Abstract
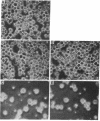
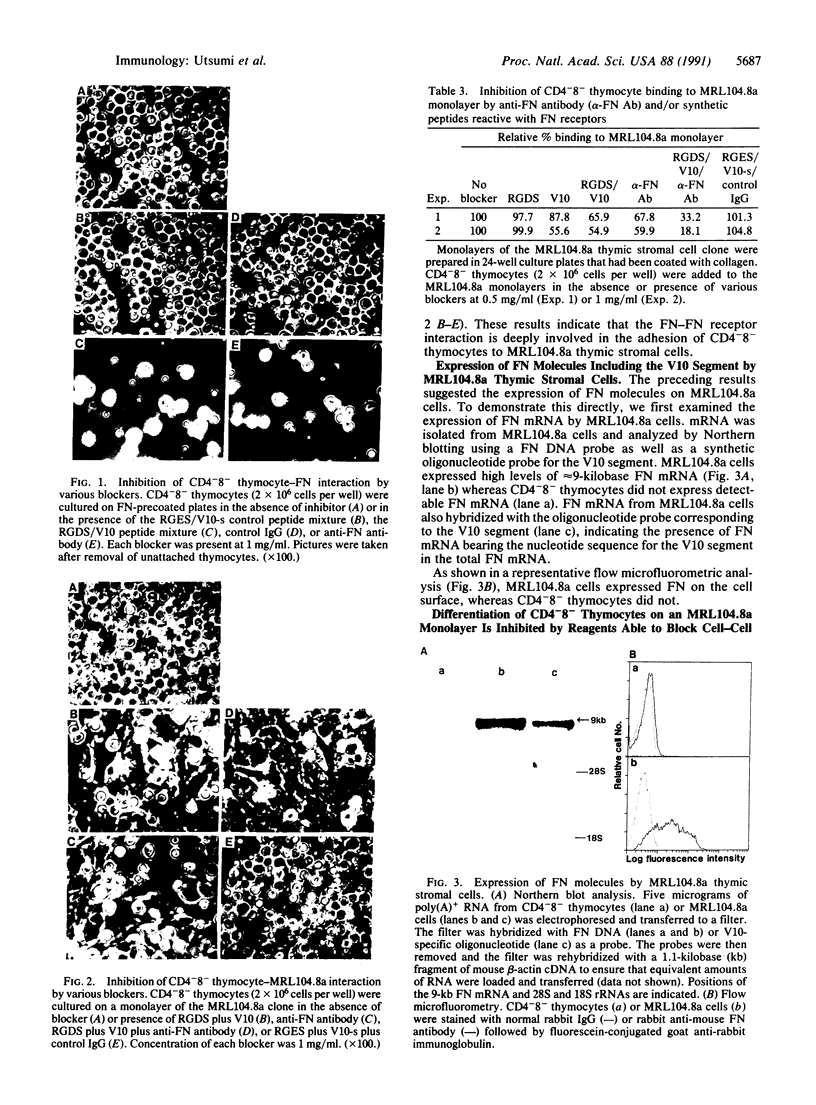
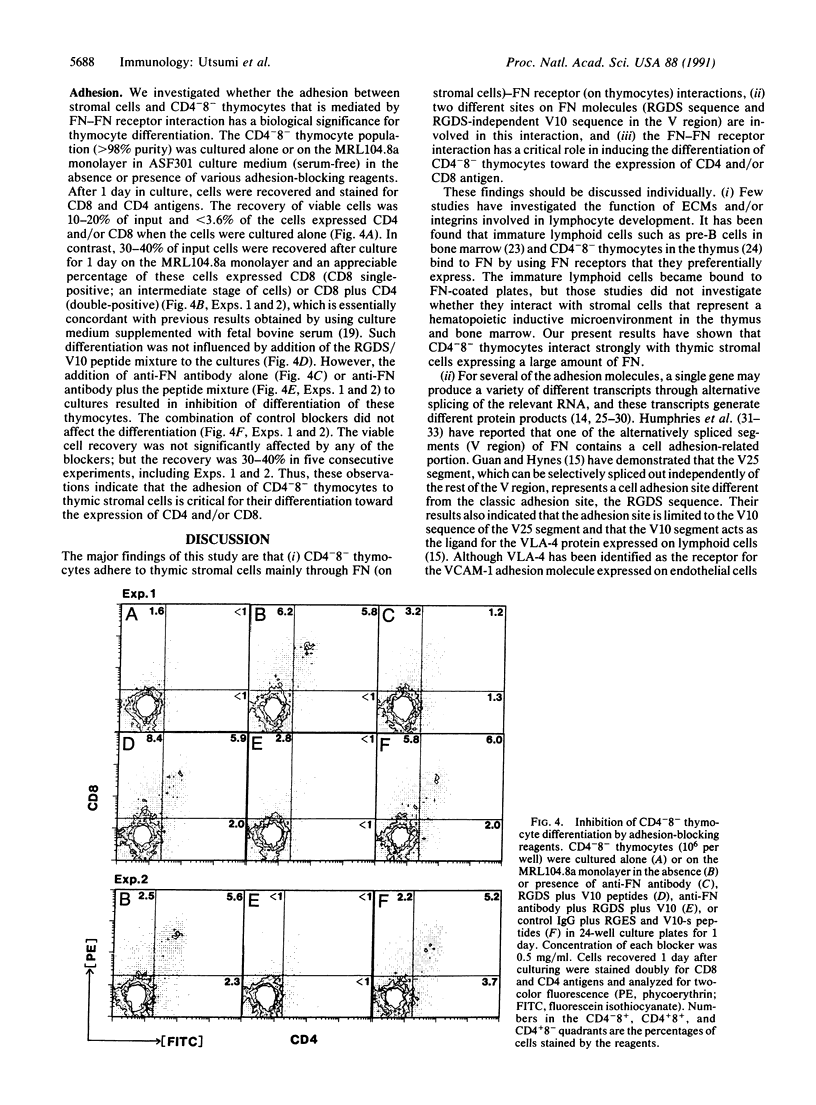
Only 10-15% of unseparated thymocytes adhered to culture plates precoated with fibronectin (FN), but 60-70% of the CD4-8- (double-negative) thymocyte population did. This population bound to FN but not to collagen, laminin, or vitronectin. Its binding to FN was inhibited by anti-FN antibody or a mixture of synthetic peptides corresponding to two different sites of FN, termed the V10 sequence and the RGDS (Arg-Gly-Asp-Ser) sequence, which interact, respectively, with the VLA-4 and VLA-5 FN receptors expressed on T-lineage cells. CD4-8- thymocytes also adhered to a monolayer of a thymic stromal cell clone, MRL104.8a, that induces growth-maintenance and differentiation of such thymocytes. The involvement of FN-FN receptor interaction in this adhesion was demonstrated by the following lines of evidence: (i) the MRL104.8a cells expressed FN molecules on their surface and (ii) the adhesion of CD4-8- thymocytes to MRL104.8a monolayers was almost completely inhibited by simultaneous addition of anti-FN antibody and a mixture of peptides (V10 plus RGDS) capable of binding to anti-FN receptors (VLA-4 and -5). Most important, blocking the adhesion of CD4-8- thymocytes to the thymic stromal cell monolayer resulted in potent inhibition of the differentiation of these thymocytes, which was otherwise induced toward the expression of CD4 and/or CD8 molecules. These results indicate that immature CD4-8- thymocytes adhere to thymic stromal cells preferentially through FN-FN receptor interaction and that such adhesion has a critical role in inducing and/or supporting the differentiation of these thymocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. Adhesion molecules and animal development. Experientia. 1990 Jan 15;46(1):2–13. doi: 10.1007/BF01955407. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Kolbe M., Weil D., Chu M. L. Human cellular fibronectin: comparison of the carboxyl-terminal portion with rat identifies primary structural domains separated by hypervariable regions. Biochemistry. 1985 May 21;24(11):2698–2704. doi: 10.1021/bi00332a016. [DOI] [PubMed] [Google Scholar]
- Bernardi P., Patel V. P., Lodish H. F. Lymphoid precursor cells adhere to two different sites on fibronectin. J Cell Biol. 1987 Jul;105(1):489–498. doi: 10.1083/jcb.105.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Brower D. L., Jaffe S. M. Requirement for integrins during Drosophila wing development. Nature. 1989 Nov 16;342(6247):285–287. doi: 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Cardarelli P. M., Crispe I. N., Pierschbacher M. D. Preferential expression of fibronectin receptors on immature thymocytes. J Cell Biol. 1988 Jun;106(6):2183–2190. doi: 10.1083/jcb.106.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Mueller S. C. Coupled expression and colocalization of 140K cell adhesion molecules, fibronectin, and laminin during morphogenesis and cytodifferentiation of chick lung cells. J Cell Biol. 1986 Sep;103(3):1073–1090. doi: 10.1083/jcb.103.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990 Aug 1;145(3):785–793. [PubMed] [Google Scholar]
- Dickson G., Gower H. J., Barton C. H., Prentice H. M., Elsom V. L., Moore S. E., Cox R. D., Quinn C., Putt W., Walsh F. S. Human muscle neural cell adhesion molecule (N-CAM): identification of a muscle-specific sequence in the extracellular domain. Cell. 1987 Sep 25;50(7):1119–1130. doi: 10.1016/0092-8674(87)90178-4. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Rocher S., Chen W. T., Yamada K. M., Thiery J. P. Cell adhesion and migration in the early vertebrate embryo: location and possible role of the putative fibronectin receptor complex. J Cell Biol. 1986 Jan;102(1):160–178. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986 Dec;103(6 Pt 2):2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Neurite extension of chicken peripheral nervous system neurons on fibronectin: relative importance of specific adhesion sites in the central cell-binding domain and the alternatively spliced type III connecting segment. J Cell Biol. 1988 Apr;106(4):1289–1297. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Pietrangeli C. E., Hayashi S., Gimble J. M. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 1989;7:111–143. doi: 10.1146/annurev.iy.07.040189.000551. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Ogata M., Hikita I., Maruo S., Sugihara S., Matsubara H., Takai Y., Hamaoka T., Fujiwara H. Model for clonal elimination in the thymus. Proc Natl Acad Sci U S A. 1989 May;86(10):3773–3777. doi: 10.1073/pnas.86.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell. 1989 Mar 24;56(6):1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- MacKrell A. J., Blumberg B., Haynes S. R., Fessler J. H. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidet C., Sémériva M., Yamada K. M., Thiery J. P. Peptides containing the cell-attachment recognition signal Arg-Gly-Asp prevent gastrulation in Drosophila embryos. Nature. 1987 Jan 22;325(6102):348–350. doi: 10.1038/325348a0. [DOI] [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Matsubara H., Takai Y., Kosaka H., Katagiri T., Sano H., Ishimura K., Fujita H., Hamaoka T., Fujiwara H. Capacities of a newly established thymic stromal cell clone to express Ia antigens and to produce interleukin-6, colony-stimulating factor, and thymic stroma-derived T-cell growth factor. J Leukoc Biol. 1989 Jan;45(1):69–78. doi: 10.1002/jlb.45.1.69. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sakata T., Iwagami S., Tsuruta Y., Teraoka H., Tatsumi Y., Kita Y., Nishikawa S., Takai Y., Fujiwara H. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J Leukoc Biol. 1990 Sep;48(3):205–212. doi: 10.1002/jlb.48.3.205. [DOI] [PubMed] [Google Scholar]
- Santoni M. J., Barthels D., Vopper G., Boned A., Goridis C., Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989 Feb;8(2):385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tatsumi Y., Kumanogoh A., Saitoh M., Mizushima Y., Kimura K., Suzuki S., Yagi H., Horiuchi A., Ogata M., Hamaoka T. Differentiation of thymocytes from CD3-CD4-CD8- through CD3-CD4-CD8+ into more mature stages induced by a thymic stromal cell clone. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2750–2754. doi: 10.1073/pnas.87.7.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- van Noesel C., Miedema F., Brouwer M., de Rie M. A., Aarden L. A., van Lier R. A. Regulatory properties of LFA-1 alpha and beta chains in human T-lymphocyte activation. Nature. 1988 Jun 30;333(6176):850–852. doi: 10.1038/333850a0. [DOI] [PubMed] [Google Scholar]