Summary
Background
Acetabular chondral defect are very frequently associated to FAI. Treatment options are still questionable.
Methods
Between 2008 and 2014, 201 patients over 583 have been arthroscopically treated with the AMIC procedure for grade III and/or IV acetabular chondral lesions. Patients age was between 18 and 50 years; acetabular chondral lesion size was between 2 and 4 cm2; radiological Tönnis degree of osteoarthritis was ≤ 2.
Results
The mean follow up of the entire group of 201 patients was 5 years (from 8 to 2). Significant improvement, as measured by the mHHS, was observed at 6 months in comparison to preoperative levels (80.3 ± 8.3) (p<0.001). Continuous improvement with respect to each previous evaluation time point was seen, reaching the highest improvement level at the three year follow-up (85.5 ± 7.2). The mean mHHS improvement recorded at the five year follow-up compared with preoperative scores was 39.1 ± 5.9.
Conclusions
AMIC is a valid procedure to repair medium-sized chondral defects on the acetabular side of the hip found during treatment of FAI and lead to long-term favourable outcomes.
Level of evidence
IV.
Keywords: hip chondral defects, AMIC (Autologous Membrane Induced Chondrogenesis), FAI (Femoro Acetabular Impingemet), hip arthroscopy
Introduction
During the past decade a dramatic change has occurred in the diagnosis and treatment of hip pathologies. While many new pathoanatomic features, such as femoroacetabular impingement (FAI), labral pathologies and developmental dysplasia have been described, hip arthroscopy has become popular as an attractive alternative to the more invasive open surgeries1.
There is actually agreement in considering chondral lesions as a consequence of other pathological features such as trauma, osteonecrosis, dysplasia, labral tears, loose bodies, dislocation, previous slipped capital femoral epiphysis and Femoro Acetabular Impingement (FAI)2,3. In recent years, FAI in particular, gained growing attention and has been indicated as a cause of osteoarthritis of the hip4,5. According to this theory, the altered morphology of the femur and/or of the acetabulum results in abnormal contact against the joint, thereby leading to stress degeneration of the labrum and cartilage.
Many options for biologic joint reconstruction as an alternative to arthroplasty exist, such as microfractures, osteochondral autograft transplantation, mosaicplasty, autologous chondrocyte implantation, etc6. Most of these techniques have a long and successful clinical history7–10.
In contrast, in the case of hip surgery, excellent results have been reported using the arthroscopic treatment of FAI, removal of symptomatic loose bodies and many other intra and extra articular hip pathologies. Nevertheless, concerns remain about the correct treatment of chondral lesions11,12.
This article discusses the innovative option for the treatment of chondral lesions in the hip with the AMIC technique.
Material and methods
Between 2008 and 2014, 201 patients over 583 treated with hip arthroscopy, underwent an AMIC procedure for the treatment of grade III and/or IV acetabular chondral lesions, according to the Outerbridge classification13. All these 201 patients included in this retrospective, non randomized study have been selected according to the following inclusion criteria: chondral lesions were located in the superior area of the acetabulum; they were consequent to FAI; Patients age was between 18 and 50 years; acetabular chondral lesion size was between 2 and 4 cm2; radiological Tönnis degree of osteoarthritis was ≤ 2. Exclusion criteria were: concomitant presence of femoral head chondral lesion; systemic rheumatoid diseases; dysplasia; femoral neck axial deviations; coxa profunda and/or protrusio acetabuli. For FAI cam-type, arthroscopic femoral head-neck resection arthroplasty was performed to restore the anatomic offset between the femoral head and neck. In case of FAI pincer-type, arthroscopic acetabular rim trimming was performed to reduce the bony overhang and to reshape the acetabulum into its normal contour. An eventual detached labrum was reattached to the superior acetabular rim with suture anchors. Intra operative dynamic tests were performed moving the hip along its full range of motion, checking the absence of any remaining bony impingement.
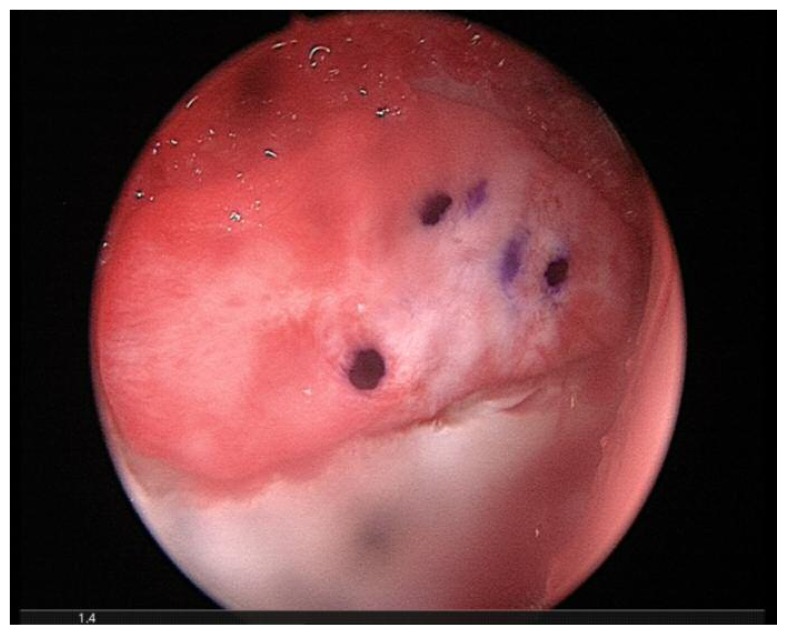
The AMIC procedure was performed arthroscopically in a single surgical stage14,15. The chondral defect was measured with an arthroscopic probe and standard microfracures (MFx) were carried out. Bone marrow bleeding from the holes was verified after removing the fluids from the joint space by continuous aspiration. Destroyed and unstable cartilage was removed using angled curettes or motorised shavers to achieve a well-contained defect. The Chondro-Gide® matrix, a resorbable bilayer collagen I/III membrane (ChondroGide®, Geistlich Pharma AG, Wolhusen, Switzerland), was cut to fit the size and the shape of the lesion and placed on the chondral defect with the porous layer facing the bone surface through an arthroscopic cannula (Figure 1). The post-operative rehabilitation programme started on the first post-op day. Patients began rehabilitation with isotonic and isometric quadriceps and gluteus contractions. Walking was allowed with the aid of two crutches with partial weight-bearing (30% of body weight) on the operated leg for three weeks. Cycling exercises started from post-operative day two, swimming was allowed after two weeks. At four weeks post-op, walking with the aid of one crutch opposite to the treated leg was allowed for seven days, then normal walking thereafter. Impact sport activity could resume at three months post-op and complete return to sport activities was allowed six months after surgery. All patients were assessed preoperatively and at follow-up after 6, 12 months and than yearly, using the modified Harris Hip Score (mHHS)16. The mHHS assesses hip function with a maximum score of 91. Our results were rated as follows: excellent (81–91), good (71–80), fair (61–70) and poor (less than 60)17. The study was approved by the IRB of the Institute and all patients gave their consent to the data collection and publication.
Figure 1.
Chondro gide membrane is applied to cover the acetabular chondral defect. The implant has been marked on its smooth surface to allow the correct placement of its rugh face in contact with the subchondral bone.
Results
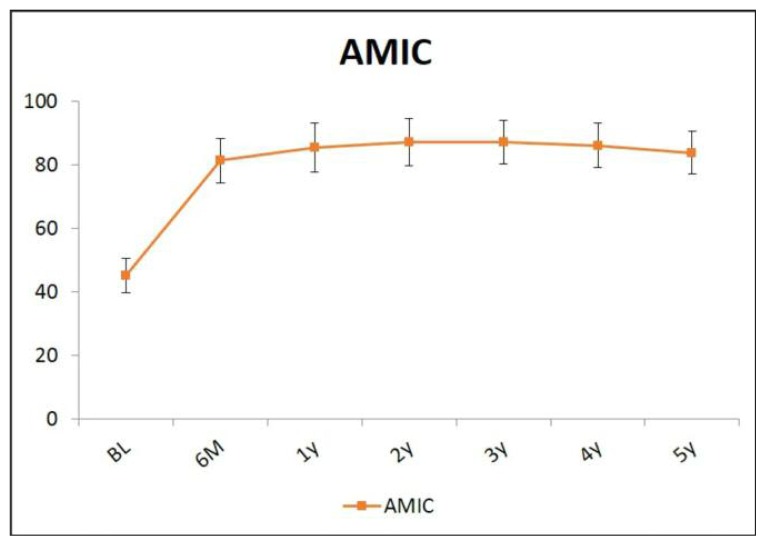
The mean follow up of the entire group of 201 patients was 5 years (from 8 to 2). The average age was 36.4±10.3 years. The mean defect size was 2.9±0.8 cm2. Pre-operative mHHS had a mean score of 44.9±5.9. Significant improvement, as measured by the mHHS, was observed at 6 months in comparison to preoperative levels (80.3±8.3) (p<0.001). Continuous improvement with respect to each previous evaluation time point was seen, reaching the highest improvement level at the three years follow-up (85.5±7.2). The mHHS then remained stable over time until the final years follow-up (Figure 2). At each of the 12, 24, 36, 48 and 60 months time points, the mHHS was significantly higher in comparison to the 6 month values. The mean mHHS improvement recorded at the 5 years follow-up compared with preoperative scores was 39.1±5.9. No patient had a poor postoperative mHHS (>60).
Figure 2.
Pre-operative mHHS and up to 5 years after AMIC.
No significant complications were reported and no failure resulting in hip arthroplasty was detected in any of these patients during the five year follow-up.
Discussion
Our study reports on long-term clinical outcomes for repair of 2–4cm2 chondral lesions with the AMIC technique, assessed by mHHS. Significant improvements were demonstrated up to the 5 years follow-up, demonstrating that AMIC can be reliably extended to 4 cm2 defects. Several arthroscopic techniques have been used to treat full-thickness chondral defects of the hip in the past few decades. However, due to the introduction of hip arthroscopy for the treatment of FAI, published outcome studies on hip cartilage repair are scarce and comprehensive evidence-based treatment guidelines for chondral lesions of the hip remain to be defined. MFx is still currently the first choice treatment for both acetabular and femoral head small chondral defects (≤ 2 cm2) as it is not invasive and very rarely has it been associated with major post-operative complications. Satisfactory clinical results after MFx of the hip2,11,18–20, including athletes9,21, have been recently reported; however, the follow-up reported for MFx in the hip did not exceed two years9,19,22. Thus, clinical data relating lesion size, treatment choice and evaluation of cartilage repair procedures beyond two years are of critical importance to determine predictable and sustainable therapy for chondral cartilage defects of the hip. Literature on the use of MACI for the treatment of medium-sized chondral defects in the hip is particularly scarce. The first case report by Akimau et al.23 described the treatment of an extensive loss of cartilage and osteonecrosis in the hip treated with bone grafting and MACI using Chondro-Gide®. Larger controlled retrospective studies, comparing arthroscopic AMIC with debridement14 and with MFx24 for the treatment of grade III/IV acetabular chondral defects larger than 2cm2, have been recently reported demonstrating very good results at 5 years of follow up in the group treated with the AMIC technique.
The AMIC technique spares donor site morbidity since effective cartilage regeneration can be stimulated in a single surgical intervention without the need for harvesting cells from a second site. AMIC exploits the regenerative potential of mesenchymal progenitor cells deriving from subchondral bone. The collagen type I/III matrix used in AMIC protects the blood clot and supplies the regenerating site with a proper microenvironment supporting cell adhesion, growth and differentiation. Collagen matrices have previously been shown to support chondrogenic differentiation of mesenchymal stem cells25 and to maintain chondrocyte phenotype26, in particular when the matrix is composed of collagen types I and III27. The AMIC technique is further beneficial because it eliminates the need for specialised centres and laboratory support to cultivate cells, in turn reducing total therapy time and overall cost, compared to two-stage procedures such as MACI. Our findings are in line with the results deriving from the treatment of cartilage defects of the knee and talus with the AMIC technique28–31. Gille et al.28 showed that patients affected by large grade IV chondral lesions experienced significant improvement in terms of five different evaluation scores at 12 months and up to 24 months after the AMIC procedure. Satisfactory outcomes were also reported for osteochondral lesions of the talus treated by AMIC32,33.
Until now, long-term outcome data for the treatment of chondral defects of the hip using the AMIC treatment were not available. One of the limitations of our study is that it is a retrospective analysis of data collected over years of treatment. Another limitation is that clinical improvement was evaluated based only on the mHHS. Although this test has high validity and reliability16, it is most suitable for assessment of functionality in elderly arthritic patients and might not be sensitive enough to assess subtle changes in function in young, otherwise healthy patients. Nevertheless, the improvement in these scores suggests that AMIC provides clinical benefits to patients affected by a chondral lesion consequent to femoral acetabular impingement.
Other parameters like age and weight of the patient and morphology of the hip and soft tissues have not been deeply evaluated in this study. Those factors can influence the the results and should be taken in consideration. The study was conducted according to international the ethical standards34.
Conclusions
In conclusion, the AMIC technique allowed a marked clinical improvement in patients affected by chondral defects due to FAI. This study suggests that AMIC is a valid procedure to repair medium-sized chondral defects on the acetabular side of the hip found during treatment of FAI and lead to long-term favorable outcomes. AMIC, due to its minimal invasiveness, single-stage procedure and proven safety, may be considered as a first choice treatment with respect to minimizing therapy time and costs.
Table l.
Baseline characteristics of the study groups
AMIC (n=201) p
|
References
- 1.Sussman PS, Ranawatt AS, Lipman J, et al. Arthroscopic versus open osteoplasty of the head-neck junction: a cadaveric investigation. Arthroscopy. 2007;23–12:1257–64. doi: 10.1016/j.arthro.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy JC, Lee J. Hip arthroscopy. Indications and technical pearls. Clin Orthop Rel Res. 2005;441:180–7. doi: 10.1097/01.blo.0000195057.27653.93. [DOI] [PubMed] [Google Scholar]
- 3.Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Current concepts. Arthroscopy. 2008;24–12:1407–21. doi: 10.1016/j.arthro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Rel Res. 2003;417:112–20. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 5.Beck M, Kalhor M, Leunig M, et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87-B:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 6.Cole BJ, Gomoll AH. Alternatives to arthroplasty. Ed Slack Inc; NJ, USA: 2009. Biologic joint reconstruction. [Google Scholar]
- 7.Lienert JJ, Rodkey WG, Steadman JR, Philippon MJ, Sekiya JK. Microfracture techniques in hip arthroscopy. Oper Tech Orthop. 2005;15:267–272. [Google Scholar]
- 8.McGill KC, Bush-Joseph CA, Nho SJ. Hip microfracture: indications, technique, and outcomes. Cartilage. 2010;1:127–136. doi: 10.1177/1947603510366028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25:327–335. doi: 10.1016/j.csm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 11.Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy. 2008;24:46–50. doi: 10.1016/j.arthro.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Leunig M, Tibor L, Naal F, Ganz R, Steinwachs M. Surgical technique: second-generation bone marrow stimulation via surgical dislocation to treat hip cartilage lesions. Clin Orthop Relat Res. 2012;470:3421–3431. doi: 10.1007/s11999-012-2466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–57. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 14.Fontana A, Bistolfi A, Crova M, Rosso F, Massazza G. Arthroscopic treatment of hip chondral defects: autologous chondrocyte transplantation versus simple debridement-a pilot study. Arthroscopy. 2012;28:322–329. doi: 10.1016/j.arthro.2011.08.304. [DOI] [PubMed] [Google Scholar]
- 15.Fontana A. A novel technique for treating cartilage defects in the hip: a fully arthroscopic approach to using autologous matrix induced chondrogenesis. Arthrosc Tech. 2012;1:e63–e68. doi: 10.1016/j.eats.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Bardakos NV, Vasconcelos JC, Villar RN. Early outcome of hip arthroscopy for femoroacetabular impingement: the role of a femoral osteoplasty in symptomatic improvement. J Bone Joint Surg Br. 2008;90:1570–1575. doi: 10.1302/0301-620X.90B12.21012. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan S, Roberts S, Griffin D. Microfracture for acetabular chondral defects in patients with femoro acetabular impingement: results at second-look arthroscopic surgery. Am J Sports Med. 2012;40:2725–2730. doi: 10.1177/0363546512465400. [DOI] [PubMed] [Google Scholar]
- 19.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br. 2010;92:629–633. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 20.Horisberger M, Brunner A, Herzog RF. Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy. 2010;26:623–629. doi: 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Singh PJ, O’Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26:743–749. doi: 10.1016/j.arthro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JW, Jones KS. Osteoarthritis caused by an inverted acetabular labrum: radiographic diagnosis and arthroscopic treatment. Arthroscopy. 2002;18:741–747. doi: 10.1053/jars.2002.32837. [DOI] [PubMed] [Google Scholar]
- 23.Akimau P, Bhosale A, Harrison PE, et al. Autologous chondrocyte implantation with bone grafting for osteochondral defect due to posttraumatic osteonecrosis of the hip-a case report. Acta Orthop. 2006;77:333–336. doi: 10.1080/17453670610046208. [DOI] [PubMed] [Google Scholar]
- 24.Fontana A, de Girolamo L. Sustained five-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. Bone Joint J. 2015 2015 May;97-B(5) doi: 10.1302/0301-620X.97B5.35076. [DOI] [PubMed] [Google Scholar]
- 25.Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62. doi: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Fuss M, Ehlers EM, Russlies M, Rohwedel J, Behrens P. Characteristics of human chondrocytes, osteoblasts and fibroblasts seeded onto a type I/III collagen sponge under different culture conditions. A light, scanning and transmission electron microscopy study. Ann Anat. 2000;182:303–310. doi: 10.1016/S0940-9602(00)80002-3. [DOI] [PubMed] [Google Scholar]
- 27.Gille J, Meisner U, Ehlers EM, Müller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37:339–348. doi: 10.1016/j.tice.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1:65–68. doi: 10.1177/1947603509360044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 30.Gille J, Behrens P, Volpi P, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133:87–93. doi: 10.1007/s00402-012-1621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC) Knee Surg Sports Traumatol Arthrosc. 2012;20:2109–2115. doi: 10.1007/s00167-011-1840-2. [DOI] [PubMed] [Google Scholar]
- 32.Walther M, Martin K. Scaffold based reconstruction of focal full thickness talar cartilage defects. Clin Res Foot Ankle. 2013;1:115. [Google Scholar]
- 33.Wiewiorski M, Miska M, Kretzschmar M, Studler U, Bieri O, Valderrabano V. Delayed gadolinium-enhanced MRI of cartilage of the ankle joint: results after autologous matrix-induced chondrogenesis (AMIC)-aided reconstruction of osteochondral lesions of the talus. Clin Radiol. 2013;68:1031–1038. doi: 10.1016/j.crad.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research: 2016 Update. MLTJ. 2016;6(1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]