Abstract
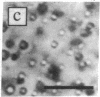
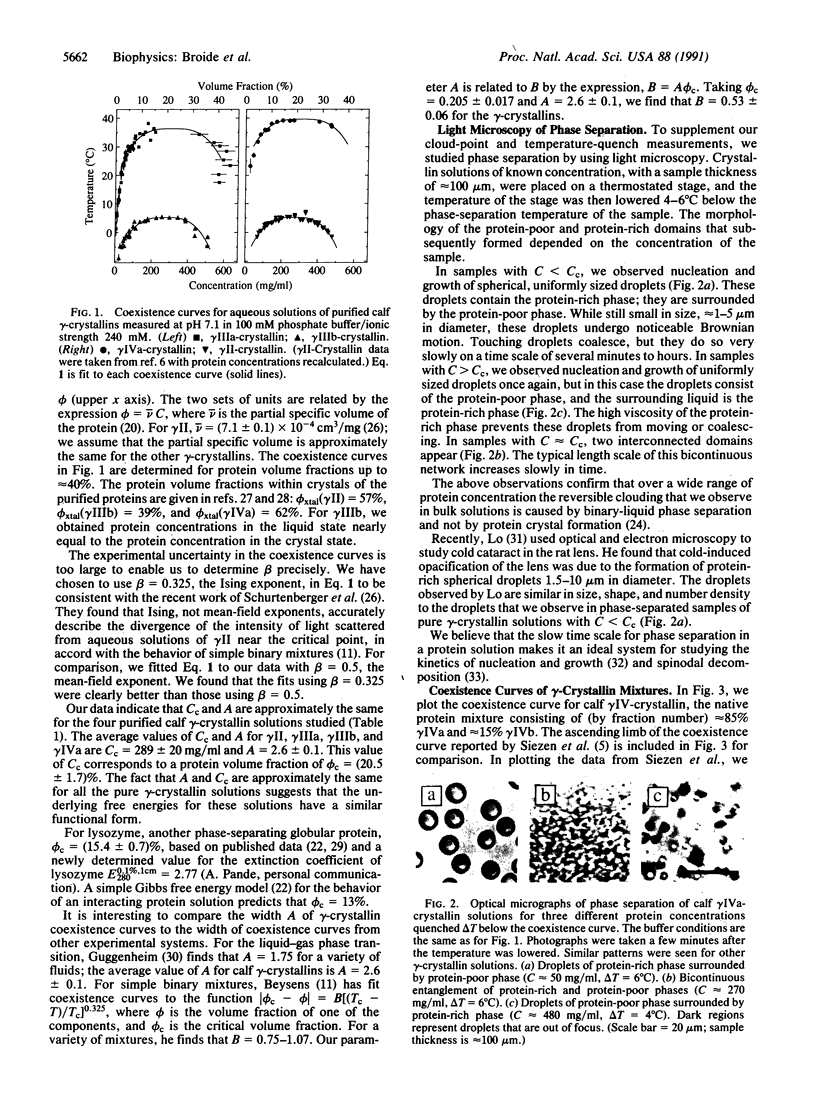
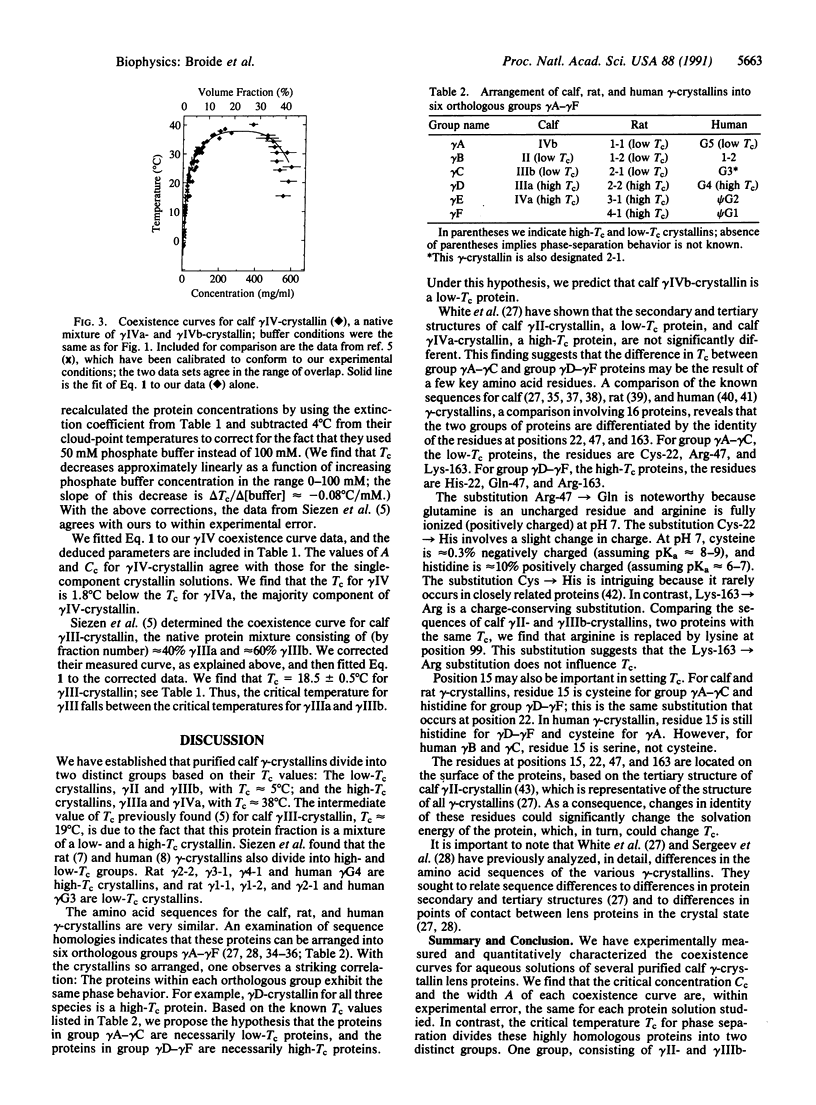
We have determined the coexistence curves (plots of phase-separation temperature T versus protein concentration C) for aqueous solutions of purified calf lens proteins. The proteins studied, calf gamma IIIa-, gamma IIIb-, and gamma IVa-crystallin, have very similar amino acid sequences and three-dimensional structures. Both ascending and descending limbs of the coexistence curves were measured. We find that the coexistence curves for each of these proteins and for gamma II-crystallin can be fit, near the critical point, to the function /(Cc-C)/Cc/ = A [(Tc - T)/Tc]beta, where beta = 0.325, Cc is the critical protein concentration in mg/ml, Tc is the critical temperature for phase separation in K, and A is a parameter that characterizes the width of the coexistence curve. We find that A and Cc are approximately the same for all four coexistence curves (A = 2.6 +/- 0.1, Cc = 289 +/- 20 mg/ml), but that Tc is not the same. For gamma II- and gamma IIIb-crystallin, Tc approximately 5 degrees C, whereas for gamma IIIa- and gamma IVa-crystallin, Tc approximately 38 degrees C. By comparing the published protein sequences for calf, rat, and human gamma-crystallins, we postulate that a few key amino acid residues account for the division of gamma-crystallins into low-Tc and high-Tc groups.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarts H. J., den Dunnen J. T., Leunissen J., Lubsen N. H., Schoenmakers J. G. The gamma-crystallin gene families: sequence and evolutionary patterns. J Mol Evol. 1988;27(2):163–172. doi: 10.1007/BF02138377. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Spector A. Complete nucleotide sequence of a cDNA derived from calf lens gamma-crystallin mRNA: presence of Alu I-like DNA sequences. DNA. 1984 Aug;3(4):287–295. doi: 10.1089/dna.1.1984.3.287. [DOI] [PubMed] [Google Scholar]
- Chirgadze Iu N., Nevskaia N. A., Sergeev Iu V., Fomenkova N. P. Evoliutsionnyi konservatizm molekuliarnoi struktury gamma-kristallinov pozvonochnykh. Mol Biol (Mosk) 1987 Mar-Apr;21(2):368–376. [PubMed] [Google Scholar]
- Clark J. I., Carper D. Phase separation in lens cytoplasm is genetically linked to cataract formation in the Philly mouse. Proc Natl Acad Sci U S A. 1987 Jan;84(1):122–125. doi: 10.1073/pnas.84.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. I., Giblin F. J., Reddy V. N., Benedek G. B. Phase separation of X-irradiated lenses of rabbit. Invest Ophthalmol Vis Sci. 1982 Feb;22(2):186–190. [PubMed] [Google Scholar]
- Cumming A, Wiltzius P, Bates FS. Nucleation and growth of monodisperse droplets in a binary-fluid system. Phys Rev Lett. 1990 Aug 13;65(7):863–866. doi: 10.1103/PhysRevLett.65.863. [DOI] [PubMed] [Google Scholar]
- Guenoun P, Gastaud R, Perrot F, Beysens D. Spinodal decomposition patterns in an isodensity critical binary fluid: Direct-visualization and light-scattering analyses. Phys Rev A Gen Phys. 1987 Nov 15;36(10):4876–4890. doi: 10.1103/physreva.36.4876. [DOI] [PubMed] [Google Scholar]
- Hay R. E., Woods W. D., Church R. L., Petrash J. M. cDNA clones encoding bovine gamma-crystallins. Biochem Biophys Res Commun. 1987 Jul 15;146(1):332–338. doi: 10.1016/0006-291x(87)90729-7. [DOI] [PubMed] [Google Scholar]
- Ishimoto C., Goalwin P. W., Sun S. T., Nishio I., Tanaka T. Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4414–4416. doi: 10.1073/pnas.76.9.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W. K. Visualization of crystallin droplets associated with cold cataract formation in young intact rat lens. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9926–9930. doi: 10.1073/pnas.86.24.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. J., Gawinowicz-Kolks M. A., Chiesa R., Spector A. The disulfide content of calf gamma-crystallin. Arch Biochem Biophys. 1988 May 1;262(2):609–619. doi: 10.1016/0003-9861(88)90413-4. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Breitman M. L., Tsui L. C. Structural and evolutionary relationships among five members of the human gamma-crystallin gene family. Mol Cell Biol. 1985 Jun;5(6):1408–1414. doi: 10.1128/mcb.5.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin S. O., Du R. P., Tsui L. C., Breitman M. L. Gamma-crystallins of the human eye lens: expression analysis of five members of the gene family. Mol Cell Biol. 1987 Aug;7(8):2671–2679. doi: 10.1128/mcb.7.8.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande J., Berland C., Broide M., Ogun O., Melhuish J., Benedek G. Suppression of phase separation in solutions of bovine gamma IV-crystallin by polar modification of the sulfur-containing amino acids. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4916–4920. doi: 10.1073/pnas.88.11.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillies GD. Comment on "Critical behavior of a binary mixture of protein and salt water". Phys Rev Lett. 1985 Sep 16;55(12):1341–1341. doi: 10.1103/PhysRevLett.55.1341. [DOI] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., RHODES C. K., HOLCOMB D. N., VAN HOLDE K. E. Physical studies of lysozyme. I. Characterization. J Biol Chem. 1962 Apr;237:1107–1112. [PubMed] [Google Scholar]
- Schurtenberger P, Chamberlin RA, Thurston GM, Thomson JA, Benedek GB. Observation of critical phenomena in a protein-water solution. Phys Rev Lett. 1989 Nov 6;63(19):2064–2067. doi: 10.1103/PhysRevLett.63.2064. [DOI] [PubMed] [Google Scholar]
- Sergeev Y. V., Chirgadze Y. N., Mylvaganam S. E., Driessen H., Slingsby C., Blundell T. L. Surface interactions of gamma-crystallins in the crystal medium in relation to their association in the eye lens. Proteins. 1988;4(2):137–147. doi: 10.1002/prot.340040207. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Fisch M. R., Slingsby C., Benedek G. B. Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1701–1705. doi: 10.1073/pnas.82.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R. J., Kaplan E. D., Anello R. D. Superior resolution of gamma-crystallins from microdissected eye lens by cation-exchange high-performance liquid chromatography. Biochem Biophys Res Commun. 1985 Feb 28;127(1):153–160. doi: 10.1016/s0006-291x(85)80138-8. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Thomson J. A., Kaplan E. D., Benedek G. B. Human lens gamma-crystallins: isolation, identification, and characterization of the expressed gene products. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6088–6092. doi: 10.1073/pnas.84.17.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R. J., Wu E., Kaplan E. D., Thomson J. A., Benedek G. B. Rat lens gamma-crystallins. Characterization of the six gene products and their spatial and temporal distribution resulting from differential synthesis. J Mol Biol. 1988 Feb 5;199(3):475–490. doi: 10.1016/0022-2836(88)90619-5. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Miller L. R. Purification and crystallization of mammalian lens gamma-crystallins. Exp Eye Res. 1983 Nov;37(5):517–530. doi: 10.1016/0014-4835(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Summers L. J., Slingsby C., Blundell T. L., den Dunnen J. T., Moormann R. J., Schoenmakers J. G. Structural variation in mammalian gamma-crystallins based on computer graphics analyses of human, rat and calf sequences. 1. Core packing and surface properties. Exp Eye Res. 1986 Jul;43(1):77–92. doi: 10.1016/s0014-4835(86)80047-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Rubin S., Sun S. T., Nishio I., Tung W. H., Chylack L. T., Jr Phase separation in rat lenses cultured in low glucose media. Invest Ophthalmol Vis Sci. 1983 Apr;24(4):522–525. [PubMed] [Google Scholar]
- Thomson J. A., Schurtenberger P., Thurston G. M., Benedek G. B. Binary liquid phase separation and critical phenomena in a protein/water solution. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7079–7083. doi: 10.1073/pnas.84.20.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. E., Driessen H. P., Slingsby C., Moss D. S., Lindley P. F. Packing interactions in the eye-lens. Structural analysis, internal symmetry and lattice interactions of bovine gamma IVa-crystallin. J Mol Biol. 1989 May 5;207(1):217–235. doi: 10.1016/0022-2836(89)90452-x. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Moormann R. J., Lubsen N. H., Schoenmakers J. G. Concerted and divergent evolution within the rat gamma-crystallin gene family. J Mol Biol. 1986 May 5;189(1):37–46. doi: 10.1016/0022-2836(86)90379-7. [DOI] [PubMed] [Google Scholar]