Hypoglycaemia. Therapeutic Advances in Endocrinology and Metabolism. 2011;2(2):81–93. doi: 10.1177/2042018811401644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 174.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–76. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 175.Morley JE, Farr SA. The role of amyloid-beta in the regulation of memory. Biochem Pharmacol. 2014;88(4):479–85. doi: 10.1016/j.bcp.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 176.Lam AR, Rodriguez JJ, Rojas A, Scheraga HA, Mukamel S. Tracking the Mechanism of Fibril Assembly by Simulated Two-Dimensional Ultraviolet Spectroscopy. The journal of physical chemistry A. 2013;117(2):342–350. doi: 10.1021/jp3101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–72. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 178.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S12–25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24(9):505–14. doi: 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 181.Becker RE, Greig NH. Lost in translation: neuropsychiatric drug development. Sci Transl Med. 2010;2(61):61rv6. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Becker RE, Greig NH. A new regulatory road-map for Alzheimer’s disease drug development. Curr Alzheimer Res. 2014;11(3):215–20. doi: 10.2174/156720501103140329210642. [DOI] [PMC free article] [PubMed] [Google Scholar]